Folate and vitamin B12 status have been directly associated with cognitive performance in cross-sectional and prospective studies( Reference Raman, Tatsioni and Chung 1 – Reference Vogel, Dali-Youcef and Kaltenbach 4 ). Some earlier case reports have observed accelerated neurological deterioration in patients with pernicious anaemia and severe vitamin B12 deficiency after treatment with folic acid( Reference Chodos and Ross 5 , Reference Israels and Wilkinson 6 ). These observations in combination with the known metabolic interrelation of folate and vitamin B12 suggest that the effects of one of these B-vitamins on cognitive performance might be modified by blood concentrations of the other B-vitamin. In line with this, cross-sectional analyses within the National Health and Nutrition Examination Survey (NHANES) study revealed that high serum folate status was generally associated with protection against cognitive impairment; however, individuals with low vitamin B12 status combined with high folate status had an increased risk of cognitive impairment compared with those with a normal status of both vitamins( Reference Morris, Jacques and Rosenberg 7 ).
It has been hypothesised that unmetabolised folic acid, which is likely to be present in individuals living in areas with mandatory folic acid fortification of food items( Reference Bailey, Mills and Yetley 8 , Reference Kalmbach, Choumenkovitch and Troen 9 ), may mask or exacerbate metabolic and clinical consequences of vitamin B12 deficiency( Reference Morris, Jacques and Rosenberg 10 ). The findings from the NHANES study that high folate concentrations were associated with an increased risk of cognitive impairment in individuals with vitamin B12 deficiency are in line with the results from the Framingham Heart Study including individuals unexposed to folic acid fortification( Reference Morris, Selhub and Jacques 11 ). In other large study populations unexposed( Reference Clarke, Birks and Nexo 12 , Reference Clarke, Sherliker and Hin 13 ) or exposed( Reference Miller, Garrod and Allen 14 ) to mandatory folic acid fortification, the NHANES findings could not be confirmed. These previous studies differed from the NHANES study in several aspects. First, they used a lower cut-off value for high folate concentrations; second, they used only one or two cognitive performance tests; third, they may have lacked power; and fourth, they measured a single marker of vitamin B12 status, either total vitamin B12 or holotranscobalamin (holoTC) II. HoloTC II refers to the fraction of circulating vitamin B12 bound to the transporter protein transcobalamin which delivers the vitamin into the cells, and it has been suggested to be a more sensitive and specific marker of vitamin B12 status than total concentrations of vitamin B12 in plasma( Reference Obeid and Herrmann 15 ).
It remains unclear whether the combination of high folate and low vitamin B12 status worsens cognitive performance when compared with that of normal folate and normal vitamin B12 status. We therefore investigated the interaction between various markers of vitamin B12 and folate status in relation to cognitive performance based on six cognitive tests in a large population-based study not exposed to mandatory or voluntary food fortification with folic acid.
Methods
Study population
The study population consisted of apparently healthy residents of Bergen (Norway) born between 1925 and 1927, who participated both in the Hordaland Homocysteine Study in 1992–3 and in the Hordaland Health Study in 1997–9 (http://www.husk.b.uib.no). A total of 2841 community-dwelling elderly individuals were invited to participate in a substudy on cognitive tests in 1997–9, of whom 2203 (77·5 %) agreed. Details of this study have been described elsewhere( 16 – Reference Ueland, Nygard and Vollset 18 ). The study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Regional Committee for Medical Research Ethics of Western Norway. Written informed consent was obtained from all the subjects.
Assessment of cognitive performance
Cognitive performance was assessed at the study location by trained nurses and included six tests( Reference Nurk, Drevon and Refsum 19 ): a modified version of the Mini-Mental State Examination (m-MMSE; global cognition, maximum score 12)( Reference Braekhus, Laake and Engedal 20 , Reference Folstein, Folstein and McHugh 21 ); a modified version of the Digit Symbol Test (perceptual speed, score reflects the number of digits recalled)( Reference Wechsler 22 ); a short form of the Block Design (visuospatial skills, maximum score 16)( Reference Wechsler 22 ); the Kendrick Object Learning Test (episodic memory, maximum score 70)( Reference Kendrick 23 ); an abridged version of the Controlled Oral Word Association Test (access to semantic memory, score reflects the number of words recalled)( Reference Benton and Hamsher 24 ); the Trail Making Test Part A (executive function, score reflects the number of seconds needed to complete the task)( Reference Reitan 25 ). For all tests, a higher score indicates better performance, except for the Trail Making Test Part A where a shorter time used indicates better performance.
Other covariates
Both in 1992–3 and 1997–9, participants underwent a brief health examination including measurements of height and weight. In addition, information on cardiovascular risk factors and lifestyle factors including smoking status (current smokers, ex-smokers or never smokers), consumption of coffee (0–1, 1–4 or more than five cups/d), alcohol use (number of glasses/week) and use of multivitamin or B-vitamin supplements was collected via self-administered questionnaires, as described previously( Reference Refsum, Nurk and Smith 17 , Reference Nygard, Vollset and Refsum 26 ). Users of folic acid supplements were included in the study. Dietary intakes of folate and vitamin B12 were measured in 1997–9 by a quantitative FFQ( Reference Nes, Andersen and Solvoll 27 ).
History of CVD was based on self-reported information on the history of myocardial infarction, angina pectoris and stroke as recorded both in 1992–3 and 1997–9, and on the history of thrombosis and phlebitis as recorded in 1992–3. Of the self-reported CVD cases, 79 % were validated with hospitalisation records used in an earlier study( Reference Nurk, Tell and Vollset 28 ), whereas the remaining 21 % were presumably less severe and did not require hospitalisation or occurred before 1992. A history of hypertension was defined as current or previous use of antihypertensive drugs, and was based on self-reported data collected in 1997–9. Diabetes was based on self-reported information collected in 1997–9. Depression score was assessed in 1997–9 by a seven-item subscale for depression from the Hospital Anxiety and Depression Scale depression subscale (HADS-D)( Reference Zigmond and Snaith 29 ). Educational level was classified as no primary school, primary school ( ≤ 9 years), vocational secondary school (10–12 years), theoretical secondary school (10–12 years), college or university ( < 4 years), and college or university ( ≥ 4 years).
Plasma measurements
Non-fasting EDTA blood samples were collected for the analyses of plasma markers of folate and vitamin B12 status. The EDTA samples were kept at 4°C until centrifugation. Samples collected in 1992–3 were stored at − 20°C for up to 10 years, whereas samples collected in 1997–9 were stored at − 80°C for up to 12 months before analyses. Plasma concentrations of folate and vitamin B12 were determined by microbiological assays( Reference Kelleher and Broin 30 , Reference O'Broin and Kelleher 31 ). A recent study has shown that folate concentrations in plasma are not stable during storage( Reference Hannisdal, Gislefoss and Grimsrud 32 ). However, folate determined as p-aminobenzoyl-glutamate (pABG) equivalents only decreases slowly during storage. We therefore measured pABG equivalents using liquid chromatography–MS/MS in 200 randomly selected samples collected in 1992–3 and 1997–9( Reference Hannisdal, Gislefoss and Grimsrud 32 ). Based on the results of these analyses, we corrected for folate degradation during storage by using separate correction factors for the samples collected at baseline (corrected folate concentration 1992–3 = 5·3373+1·4045 × folate concentration measured in 1992–3) and those collected at follow-up (corrected folate concentration 1997–9 = 8·051+1·101 × folate concentration measured in 1997–9).
Plasma concentrations of methylmalonic acid (MMA), an inverse marker for vitamin B12 status( Reference Lindenbaum, Savage and Stabler 33 , Reference Moelby, Rasmussen and Jensen 34 ), were determined by a modified GC–MS method based on ethylchloroformate derivatisation( Reference Husek 35 ), and plasma concentrations of holoTC II were analysed by microbiological assays( Reference Refsum, Johnston and Guttormsen 36 ). These indicators of vitamin B12 status were only measured in the samples collected in 1997–9.
Plasma total homocysteine (tHcy) concentration was determined using an automated HPLC assay( Reference Fiskerstrand, Refsum and Kvalheim 37 , Reference Refsum, Ueland and Svardal 38 ).
Within-day CV for plasma measurements were < 5 % for concentrations of folate, vitamin B12, MMA, holoTC II and tHcy.
Serum creatinine levels were analysed in the samples collected in 1997–9 by a modification of a liquid chromatography–MS/MS procedure( Reference Holm, Ueland and Kvalheim 39 ). ApoE-ɛ4 genotypes (0, 1 or 2 apoE-ɛ4 alleles) were determined using a one-stage PCR method( Reference Wenham, Price and Blundell 40 ), and methylenetetrahydrofolate reductase genotyping (677C → T) was performed by a real-time PCR( Reference Ulvik and Ueland 41 ).
Statistical analyses
Descriptive analyses
Plasma concentrations of folate and vitamin B12 measured in 1992–3 and 1997–9 were compared with a paired sample t test. Relationships between the different markers of vitamin B12 status measured in 1997–9 were evaluated with Spearman's correlation tests.
Principal component analysis (PCA) was used to create a summary score for cognitive performance that accounted for the correlations between the different cognitive performance tests and, thereby, maximised the explained variance. The number of components to be retained was determined according to two criteria: (1) eigenvalues >1 and (2) Cattell's scree plot which shows the total variance related to each component. For comparison of cognitive performance on the individual tests across the quartiles of the cognitive performance component created with PCA, univariate ANOVA was used.
Quantile regression analyses
In order to investigate the interactions between folate and markers of vitamin B12 status in relation to cognitive performance, multivariate quantile regression and ordinary least-squares regression were used including the cognitive performance component extracted with PCA as the dependent variable. Interactions between folate and markers of vitamin B12 status in relation to cognitive performance were assessed with models including folate, a marker for vitamin B12 status, and their product term reflecting interaction, as continuous, independent variables. The quantile regression technique was used to provide distribution-free tests of whether the associations between folate, vitamin B12 markers and their interactions vary along the cognitive performance distribution. Plasma concentrations of folate and markers of vitamin B12 status were expressed as standardised z-scores to provide comparable associations per sd increase.
Logistic regression analyses
We further studied the interaction by estimating OR for cognitive impairment according to categories of combined folate and vitamin B12 status using logistic regression analyses. Cognitive impairment was defined as the lowest 10th percentile of the combined cognitive performance component as derived from the PCA. We determined quartiles for the markers of folate and vitamin B12 status, and defined the first quartile as ‘low’, the fourth quartile as ‘high’ and the two middle quartiles as ‘normal’. Categories were created in which we combined ‘low’, ‘normal’ or ‘high’ folate status with ‘low’, ‘normal’ or ‘high’ vitamin B12 status. The combination of normal folate and normal vitamin B12 status was used as the reference group. All analyses were adjusted for sex, education level, history of CVD/hypertension, apoE-ɛ4 genotype and creatinine. These covariates were strong predictors for cognitive performance or associated with both B-vitamin levels and cognitive performance, as demonstrated with ANOVA or Pearson's correlation coefficients. BMI, smoking status, consumption of coffee, alcohol use, methylenetetrahydrofolate reductase 677 C → T genotype, diabetes and depression score were associated with either plasma folate or the markers of vitamin B12 or with cognitive performance, but adjusting for these biological and lifestyle factors did not markedly change the results of the analysis, and are therefore not included in the final model.
Descriptive analyses and PCA were performed using SAS version 9.2 (SAS Institute Inc.). Quantile regression analyses were performed in R version 2.13.1 (R Foundation for Statistical Computing) using the package ‘quantreg’. Multiple imputation of missing values was carried out by chained equations with fully conditional specifications using the package ‘mice’. P values < 0·05 were considered as statistically significant.
Results
Characteristics of the study population
The characteristics of the study population in 1997–9 are presented in Table 1. The mean age of the participants was 72·5 years and 44·9 % were men. Of the participants, about one-half (51 %) reported a history of CVD, 17 % suffered from depressive symptoms as indicated by a HADS-D score ≥ 8 and 14 % were current smokers. The median plasma folate concentration measured in 1992–3 was 12·5 (5th–95th percentile 8·7–20·9) nmol/l after correction for folate degradation during storage, which was lower than the concentration measured in 1997–9 (median 15·8 (5th–95th percentile 12·0–34·0) nmol/l) (P for difference < 0·0001). The median plasma vitamin B12 concentration in 1992–3 was 338 (5th–95th percentile 196–595) pmol/l and comparable with the concentration measured in 1997–9 (median 339 (5th–95th percentile 192–651) pmol/l) (P for difference = 0·12). In 1997–9, 4·9 % of the participants had vitamin B12 deficiency, defined as plasma concentrations of vitamin B12 < 150 pmol/l or MMA >0·37 μmol/l. Plasma folate concentrations >30, >45 and >59 nmol/l were observed in 6·6, 1·6 and < 1 % of the participants, respectively. Among the participants with vitamin B12 deficiency, only three had a folate concentration >30 nmol/l.
Table 1 Characteristics of the study population in 1997–1999 (Number of subjects and percentages; mean values and 95 % confidence intervals; median values and 5th and 95th percentiles)

MTHFR, methylenetetrahydrofolate reductase; HADS-D, Hospital Anxiety and Depression Scale depression subscale; MMA, methylmalonic acid; holoTC II, holotranscobalamin; m-MMSE, modified version of the Mini-Mental State Examination; m-DST, modified version of the Digit Symbol Test; m-BD, short form of the Block Design; KOLT, Kendrick Object Learning Test; COWAT, abridged version of the Controlled Oral Word Association Test; TMT-A, Trail Making Test Part A.
* Sample numbers may vary across the different variables due to different numbers of missing data.
† Based on self-reported CVD (myocardial infarction, angina pectoris, stroke, thrombosis and phlebitis) or hypertension at baseline or follow-up.
‡ Based on self-reported diabetes.
§ E2/E4 and E3/E4 genotypes.
∥ Folate concentrations are corrected for degradation during storage( Reference Hannisdal, Gislefoss and Grimsrud 32 ) as explained in the Methods section.
Spearman's correlations (r) between plasma concentrations in 1992–3 and 1997–9 were 0·41 (P< 0·0001) for folate and 0·63 (P< 0·0001) for vitamin B12. Plasma vitamin B12 (in 1997–9) correlated significantly with holoTC II (r 0·66, P< 0·0001) and MMA (r − 0·20, P< 0·0001), and holoTC II also correlated significantly with MMA (r − 0·24, P< 0·0001). Furthermore, in individuals with vitamin B12 concentrations < 274 pmol/l, holoTC II concentrations were decreased and MMA and tHcy concentrations were elevated compared with individuals with vitamin B12 concentrations >274 pmol/l (Table S1, available online). tHcy concentrations were not elevated in participants with high folate concentrations (>18·5 nmol/l) compared with those with folate concentrations ≤ 18·5 nmol/l.
Cognitive performance factors
Median (5th–95th percentile) scores for the six cognitive performance tests are presented in Table 1. Based on m-MMSE scores ≤ 10( Reference Nurk, Drevon and Refsum 19 ), 36 % of the study population suffered from mild cognitive impairment. Eigenvalues and Cattell's scree plot revealed that one component derived by the PCA should be retained, explaining 40·5 % of the total variance, whereas factors 2–4 explained less than 14·6 % each. The factor loading matrix is presented in Table S2 (available online). The first component strongly correlated with all six cognitive tests performed and is further referred to as ‘overall cognitive performance’. Overall cognitive performance scores ranged from − 4·80 to 2·49 with a mean of 0 and a sd of 1. Across the increasing quartiles of overall cognitive performance, the subjects performed better on each individual cognitive test (for all tests, P for difference < 0·0001; data not shown).
Cross-sectional associations of plasma concentrations of folate and vitamin B12 markers with overall cognitive performance assessed in 1997–9
Fig. 1 shows that plasma vitamin B12 is directly associated with cognitive performance at the lower quantiles of the cognitive performance distribution and inversely associated at the upper quantiles of the distribution. However, overall, this association is not significantly different from zero. Furthermore, Fig. 1 indicates a significant direct association of plasma folate, and a significant inverse interaction between plasma concentrations of folate and vitamin B12 in relation to cognitive performance. The associations of sex (male v. female), education (direct), history of CVD/hypertension (inverse) and apoE-ɛ4 status (inverse) with overall cognitive performance were as expected( Reference Anstey and Christensen 42 ). Notably, the associations of folate, education and apoE-ɛ4 with overall cognitive performance were asymmetric with the strongest effects in the lowest ranges of the overall cognitive performance scores.

Fig. 1 Changes in cognitive score according to plasma vitamin B12, folate and other determinants by quantile regression. The y-axis represents the associations between independent variables and the summary score for cognitive performance (based on six cognitive performance tests) extracted with the principal component analysis. The x-axis represents the quantiles of the cognitive performance distribution as observed within the present study population. The direction (positive and negative) and strength of the association are given by the number on the y-axis, whereas the ‘slope’ of the graph demonstrates the asymmetry or tail effects. The black points represent quantile regression fits, the dark-shaded grey zones represent 95 % CI for the estimates. The black horizontal lines at y= 0 indicate no (zero) changes in cognition. An upward or downward slope indicates the highest or lowest response at the upper or lower tail, respectively, of the distribution of the cognitive scores, whereas a horizontal graph below or above zero indicates similar effects through the whole distribution. The red horizontal solid lines represent the ordinary least-squares estimates of the conditional mean effects, and the red horizontal dotted lines represent the conventional 95 % CI for the least-squares estimates. (a) Intercept. (b) Plasma vitamin B12 and (c) folate were given as z-scores; (d) sex categorised as (1) men and (2) women; (e) education as (0) no primary school, (1) primary school ( ≤ 9 years), (2) vocational secondary school (10–12 years), (3) theoretical secondary school (10–12 years), (4) college or university ( < 4 years) and (5) college or university ( ≥ 4 years); (f) history of CVD/hypertension as (1) yes or (0) no; (g) apoE-ɛ4 genotype as 0, 1 or 2 apoE-ɛ4 alleles; (h) creatinine was given as μmol/l and (i) interaction (vitamin B12× folate).
When including holoTC II or MMA instead of plasma vitamin B12 in the model, the quantile regression plots showed non-significant associations between holoTC II and MMA, a non-significant interaction between plasma folate and vitamin B12 markers, but similar associations of the other covariates (data not shown).
Table 2 presents the multivariate-adjusted ordinary least-squares regression estimates for the associations of plasma folate concentrations and the markers of vitamin B12, as well as the interaction between plasma folate concentrations and the markers of vitamin B12 in relation to the overall cognitive performance score. In agreement with Fig. 1, plasma folate was directly associated and plasma vitamin B12 was not associated with the overall cognitive performance score. The negative interaction between plasma concentrations of folate and vitamin B12 indicates that the linear association between plasma folate and the overall cognitive performance score changes at different plasma concentrations of vitamin B12 and vice versa. The observed association for the interaction term suggests that, among the elderly with vitamin B12 concentrations in the lower range of the population, for example 2 sd below the mean, the association between plasma folate and the overall cognitive performance changes from β = 0·097 to β = 0·097 − 2 × ( − 0·058) = 0·213, indicating that the positive effect of plasma folate concentrations on cognition becomes stronger at lower vitamin B12 concentrations. In contrast, among the elderly with vitamin B12 concentrations, 2 sd above the mean, the significant positive association between plasma folate and the overall cognitive performance changes from β = 0·097 to β = 0·097+2 × ( − 0·058) = − 0·019, indicating that the positive effect of plasma folate concentrations on cognition is slightly reduced. The vitamin B12 markers MMA and holoTC II did not show significant associations with the overall cognitive performance factor, nor were any of the interactions with folate significant (Table 2).
Table 2 Cross-sectional associations between plasma concentrations of folate, markers of vitamin B12 status and their interactions in relation to overall cognitive performance measured in 1997–9* (β-Coefficients and standard errors)

HoloTC, holotranscobalamin; MMA, methylmalonic acid.
* Ordinary least-squares regression coefficients adjusted for sex, education, apoE-ɛ4 status, history of CVD/hypertension and creatinine.
† Standardised B-vitamin concentrations (z-scores).
‡ The coefficient for the product term indicates how the association between folate status and the cognitive performance score changes when the concentration of the marker for vitamin B12 status increases.
§ Education level is defined as follows: no primary school; primary school ( ≤ 9 years); vocational secondary school (10–12 years); theoretical secondary school (10–12 years); college or university ( < 4 years); college or university ( ≥ 4 years).
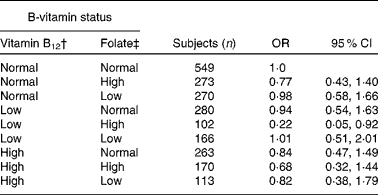
We further evaluated the observed interaction between plasma concentrations of folate and vitamin B12 using logistic regression analysis (Table 3). Plasma vitamin B12 concentrations in the lowest quartile of its distribution ( < 274 pmol/l) in combination with plasma folate concentrations in the highest quartile (>18·5 nmol/l) (n 102) when compared with normal plasma concentrations in the second and third quartiles of both vitamins (n 549) were associated with a reduced risk of cognitive impairment (OR 0·22, 95 % CI 0·05, 0·92).
Table 3 Risk of cognitive impairment according to vitamin B12 and folate status by logistic regression* (Number of subjects, odds ratios and 95 % confidence intervals)
* Adjusted for sex, education, apoE-ɛ4 status, history of CVD/hypertension and creatinine.
† The categories ‘low’, ‘normal’ and ‘high’ plasma vitamin B12 were based on quartiles (Q) and defined as follows: < 274 pmol/l (Q1), 274–432 pmol/l (Q2 and Q3) and >432 pmol/l (Q4), respectively.
‡ The categories ‘low’, ‘normal’ and ‘high’ plasma vitamin folate were based on quartiles (Q) and defined as follows: < 14·1 nmol/l (Q1), 14·1–18·5 nmol/l (Q2 and Q3) and >18·5 nmol/l (Q4), respectively.
Prospective associations of plasma concentrations of folate and total vitamin B12 in 1992–3 with cognitive performance assessed in 1997–9
Multivariate quantile regression and ordinary least-squares regression revealed no significant associations of plasma folate (β = 0·019, se= 0·031, P= 0·540) or plasma vitamin B12 (β = 0·020, se= 0·033, P= 0·541) measured in 1992–3 along the distribution of overall cognitive performance. In addition, the interaction between plasma folate and vitamin B12 sampled 6 years before the measurement of overall cognitive performance was not significant (β = 0·024, se= 0·028, P= 0·394; data not shown).
Discussion
The present population-based study investigated the hypothesis that high folate status in combination with low vitamin B12 status increased the risk for cognitive impairment, as was observed within the NHANES study( Reference Morris, Jacques and Rosenberg 7 ), and was conducted in a population that was not exposed to mandatory or voluntary fortification of food items with folic acid. The study, which included 2203 elderly people aged 71–74 years, revealed that plasma folate, but not plasma vitamin B12, was associated with better cognitive performance. Although the subgroup was rather small, a combination of plasma folate >18·5 nmol/l with vitamin B12 < 274 pmol/l was associated with a reduced risk of cognitive impairment when compared with having normal concentrations of both vitamins.
Methodological considerations
It has been suggested that folate and vitamin B12 are related to different cognitive outcomes, and that there may even also be a difference in outcomes related to indicators of functional vitamin B12 status (MMA and holoTC II) v. total concentrations of vitamin B12 in plasma( Reference Morris 43 ). A major strength of the present study is the use of an extensive cognitive test battery covering global cognition, perceptual speed, visuospatial skills, episodic memory, access to semantic memory and executive function. We derived a cognitive performance component by the PCA, which is a robust measure for cognitive performance that reduces the possibility of measurement error and chance findings( Reference Barnes, Wilson and Schneider 44 , Reference Visser 45 ).
There is currently no ‘gold standard’ to define vitamin B12 deficiency with respect to potential markers and cut-off points to be used( Reference Clarke, Sherliker and Hin 46 , Reference Hoey, Strain and McNulty 47 ), although it has been recommended to include holoTC II and MMA as additional markers of vitamin B12 status( Reference Carmel 48 ). Fedosov( Reference Fedosov 49 ) presented a mathematic model combining the concentrations of vitamin B12, MMA, holoTC II and tHcy to classify vitamin B12 status without the use of pre-defined cut-off levels. In addition, single markers were examined for their potential to predict vitamin B12 deficiency. They showed concentrations of holoTC II and MMA to be more reliable than vitamin B12 or tHcy concentrations for predicting vitamin B12 deficiency. Vogiatzoglou et al. ( Reference Vogiatzoglou, Oulhaj and Smith 50 ) observed that vitamin B12 concentrations below 400 pmol/l were associated with elevated concentrations of tHcy and MMA in the Hordaland Health cohort. In line with this, it has been suggested that desirable blood vitamin B12 concentrations may be higher than the current clinical recommendations since many studies have observed associations between ‘low–normal’ vitamin B12 and several negative health outcomes( Reference Smith and Refsum 51 ). To avoid the use of cut-off levels for low vitamin B12 status, elevated folate status and cognitive impairment, as for the latter two, a consensus definition is lacking as well, we performed the present analyses by quantile regression, in which we plotted the associations between B-vitamins and the overall cognitive score through the distribution of the overall cognitive score. To facilitate comparison with the literature, we additionally created population-based quartile categories to define high and low concentrations of B-vitamins.
Our study group previously demonstrated that folate is degraded in stored samples to compounds that are almost completely recoverable as pAGB( Reference Hannisdal, Gislefoss and Grimsrud 32 ). In the present study, we used the pABG assay( Reference Hannisdal, Ueland and Eussen 52 ) in a subsample and subsequently corrected for folate degradation in the full cohort. Nevertheless, the concentrations measured in 1992–3 were still lower than those measured in 1997–9. This may indicate either improved folate status over time or insufficient correction for folate degradation. Although, the pABG assay has been demonstrated to be the best available method to determine folate status in samples stored for a longer time, about 1 % of folate measured as pABG is lost per year( Reference Hannisdal, Gislefoss and Grimsrud 32 ). Therefore, actual folate concentrations of the participants at the time of blood withdrawal may have been underestimated, and differences between folate concentrations in 1992–3 and 1997–9 may have been smaller than observed. However, it seems unlikely that this would have changed the present results.
Comparison with the literature
The present main observation that a combination of plasma folate >18·5 nmol/l with plasma vitamin B12 < 274 pmol/l was associated with better cognitive performance contrasts the findings from the NHANES( Reference Morris, Jacques and Rosenberg 7 ) and the Framingham Heart Study( Reference Morris, Selhub and Jacques 11 ). The NHANES showed worse cognitive performance in individuals with a combination of high plasma folate (>59 nmol/l) with low vitamin B12 (plasma vitamin B12 < 148 pmol/l or MMA >0·21 μmol/l)( Reference Morris, Jacques and Rosenberg 7 ). Several aspects related to study design might explain this discrepancy. First, the NHANES was conducted in an area of mandatory folic acid fortification, and the use of supplements containing folic acid was common, which led to increased concentrations of serum folate( Reference Choumenkovitch, Jacques and Nadeau 53 ) and unmetabolised folic acid( Reference Yang, Cogswell and Hamner 54 ). In contrast, supplement use was uncommon among Norwegian elderly, and mean folate intake including folate from supplements did not differ from folate intake from foods (Table 1). As a result, very high folate concentrations (>59 nmol/l) were present in 20·7 % of the NHANES study population (n 1459), compared with less than 1 % in the present study.
Second, the Norwegian elderly population had better vitamin B12 status, with only 1·5 % suffering from vitamin B12 deficiency ( ≤ 148 pmol/l), which was used as a cut-off by the NHANES. The unique combination of very high folate with very low vitamin B12 status was present in a relatively small subgroup of forty-two individuals in the NHANES study, and even lower in the present study population (n 3). The better vitamin B12 status of the present study population may be explained by an adequate dietary intake of vitamin B12 (total mean intake 6·73 μg/d), probably related to the high intake of milk and fish in this population as shown previously( Reference Vogiatzoglou, Smith and Nurk 55 ).
The present findings also differed from the results of the Framingham Heart Study, unexposed to mandatory folic acid fortification, that showed a faster rate of decline in MMSE scores associated with having low vitamin B12 plasma concentrations ( < 257 pmol/l) at high folate levels (>20·2 nmol/l) (P interaction < 0·001)( Reference Morris, Selhub and Jacques 11 ). Cut-off levels used to define ‘low’ vitamin B12 and ‘high’ folate status were similar to the present study; however, the Framingham Heart Study population showed more variation in plasma concentrations of both vitamin B12 (18·6–695 pmol/l) and folate (0·54–149 nmol/l), which may explain the discrepant findings.
Overall, the contrasting findings between the NHANES study( Reference Morris, Jacques and Rosenberg 7 ) and the present study, supported by the findings of the Framingham Heart Study( Reference Morris, Selhub and Jacques 11 ), suggest that only supraphysiologic folate status, resulting from fortification or supplemental folic acid intake, may exacerbate adverse cognitive consequences of low plasma vitamin B12 status. This hypothesis is supported by recent data published by Morris( Reference Morris 43 ).
Metabolic effects of a combination of high folate and low vitamin B12 status
Some( Reference Morris, Jacques and Rosenberg 7 , Reference Miller, Garrod and Allen 14 , Reference Selhub, Morris and Jacques 56 , Reference Selhub, Morris and Jacques 57 ), but not all( Reference Clarke, Sherliker and Hin 13 ) studies have shown that elderly individuals with high folate and low vitamin B12 status have a higher prevalence of anaemia, and higher concentrations of tHcy and MMA. It has been proposed that high folate concentrations may exacerbate the negative consequences of vitamin B12 deficiency. It is possible that subjects with a combination of low vitamin B12 and high folate status in these studies( Reference Morris, Jacques and Rosenberg 7 , Reference Clarke, Sherliker and Hin 13 , Reference Miller, Garrod and Allen 14 , Reference Selhub, Morris and Jacques 56 , Reference Selhub, Morris and Jacques 57 ) suffered from severe vitamin B12 deficiency due to disorders that affected vitamin B12 absorption, such as pernicious anaemia( Reference Berry, Carter and Yang 58 , Reference Carmel 59 ). A recent study in healthy young adults without any medical conditions that could induce anaemia or affect folate or vitamin B12 absorption did not observe any adverse effects of high folate concentrations on biochemical markers related to vitamin B12 deficiency( Reference Mills, Carter and Scott 60 ). In our population, MMA concentrations were highest in participants with plasma vitamin B12 in the lowest quartile and folate status in the highest quartile, which is in line with previous findings( Reference Miller, Garrod and Allen 14 , Reference Selhub, Morris and Jacques 57 ). In contrast, tHcy concentration was substantially lower in subjects with folate concentration in the highest quartile (>18·5 nmol/l) when compared with the other quartiles, independent of vitamin B12 status.
The view prevails that low vitamin B12 status is associated with cognitive impairment( Reference Smith and Refsum 3 ). For instance, six decades ago, it was proposed that high folate status in vitamin B12-deficient subjects may deteriorate cognitive performance( Reference Chodos and Ross 5 , Reference Israels and Wilkinson 6 ). The adverse effect of high folate status may be confined to subjects with severe vitamin B12 deficiency, leading to the methylfolate trap( Reference Scott and Weir 61 ). The Hordaland Health population was replete with vitamin B12, and only 4·9 % had plasma concentrations of vitamin B12 < 150 pmol/l or MMA >0·37 μmol/l. Under these conditions, high folate levels may accelerate methionine synthesis, thereby increasing biological methylation including compounds involved in neurotransmission( Reference Weir and Scott 62 ). This is in line with the present observation that folate is associated with better cognitive performance, which remains significant even at vitamin B12 concentrations < 274 pmol/l. We did not observe an interaction of folate with holoTC II and MMA. The utility of the vitamin B12 markers MMA and holoTC II in relation to cognitive performance has been evaluated in some observational studies( Reference Smith and Refsum 3 , Reference Doets, van Wijngaarden and Szczecinska 63 ). The discrepancy between total vitamin B12 and the other markers in the present study could perhaps be related to the fact that total vitamin B12 may be a less sensitive indicator of vitamin B12 status at concentrations above the traditional clinical cut-off level of 150 pmol/l due to buffering capacities of vitamin B12 body stores( Reference Carmel 48 ). Furthermore, similar to the present study, previous studies have observed poor correlations between the different indicators of vitamin B12 status( Reference Carmel, Green and Rosenblatt 64 – Reference Miller, Garrod and Rockwood 67 ). The optimal combinations of markers, cut-off levels and assays for vitamin B12 status assessment have been the topic of debate since many years. Mathematic models combining the results of different markers may provide the best alternative for assessing vitamin B12 status in the future( Reference Fedosov 49 ).
Conclusion
In conclusion, the present large study population unexposed to mandatory or voluntary folic acid fortification showed that plasma folate, but not plasma vitamin B12, was associated with cognitive performance. Among the elderly participants with vitamin B12 concentrations in the lower range, the association between plasma folate and cognitive performance was strongest. No interaction between folate and vitamin B12 status was observed when considering more sensitive markers of vitamin B12 status, MMA and holoTC II. Therefore, the clinical relevance of these observations is uncertain.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S000711451300336X
Acknowledgements
The present study was supported by the Norman Collisson Foundation, UK. The laboratory measurements were supported by the Foundation to Promote Research into Functional Vitamin B12 Deficiency, Norway. Both the Norman Collisson Foundation and the Foundation to Promote Research into Functional Vitamin B12 Deficiency had no role in the design, analysis or writing of this article.
We thank Lisette de Groot for her financial contribution to perform additional analysis on the folate samples. Lisette de Groot contributed to the preparation of the manuscript.
The authors' contributions were as follows: E. L. D. and S. J. P. M. E. designed the research; E. L. D. conducted the research; G. S. T., A. D. S. and H. R. provided the essential materials; E. L. D., S. J. P. M. E. and P. M. U. analysed the data and performed the statistical analysis; E. L. D. and S. J. P. M. E. wrote the paper; P. M. U., G. S. T., S. E. V., O. K. N., P. v. V., L. C. P. G. M. d. G., E. N., H. R. and S. J. P. M. E. provided a critical review of the manuscript; E. L. D. had primary responsibility for the final content. All authors read and approved the final manuscript.
None of the authors has any conflict of interest.






