Introduction
Cribrilinidae Hincks, Reference Hincks1879 is an extremely large family of cheilostome bryozoans including 127 genera and more than 700 living and fossil species to date, accounting for ~3% of total bryozoan diversity (Bock, Reference Bock2020). First appearing ca. 100 Ma, in the Cenomanian, Cribrilinidae underwent a peak of diversification during the Santonian, greatly contributing to the radiation of cheilostomes in the Late Cretaceous (Cheetham, Reference Cheetham1971; Jablonski et al., Reference Jablonski, Lidgard and Taylor1997 and references therein). This family is one of the most species-rich in the present-day Mediterranean (Rosso and Di Martino, Reference Rosso and Di Martino2016), as well as in other regions of the world (e.g., Gordon et al., Reference Gordon, Bock, Souto-Derungs and Reverter-Gil2019). Cribrilinids exhibit a typical and distinctive costate frontal shield, but also high morphological variability, including different types of heteromorphs (avicularia, kenozooids, articulated and non-articulated spines, etc.) and ovicell structures. A future subdivision of Cribrilinidae into several families or subfamilies is very likely. A more accurate definition of certain genera will, however, require a thorough re-examination of the original material, particularly of the numerous Cretaceous representatives (e.g., Taylor and McKinney, Reference Taylor and McKinney2006; Rosso et al., Reference Rosso, Beuck, Vertino, Sanfilippo and Freiwald2018), as well as phylogenetic analyses. Genus and species identification are often based on subtle morphological characters, such as those associated with the zooidal orifice and the suboral bar (e.g., Harmelin, Reference Harmelin1970, Reference Harmelin1978, Reference Harmelin2001, Reference Harmelin2006; Bishop and Househam, Reference Bishop and Househam1987), which require scanning electron microscopy (SEM), still lacking in the descriptions of numerous taxa. In fossil material, identification of taxa is also jeopardized by taphonomic filters, with abrasion, corrosion, partial dissolution and recrystallization obliterating fine diagnostic characters. This is particularly true for species introduced in old publications, normally including only brief descriptions and often lacking proper illustrations. Descriptions and revisions of fossil cribrilinids based on detailed illustrations are scarce in the modern literature, especially for specific stratigraphic intervals (Berning, Reference Berning2006; Taylor and McKinney, Reference Taylor and McKinney2006; Di Martino and Rosso, Reference Di Martino and Rosso2015). In this context, this paper aims to: (1) document cribrilinid associations from Pleistocene deep-water habitats of southern Italy; (2) illustrate fossil representatives of some established species; (3) describe three new species; (4) amend the diagnosis of the genus Figularia Jullien, Reference Jullien1886, and provide a comparative morphological analysis of species currently assigned to this genus; and (5) propose new combinations for two species of Puellina and two species of Figularia.
Geological setting
North-eastern Sicily is part of the north Sicily Chain, which, in this sector, includes the Kabilo-Calabride crystalline basement (Paleozoic rocks of different metamorphic grade) and its sedimentary cover (i.e., discontinuous upper Miocene sediments unconformably covered by Plio-Pleistocene deposits; Barrier, Reference Barrier1987). The Plio-Pleistocene succession starts with lower Pliocene deep-water whitish foraminiferal marls, marly limestones, and coarser sediments including breccias, overlaid with middle Pliocene to middle Pleistocene sediments, usually in thin discontinuous, often laterally heteropic bodies. Those bodies can be grouped in: (1) a middle Pliocene–middle Pleistocene “Bathyal Facies Association” (PP), and (2) a middle Pleistocene “Circalittoral-Infralittoral Facies Association” (mP) (Barrier, Reference Barrier1987; Barrier et al., Reference Barrier, Di Geronimo and Montenat1987a; Vertino, Reference Vertino2003). PP includes carbonate-dominated and siliciclastic-dominated facies. The former facies mainly consist of coral-rich rudstones, with the frame-building deep-water scleractinians Madrepora oculata Linnaeus, Reference Linnaeus1758, Desmophyllum pertusum (Linnaeus, Reference Linnaeus1758), and D. dianthus (Esper, Reference Esper1794), interfingered with calcarenites and carbonate sands containing scattered isidid octocorals, and locally truncated by erosional surfaces and overlaid with debris-flow deposits. The siliciclastic-dominated facies are mainly characterized by marly and silty clays, sometimes embedding coral rudstone boulders that are often encrusted by corals, bivalves, serpulids, and bryozoans (Barrier, Reference Barrier1986, Reference Barrier1987; Barrier et al., Reference Barrier, Di Geronimo, La Perna, Rosso, Sanfilippo, Zibrowius, Meléndez, Blasco and Pérez1996). Facies mP includes the “upper gravels and sands” with fossils of infralittoral–upper circalittoral origin and, locally, large blocks encrusted by circalittoral organisms, and Gilbert-type delta deposits regionally known as the “Messina Formation.” The succession is erosionally capped by Upper Pleistocene fluvio-marine terraces.
At Capo Milazzo, the so-called “yellow calcareous marl” crops out along the south-western and the north-eastern coast. The sandy-silty sediments unconformably lie on erosive surfaces of the pre-Messinian basement (Paleozoic metamorphites to upper Miocene shallow-water deposits), constituting discontinuous sedimentary bodies filling small depressions (Fois, Reference Fois1990). Sediment deposition, previously dated as late Pliocene, occurred during the MPl5 and MPl6 zones, largely overlapping with the Gelasian Stage of Rio et al. (Reference Rio, Sprovieri and Di Stefano1994), and now considered as the basal part of the Pleistocene (Gibbard et al., Reference Gibbard, Head and Walker2010; Violanti, Reference Violanti2012). Deposition in epibathyal environments is indicated by both macrofaunal associations, including brachiopods, corals, serpulids, and, occasionally, mollusks (e.g., Gaetani and Saccà, Reference Gaetani and Saccà1984; Langer, Reference Langer1989), as well as microfaunas, including foraminiferans and ostracodes (e.g., Violanti, Reference Violanti1988; Sciuto, Reference Sciuto2014a, b). Bryozoans are common, but hardly detectable in the field owing to the small size of their colonies and/or colony fragments. Bryozoan assemblages are very diverse, including up to 60 species, some exclusively found in these deposits (Rosso, Reference Rosso2002a, Reference Rossob, Reference Rosso2005; Rosso and Braga, Reference Rosso and Braga2013; Rosso and Di Martino, Reference Rosso and Di Martino2015; Rosso and Sciuto, Reference Rosso and Sciuto2019).
Scoppo is located immediately west of the city of Messina, in the Messina Strait area, where Pleistocene bathyal sediments discontinuously occur (Barrier, Reference Barrier1984; Barrier et al., Reference Barrier, Di Geronimo and Montenat1987a; Vertino, Reference Vertino2003). At Scoppo, these sediments unconformably lie on Messinian brecciated evaporitic limestone. They consist of basal rudstones rich in fragments of cold-water corals (i.e., M. oculata, D. pertusum, and D. dianthus) that are overlain by poorly cemented white marls with sparse corals and plates of the cirriped Scillaelepas Seguenza, Reference Seguenza1876. These macrofossils, and ostracodes, point to deposition in bathyal environments (Vertino et al., Reference Vertino, Titschack, Rosso, Di Geronimo and Pino2013; Sciuto, Reference Sciuto2016) in the MNN19b–19c biozones (A. Baldanza, personal communication, 2015), corresponding to the early Calabrian (=Santernian).
Materials and methods
Studied material originates from deep-water sediments cropping out in two different localities near Messina in north-eastern Sicily: Capo Milazzo Peninsula (two outcrops: Cala Sant'Antonino and Punta Mazza) and Scoppo (Fig. 1; see Geological setting for details). Additional material used for comparison derives from a present-day submarine sample collected at the Apollo Bank off Ustica Island in the Tyrrhenian Sea (Fig. 1).
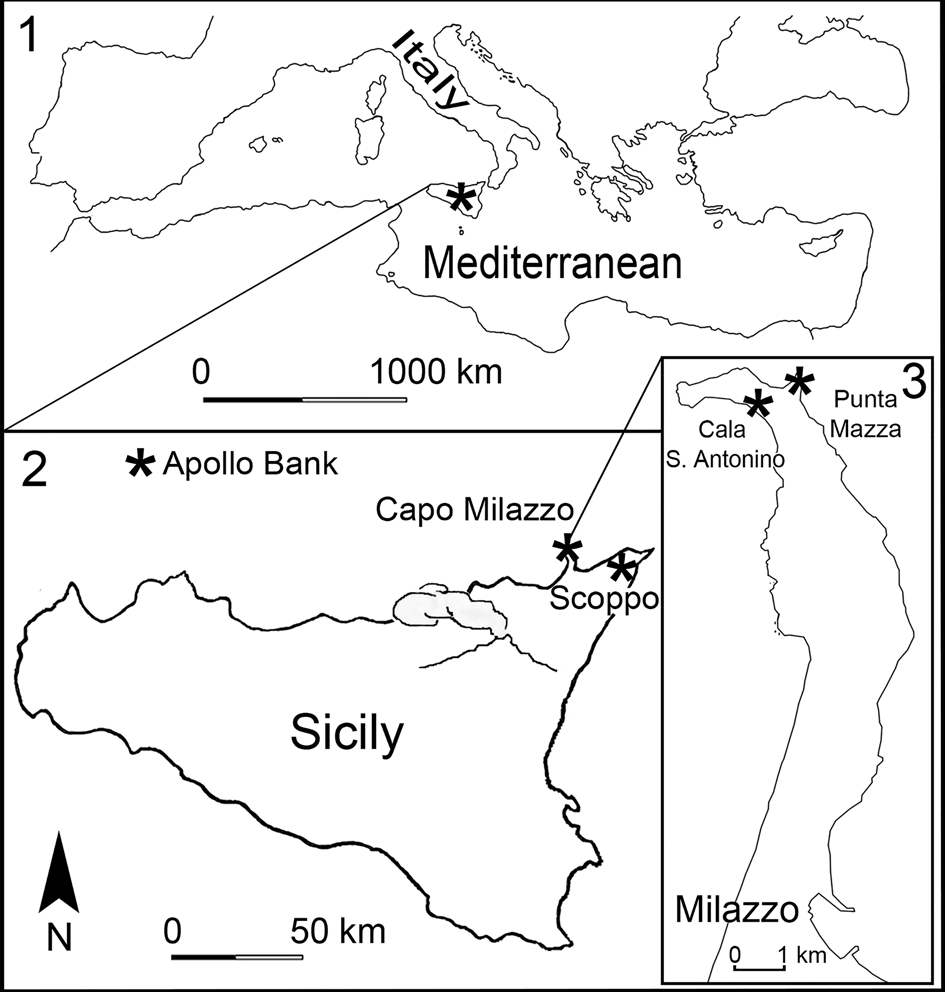
Figure 1. Location of (1) Sicily in the Mediterranean Sea and (2) the study area in northeastern Sicily with sampling localities (Capo Milazzo, Scoppo, and the Apollo Bank, see asterisks); (3) shows Cala Sant’Antonino and Punta Mazza sections at Capo Milazzo. Modified from Rosso and Sciuto (Reference Rosso and Sciuto2019).
At Capo Milazzo, cribrilinid bryozoans were found in “sample 1 (1999)” collected near the top of the layers exposed at Cala Sant'Antonino West; “sample 17 (2000)” and “sample 2015” collected in the central part of Cala Sant'Antonino outcrop; and “sample 4” and “sample 5” collected in biogenic layers near the base of Punta Mazza section, corresponding to “sample 12” and “sample 11” of Sciuto (Reference Sciuto2014b), respectively. Further information on these samples can be found in Sciuto (Reference Sciuto2014b) and Rosso and Sciuto (Reference Rosso and Sciuto2019). At Scoppo, cribrilinids were found in a test sample associated with a Scillaelepas-rich layer, and in the sample “Scoppo 24 top” coming from uncemented marly sediment.
At the Apollo Bank, coarse sediments associated with the kelp Laminaria rodriguezii Bornet, Reference Bornet1888 were collected at about 60 m depth. Living and dead bryozoan associations were characterized by high species richness, but delivered only one colony (now fragmented) of Figularia figularis (Johnston, Reference Johnston1847) (Di Geronimo et al., Reference Di Geronimo, Giacobbe, Rosso and Sanfilippo1990).
Sediment was routinely treated (washed, sieved, and dried) at the Paleoecological Laboratory of the University of Catania. All bryozoans were picked from residues larger than 0.5 mm. After preliminary identification under a stereomicroscope, selected uncoated specimens were mounted for scanning electron microscopy (SEM) using a TESCAN VEGA 2 LMU in backscattered-electron/low-vacuum mode at the Microscopical Laboratory of the University of Catania. For the attribution of the specimens to the genera Cribrilaria Canu and Bassler, Reference Canu and Bassler1929 and Glabrilaria Bishop and Househam, Reference Bishop and Househam1987, we followed the diagnoses in Rosso et al. (Reference Rosso, Beuck, Vertino, Sanfilippo and Freiwald2018) summarized herein: Cribrilaria has totally calcified non-pseudoporous ooecia produced by the distal autozooid or kenozooid, interzooidal avicularia of variable size and shape, usually five (4–8) oral spines, and relatively large uncalcified windows of pore-chambers; Glabrilaria has non-pseudoporous ooecia that are exclusively produced by the distal kenozooid, erect or semi-erect avicularia, 6–7 (rarely five) oral spines, small to moderately sized uncalcified windows of pore-chambers. Measurements were obtained from SEM images using the image processing program ImageJ (Schneider et al., Reference Schneider, Rasband and Eliceiri2012). Measurements were tabulated and provided in micrometers. The complete range is given first, followed by the mean value plus/minus standard deviation and the number of measurements taken. In specimens of Glabrilaria, zooidal boundaries were obliterated by recrystallisation with bands of crystals filling the interzooidal grooves. To estimate zooidal size, length was measured from the distal end of the orifice to the mid-point of the crystal band located proximally, while width was measured from mid-point to mid-point of the crystal bands located laterally.
Repositories and institutional abbreviations
All specimens described and illustrated in this work are part of the Rosso Collection deposited at the Museum of Paleontology of the University of Catania (PMC) under the catalogue numbers reported in the “Systematic paleontology” section. Other abbreviations: MNHN, Muséum national d'Histoire naturelle, Paris; NHMUK, Natural History Museum, London; NMNH, National Museum of Natural History, Smithsonian Institution, Washington DC.
Systematic paleontology
Phylum Bryozoa Ehrenberg, Reference Ehrenberg1831
Order Cheilostomatida Busk, Reference Busk and MacGillivray1852
Suborder Flustrina Smitt, Reference Smitt1868
Superfamily Cribrilinoidea Hincks, Reference Hincks1879
Family Cribrilinidae Hincks, Reference Hincks1879
Genus Cribrilaria Canu and Bassler, Reference Canu and Bassler1929
Type species
Eschara radiata Moll, Reference Moll1803, by original designation.
Cribrilaria profunda new species
Figures 2, 3; Table 1
- Reference Harmelin and Aristegui1988
Puellina (Cribrilaria) scripta; Harmelin and Aristegui, p. 526, figs. 18–19, 24.
- Reference Harmelin and d'Hondt1993
Puellina scripta; Harmelin and d'Hondt, fig. 5.

Figure 2. Cribrilaria profunda n. sp., Capo Milazzo, Gelasian. (1–4): PMC. B27.10.10.2019a, holotype with slightly recrystallized zooids, Cala Sant'Antonino center, sample 2015: (1) group of autozooids, some ovicellate, and interzooidal avicularia (ooecium shows no median carina); (2) distal part of an ovicellate zooid with four spine bases situated laterally to the orifice, the suboral bar, and intercostal lacunae; (3) close-up of autozooidal orifice with five spine bases; (4) close-up of an avicularium and a kenozooid. (5–9) PMC. B27.10.10.2019b, same details as the holotype; one of the largest paratype specimens: (5) general view (note different zooidal shapes); (6) ovicellate zooid tilted to show the median carina of the ooecium; (7) autozooids and an avicularium; (8) an autozooid with recrystallized hidden margins; (9) autozooids of different shapes. Scale bars: (1) 500 μm; (2, 3) 100 μm; (4, 6–9) 200 μm; (5) 1 mm.
Table 1. Measurements (in μm) of Cribrilaria profunda n. sp. Abbreviations: L: length; W: width.

Holotype
PMC. B27.10.10.2019a. Capo Milazzo Peninsula: Cala Sant'Antonino center, sample 2015: one small fragment including ovicellate zooids and interzooidal avicularia.
Paratypes
PMC. B27.10.10.2019b. Additional specimens from Capo Milazzo Peninsula: Cala Sant'Antonino West, sample 1 (1999: surface): one specimen; Cala Sant'Antonino center, sample 17 (2000): three specimens; sample 2015: 12 specimens in addition to the holotype. PMC. B27.10.10.2019c. Scoppo: sample 24 top: two specimens.
Diagnosis
Colonies encrusting, multiserial. Autozooids nearly flat, oval to irregularly polygonal. Basal pore-chambers present. Gymnocyst visible along the zooidal margins. Frontal shield consisting of 14–25 costae with 4–11 intercostal pores/lacunae. Suboral bar formed by the first pair of widest costae with blunt median prominence and proximal pore. Orifice transversely D-shaped with five (occasionally 6–7) oral spines, four in ovicellate zooids. Interzooidal avicularia with elongate, triangular or parallel-sided, raised rostrum, crossbar lacking. Ovicell hyperstomial, presumably cleithral. Ooecium formed by distal autozooid, with a longitudinal median carina. Kenozooids rare.
Occurrence
Cribrilaria profunda n. sp. is presently known from the early Pleistocene deep-water deposits of southern Italy (Gelasian of Capo Milazzo Peninsula and early Calabrian of Scoppo, Messina), in the Recent Ibero-Moroccan Gulf (223–990 m depth), the Gibraltar Strait (580 m depth) (Harmelin and Aristegui, Reference Harmelin and Aristegui1988), and in the Alboran Sea (205 m) (Harmelin and d'Hondt, Reference Harmelin and d'Hondt1992, Reference Harmelin and d'Hondt1993).
Description
Colonies encrusting, multiserial, unilaminar, the largest observed fragment including a dozen zooids. Zooids large and nearly flat, slightly longer than wide (L/W = 1.15: Scoppo; 1.29: Milazzo), oval to rhomboidal or rarely irregularly polygonal in shape, wider in their proximal half; zooidal boundaries marked by shallow grooves (Figs. 2.1, 2.4, 2.7, 3.1, 3.6). Gymnocyst exposed all along the zooidal margins, usually wider laterally to the orifice and at triple zooid junctions (Figs. 2.1, 3.1, 3.2). Interzooidal communication through basal pore-chambers with windows (~70 × 20 μm), visible only in some zooids at colony periphery (Fig. 3.5). Frontal shield flat (Figs. 2.1, 2.7–2.9, 3.1, 3.2), consisting of 14–25 wedge-shaped costae (including suboral), narrowing and tapering towards the center of the zooid (maximum basal width 32–65 μm), converging toward a median point or along a median longitudinal, transverse, or trifurcate midline. Costae connected by several intercostal bridges leaving 4–11, regularly spaced, subrectangular lacunae, 8–16 μm long; peripheral pores the largest. Intercostal pores reduced to 4–5 proximally to the first suboral pair of costae (Fig. 2.2). These are shorter and larger than the other pairs, and merge along the zooidal midline leaving a suture with a median pore, and often forming a more or less elevated prominence distally, adjacent to the pore (Figs. 2.2, 3.3, 3.4). Orifice transversely D-shaped, outlined by a raised rim. Orifice bearing five (occasionally up to 7) equally spaced, articulated oral spines (Figs. 2.3, 3.3, 3.4), four persisting in ovicellate zooids (Fig. 2.2). Interzooidal avicularia common, directed laterally or rarely distolaterally, with a variably shaped (often triangular) cystid and an elongate triangular to almost parallel-sided rostrum, raised above or positioned between the costate shield of adjacent autozooids, no crossbar (Figs. 2.1, 2.4, 2.7, 2.9, 3.5, 3.6). Ovicell hyperstomial, presumably cleithral. Ooecium formed by the distal autozooid. Ectooecium smooth, with a longitudinal median elevated carina (Figs. 2.1, 2.2, 2.6, 3.6). A single kenozooid with costate frontal shield numbering 13 costae was observed (Fig. 2.4). Ancestrula not seen.

Figure 3. Cribrilaria profunda n. sp., Scoppo, sample 24 top, early Calabrian, MNN19b-19c biozones, PMC. B27.10.10.2019c, paratype. (1) The largest fragment; (2) general view of an autozooid; (3) close-up of an orifice with unusual L/W ratio and seven oral spine bases; (4) orifice with five oral spine bases; (5) colony margin showing basal pore-chambers and interzooidal avicularium; (6) ovicellate zooid, avicularium, and ooecium showing longitudinal carina. Scale bars: (1) 500 μm; (2, 6) 200 μm; (3–5) 100 μm.
Etymology
From the Latin profundus, alluding to its deep-water distribution.
Remarks
Specimens from Capo Milazzo and Scoppo are very similar in general appearance, including the occurrence of some irregularly polygonal autozooids with a somewhat trifurcate suture in the costate shield. Measurements also largely overlap, although Capo Milazzo material shows more variability. Yet, some specimens from Scoppo show a slightly convex costate shield with fewer costae, a more raised suboral prominence, and more (occasionally 6–7) oral spine bases. Variability in the number of oral spines within the same species is known in other cribrilinids, such as Cribrilaria pseudoradiata Harmelin and Aristegui, Reference Harmelin and Aristegui1988. Specimens reported as Cribrilaria scripta (Reuss, Reference Reuss1848) by Harmelin and Aristegui (Reference Harmelin and Aristegui1988) and Harmelin and d’Hondt (Reference Harmelin and d'Hondt1993) share their characters with the Capo Milazzo material and are here considered conspecific (see below).
Cribrilaria profunda n. sp. is very similar to the Recent C. saginata Winston, Reference Winston2005 from off Bahia Honda (Cuba) (Winston, Reference Winston2005) and the Bahama Bank (Rosso et al., Reference Rosso, Beuck, Vertino, Sanfilippo and Freiwald2018). However, C. saginata differs in having a distinctly more extensive proximal gymnocyst, a shorter and squatter orifice (orifice length/orifice width 0.42–0.55 in C. saginata vs. 0.64–0.69 in C. profunda n. sp.), five constant oral spines, and carinated suboral costae. Hincks (Reference Hincks1884), and later Neviani (Reference Neviani1900), also suggested conspecificity between C. saginata, as C. radiata (Moll, Reference Moll1803) from Florida, and the middle Miocene (Langhian) Lepralia elegantissima Seguenza, Reference Seguenza1880 from southern Calabria (Italy), which is, however, extremely unlikely owing to the great geographic and temporal distance between the two populations. In addition, the only illustration available for L. elegantissima (Seguenza, Reference Seguenza1880, pl. 8, fig. 11) is a drawing showing a very distinctive morphology for this species, with ovoidal zooids having a wide and prominent frontal median keel, and seemingly 3–5 suboral tubercles alternating with lacunae.
Cribrilaria scripta and C. radiata, although similar in appearance to C. profunda n. sp., have smaller zooidal dimensions and larger interzooidal avicularia, and four oral spines occur in most zooids in the latter species (Harmelin, Reference Harmelin1970; Bishop and Househam, Reference Bishop and Househam1987). Recent specimens of C. scripta sensu Harmelin and Aristegui (Reference Harmelin and Aristegui1988) from deep waters of the Ibero-Moroccan Bay and Gibraltar Strait, are here attributed to C. profunda n. sp. based on the measurements, the presence of generally five oral spines, and presence of a robust and smooth pair of suboral costae forming a median prominence.
In addition, specimens from the early Messinian of Carboneras (SE Spain) identified by J.-G. Harmelin as Puellina (Cribrilaria) scripta and mentioned in Barrier et al. (Reference Barrier, Zibrowius, Lozouet, Montenat, Ott d'Estevou, Serrano and Soudet1992), without description or illustrations, might belong to C. profunda n. sp.
The Recent Cribrilaria pseudoradiata from the upper bathyal Atlanto-Mediterranean region is also similar to C. profunda n. sp., but has smaller dimensions and lacks interzooidal avicularia.
Cribrilaria profunda n. sp. could possibly correspond to Lepralia planicosta Seguenza, Reference Seguenza1880, a cribrimorph species reported from Plio-Pleistocene sediments of the Messina Strait area. Seguenza (Reference Seguenza1880) distinguished his species from C. scripta, adducing that autozooids were irregularly shaped, with a flat costate shield consisting of several costae, as in C. profunda n. sp. Unfortunately, Lepralia planicosta, supposedly corresponding to Lepralia scripta sensu Manzoni (Reference Manzoni1875) from the early Pliocene of Castrocaro, was not figured and the type material was lost in 1908 during the Messina earthquake. We refrain from selecting our material as the neotype of L. planicosta because the original description of this species seems insufficient to ensure their conspecificity, and the type localities, although geographically close, are not exactly the same, and neither are the geologic horizons. Seguenza (Reference Seguenza1880) abstained from illustrating his new species and referred to drawings of L. scripta sensu Manzoni (Reference Manzoni1875, figs. 25, 25a). Manzoni's specimens, held in the collection of the Museo di Storia Naturale, Geologia e Paleontologia of Florence, should be located and examined before selecting a neotype for this species.
Genus Glabrilaria Bishop and Househam, Reference Bishop and Househam1987
Type species
Puellina pedunculata Gautier, Reference Gautier1956, by original designation.
Glabrilaria cf. G. pedunculata (Gautier, Reference Gautier1956)
Figure 4; Table 2
- cf. Reference Gautier1956
Puellina pedunculata Gautier, p. 203, fig. 20.
- cf. Reference Prenant and Bobin1966
Colletosia pedunculata; Prenant and Bobin, p. 596, fig. 207 III.
- cf. Reference Harmelin1970
Cribrilaria pedunculata; Harmelin, p. 93, fig. lg, h, pl. 2, fig. 6.
- cf. Reference Bishop and Househam1987
Puellina (Glabrilaria) pedunculata; Bishop and Househam, figs. 95–97, tab. 13.
- cf. Reference Harmelin1988
Puellina (Glabrilaria) pedunculata; Harmelin, p. 31, figs. 9–11.
- cf. Reference Rosso, Di Martino, Sanfilippo and Di Martino2013a
Puellina (Glabrilaria) pedunculata; Rosso et al., tab. 17.1.
- cf. Reference Sanfilippo, Rosso, Guido, Mastandrea, Russo, Ryding and Taddei-Ruggiero2015
Puellina (Glabrilaria) pedunculata; Sanfilippo et al., tab. 2, fig. 5f.
- cf. Reference Rosso, Gerovasileiou, Sanfilippo and Guido2019a
Glabrilaria pedunculata; Rosso et al., fig. 5e, f.

Figure 4. Glabrilaria cf. G. pedunculata Gautier, Reference Gautier1956, Capo Milazzo, Gelasian, Rosso Collection collective code PMC I. Pl. B.81a. (1–5) Cala Sant'Antonino center, sample 2015: (1) small fertile colony, with autozooids radiating from an apparent central ancestrula, seemingly regenerated as a miniature autozooid; (2) close-up of the three zooids on the top left of (1); note the carinate ooecia; (3) frontal view of autozooid with the transversely D-shaped orifice, seven oral spines, and a recrystallized suboral area; (4, 5) inclined views of an ovicellate zooid with four oral spines and ooecium formed by the distal kenozooid with small costal shield; arrows indicate the basal pore chambers potentially producing the avicularia lateral to the ovicell; (6) Cala Sant'Antonino center, sample 17 (2000), part of a large worn colony on a bioclast; abundant kenozooids with eight costae are seen between autozooids. Scale bars: (1, 2, 6) 200 μm; (3–5) 100 μm.
Table 2. Measurements (in μm) of Glabrilaria cf. G. pedunculata Gautier, Reference Gautier1956 and Glabrilaria transversocarinata n. sp. L: length; W: width.

Holotype
MNHN-IB-2008-10384, Grand Conclu de Riou (Golfe de Marseille), Mediterranean, Recent.
Occurrence
Glabrilaria pedunculata is an endemic Mediterranean species, widespread throughout the basin, from the Gulf of Lion to the Aegean Sea. Its presence in the Atlantic is restricted to areas swept by Mediterranean outflow water (Harmelin and d’Hondt, Reference Harmelin and d'Hondt1992). It has been reported from: (1) shallow-water submarine caves in the Provençal area (Harmelin, Reference Harmelin1969, Reference Harmelin1970, Reference Harmelin1988, Reference Harmelin2003), in the Ionian sea (Rosso et al., Reference Rosso, Di Martino, Sanfilippo and Di Martino2013a, Reference Rosso, Sanfilippo, Taddei-Ruggiero and Di Martinob; Sanfilippo et al., Reference Sanfilippo, Rosso, Guido, Mastandrea, Russo, Ryding and Taddei-Ruggiero2015) and Aegean sea (Crete: Harmelin, Reference Harmelin1988; Lesvos: Rosso et al., Reference Rosso, Gerovasileiou, Sanfilippo and Guido2019a); (2) cryptic microhabitats from shallow waters (Harmelin, Reference Harmelin2003), mid-shelf сoralligenous cliffs, and outer shelf “Coralligène de Plateau,” at 100–140 m depth off Lybia and near Santorini (Harmelin, Reference Harmelin1988); and (3) at bathyal depths, ~700 m in the Sicily Strait (Harmelin, Reference Harmelin1979, Reference Harmelin1988), ~280 m in the southern Adriatic Sea (D'Onghia et al., Reference D'Onghia, Capezzuto, Cardone, Carlucci, Carluccio, Chimienti, Corriero, Longo, Maiorano, Mastrototaro, Panetta, Rosso, Sanfilippo, Sion and Tursi2015), and ~500 m at Leuca, northeastern Ionian Sea (Mastrototaro et al., Reference Mastrototaro, D'Onghia, Corriero, Matarrese, Maiorano, Panetta, Gherardi, Longo, Rosso, Sciuto, Sanfilippo, Gravili, Boero, Taviani and Tursi2010), usually associated with cold-water coral habitats. Specimens from the Gelasian of Sicily represent the first fossil record for this species, suggesting its persistence, at least in deep-water settings, in the Mediterranean since the early Pleistocene.
Description
Colony encrusting, multiserial, unilaminar (Fig. 4.1, 4.6), the largest specimen including at least 50 zooids. Zooids oval, longer than wide (L/W = 1.28), convex, outlined by furrows filled by incipient re-crystallization (Fig. 4). Interzooidal communication through basal pore-chambers, more than 10 visible only in some marginal zooids, with longitudinally elongate windows ~10 × 20 μm (Fig. 4.4). Gymnocyst narrow, steeply sloping. Costate frontal shield oval and extensive, formed by 13–17 (including suboral) wedge-shaped, prominent costae, 27–45 μm wide at the base, converging towards the midline and forming a slightly raised carina (Fig. 4.4, 4.5). Costae joined by regularly spaced intercostal bridges leaving 6–7 slit-like intercostal pores, ~7–8 μm long (Fig. 4.5). Only four intercostal spaces occur proximally to the suboral pair of costae, which are flat and merge at the midline forming a triangular shelf, possibly leaving a single round pore (Fig. 4.3, 4.6). Orifice transversely D-shaped (Fig. 4.1, 4.3, 4.6), marked by a raised rim, provided with 6–7 closely spaced, articulated oral spines (Fig. 4.1, 4.3), four persisting in ovicellate zooids (Fig. 4.2, 4.5). Ovicells hyperstomial, presumably cleithral. Ooecium formed by distal kenozooid, with frontally visible small costate shield consisting of three costae (Fig. 4.4); ectooecium smooth, with elevated longitudinal carina (Fig. 4.2, 4.4, 4.5). Avicularia not observed. Abundant small kenozooids recorded in larger colonies, interspersed between autozooids, seemingly polygonal, with boundaries obliterated by recrystallisation, with extensive gymnocyst and costate frontal shield of 6–8 costae (Fig. 4.6). The only ancestrula found seemingly regenerated as a miniature autozooid (Fig. 4.1).
Materials
Rosso-Collection, collective code: PMC I. Pl. B.81a: Capo Milazzo Peninsula: Cala Sant'Antonino center: sample 2015: three specimens; sample 17 (2000): one specimen; Punta Mazza: sample 4: two specimens; sample 5: one specimen.
Remarks
The available specimens are worn and recrystallized, preventing recognition of some diagnostic characters. However, the morphology and morphometrics of autozooids, ooecia, and kenozooids are closely reminiscent of Glabrilaria pedunculata Gautier, Reference Gautier1956, although with a few small differences. The present-day Mediterranean species invariably shows six oral spines and two median pores in the triangular shelf distal to the suboral costae (Bishop and Househam, Reference Bishop and Househam1987, fig. 97; Harmelin, Reference Harmelin1988, fig. 17a, c; Rosso et al., Reference Rosso, Gerovasileiou, Sanfilippo and Guido2019a, fig. 5e, f). However, both the variability in the number of oral spines and the presence/absence of median pores are considered to be in the range of intraspecific variability in cribrilinids (e.g., C. pseudoradiata Harmelin and Aristegui, Reference Harmelin and Aristegui1988 and G. orientalis Harmelin, Reference Harmelin1988). The long-stalked (=pedunculate) avicularia, originating from basal pore chambers in both autozooids and kenozooids, which are typical of G. pedunculata, were not observed in our fossil specimens. This is likely a taphonomic bias, because such avicularia can be easily detached even in living colonies, as observed in Glabrilaria hirsuta Rosso in Rosso et al., Reference Rosso, Beuck, Vertino, Sanfilippo and Freiwald2018 from the Bahama Bank. In our fossil specimens, zooidal boundaries are mostly covered by neomorphic calcite crystals that prevent the detection of the basal pore chambers from which the pedunculate avicularia are budded. However, in Figure 4.4 and 4.5 (see arrows) the pores potentially producing the avicularia lateral to the ovicell are visible.
Seven oral spines were described in Glabrilaria corbula Bishop and Househam, Reference Bishop and Househam1987 and Glabrilaria orientalis lusitanica Harmelin, Reference Harmelin1988, two closely related extant species reported from the Atlanto-Mediterranean region and the Gibraltar Strait area, respectively. However, the former species shows an ooecium that is formed by a distal kenozooid which is not distinguishable in frontal view, has 4–6 costae-like ridges arranged in a radial pattern, a flatter autozooidal shield with somewhat carinate costae that are sometimes with a pelma, and two large pores in the suboral shelf (Bishop and Househam, Reference Bishop and Househam1987; Harmelin, Reference Harmelin1988), while the latter species lacks midline pores in the suboral shelf (Harmelin, Reference Harmelin1988). Glabrilaria orientalis lusitanica also has semi-erect interzooidal avicularia (Harmelin, Reference Harmelin1988) backed against the ooecium. Six to seven oral spines also occur in Glabrilaria africana (Hayward and Cook, Reference Hayward and Cook1983), but this species has numerous variably sized pores in the suboral shelf in addition to semi-erect avicularia associated with the ooecium and squeezed between autozooids.
Glabrilaria transversocarinata new species
Figure 5; Table 2

Figure 5. Glabrilaria transversocarinata n. sp., Scoppo, sample 24 top, early Calabrian, MNN19b-19c biozones, PMC. B28.10.10.2019a, holotype. (1) The largest specimen consisting of partly superimposed colony layers; (2) group of zooids at the colony margin showing intercostal spaces; (3) cluster of ovicellate and non-ovicellate zooids; arrow indicates a small kenozooid with five costae (note the elevated bases of oral spines and the transversely oriented crest located in the middle of the ooecium and the possible persistence of four oral spines); (4) two ovicellate zooids (note the prominent bifid suboral mucro and flat shield composed of somewhat tuberculate costae). Scale bars: (1) 500 μm; (2–4) 200 μm.
Holotype
PMC. B28.10.10.2019a: colony consisting of ~20 autozooids, some ovicellate. Scoppo, sample 24 top, early Calabrian, MNN19b-19c biozones.
Paratype
PMC. B28.10.10.2019b: small colony fragment including seven autozooids, two ovicellate. Scoppo: sample 24 top, early Calabrian, MNN19b-19c biozones.
Diagnosis
Colony encrusting, multiserial. Autozooids convex. Gymnocyst narrow. Frontal shield consisting of 12–14 prominent and tuberculate costae with 3–7 intercostal spaces. Suboral pair of costae forming a bifid mucro. Orifice transversely D-shaped with six oral spines, four persisting in ovicellate zooids. Ovicells subimmersed. Ooecium formed by distal kenozooid, surface smooth, with transverse rib. Avicularia not observed. Kenozooids rare.
Occurrence
Only known from the early Calabrian of Scoppo, Messina.
Description
Colony encrusting, multiserial, unilaminar, but including superimposed lobes (Fig. 5.1), the largest observed fragment consisting of ~20 zooids. Zooids oval, longer than wide (L/W = 1.44), convex, the outline hidden by incipient recrystallization (Fig. 5.4). Interzooidal communication through basal pore-chambers visible in some peripheral zooids, with slightly longitudinally elongate windows ~21 × 18 μm (Fig. 5.2). Gymnocyst very narrow, except for proximal and, occasionally, lateral extensions wedged between neighboring zooids (Fig. 5.2, 5.3). Frontal shield oval and extensive, formed by 14–16 (including suboral) wedge-shaped, prominent, tuberculate costae, 26–47 μm wide at the base, converging towards the midline (Fig. 5.2–5.4). Costae joined by intercostal bridges apparently leaving 6–7 intercostal pores (Fig. 5.2), seemingly reduced to 3–4 proximally to the suboral pair of costae. These are shorter and more robust than the other costae and raised at the midline, forming a bifid mucro (Fig. 5.2, 5.3). Orifice transversely D-shaped, provided with six closely spaced, articulated oral spines (Fig. 5.2), four persisting in ovicellate zooids (Fig. 5.3, 5.4). Ovicells subimmersed. Ooecium formed by the distal kenozooid, with frontally visible costate (4–5 costae) shield and distal band of gymnocyst (Fig. 5.3, 5.4); ooecium with prominent, transverse, straight to slightly arched rib possibly with protruding spikes (lost) (Fig. 5.3, 5.4); an additional thinner and lower longitudinal carina was observed in a single ooecium (Fig. 5.3). Avicularia not observed. Only one kenozooid was observed. It was small, polygonal, with a relatively narrow gymnocyst and costate frontal shield of five radial costae (Fig. 5.3). Ancestrula not observed.
Etymology
From the Latin transversus, meaning transversely placed, and carina alluding to the typical median crest of the ooecium.
Remarks
The co-occurrence of a prominent transverse ridge on the ooecium and a bifid suboral mucro is distinctive of this species. Ooecia with a transverse ridge are known in a few species only. One is the extant Glabrilaria hirsuta Rosso in Rosso et al., Reference Rosso, Beuck, Vertino, Sanfilippo and Freiwald2018 from the Bahama Bank, in which the ridge is, however, very arched to subtriangular and equipped with prominent spine-like processes (Rosso et al., Reference Rosso, Beuck, Vertino, Sanfilippo and Freiwald2018). Furthermore, in G. hirsuta, the number of oral spines (six, four persisting in ovicellate zooids) occasionally increases to seven, the costae have more obvious spine-like processes at the periphery of the frontal shield, the suboral costae form a transverse spiny crest proximal to the orifice, and kenozooids arranged in rows or clusters are very common (Rosso et al., Reference Rosso, Beuck, Vertino, Sanfilippo and Freiwald2018). In the extant Glabrilaria cristata (Harmelin, Reference Harmelin1978) from the Hyères and Meteor banks south of the Azores, the ooecial ridge is extremely protruding and situated more proximally towards the orifice, contributing to form a sort of spiny collar around the orifice together with the second pair of suboral costae. These costae bear cockscomb-like spines that are still present but smaller than those on the other pairs (Harmelin, Reference Harmelin1978). Oral spines are invariably seven in this species.
Occasionally, transverse ornamentation has been reported in the ooecia of other cribrilinid genera. A succession of ribs adds to a longitudinal carina in Puellina cassidainsis Harmelin, Reference Harmelin1984 from the 3PP submarine cave in the Mediterranean French coast (see Harmelin, Reference Harmelin1984, fig. 7b). A cruciform pattern can develop in the ooecia of Cribrilaria macaronensis (Harmelin, Reference Harmelin2006), and transverse ridges or wrinkles in Cribrilaria atlantis (Harmelin, Reference Harmelin2006), both species previously assigned to Puellina (see Harmelin, Reference Harmelin2006, fig. 1).
Measurements of Glabrilaria transversocarinata n. sp. generally overlap with those of G. cf. G. pedunculata from Capo Milazzo (Table 2), but tend towards the higher values, sometimes exceeding the upper limit. The only exception is the size of the kenozooid, which seems to be smaller, although only based on a single measurement. However, morphological differences, including the number of oral spines, shape of costae, suboral lacuna and ooecia, and the rarity of kenozooids, distinguish the two species.
The two colony fragments available are detached from the substratum, a common feature for bryozoan specimens found in the Capo Milazzo “yellow marl.” This may indicate either that the substratum was organic or that selective aragonitic dissolution took place during/before fossilization.
Genus Figularia Jullien, Reference Jullien1886
Type species
Lepralia figularis Johnston, Reference Johnston1847, by original designation.
Amended diagnosis
Colony commonly encrusting, but erect, fan-shaped, or developing erect lobes in some species. Autozooids with variably developed gymnocyst, usually wider proximally; costate shield formed by few to numerous (up to 30) costae, each bearing a pelma (circular to drop-shaped or transversely elongated) varying in size and position. Orifice with well-developed poster and condyles, dimorphic and typically larger in ovicellate zooids. Oral spines absent. Avicularia, when present, vicarious, elongate, and often spatulate, with complete crossbar. Ovicells hyperstomial or subimmersed, cleithral. Ooecium formed by the distal autozooid or kenozooid (sometimes in the same colony), bilobate, consisting of two very large, modified costae, arched and meeting in the midline to form a suture and/or carina; each costa with a wide fenestra. Interzooidal communication via mural pore chambers in the transverse walls and multiporous septula in the lateral walls. Ancestrula only observed in the type species, wider than autozooids, subcircular, with narrow gymnocyst encircling an extensive opesia with differentiated orifice; no spines.
Remarks
The finding of a new species having morphological skeletal features fitting into the genus Figularia Jullien, Reference Jullien1886, but characterized by erect colony form and a very distinctive and large ooecium formed by a distal kenozooid, led to the examination of species currently placed in this genus (Tables 3, 4).
Table 3. List of species currently belonging to the genus Figularia with description of the main skeletal morphological characters. These species conform to the diagnosis of the genus. Abbreviations: Dim Or, Dimorphic orifice; Distr, Stratigraphic distribution; E, Eocene; M, Miocene; N, number; O, Oligocene; Orig, Origin; P, Pliocene; Pl, Pleistocene; R, Recent; ZL: autozooidal length; ZW: autozooidal width; Transv. = transversal; Longit. = longitudinal; Or. = orifice. Symbols in the column Orig: *ooecium formed by the distal autozooid; § ooecium formed by the distal kenozooid; ? uncertain. In the columns Suture and Dim Or the asterisk indicates the occurrence of the feature. Information is mostly compiled from the original descriptions.

Table 4. List of doubtful species currently attributed to the genus Figularia. New combinations are suggested for two species, while attribution of the remaining species awaits examination of the type material. Abbreviations: Dim Or, Dimorphic orifice; Distr, Stratigraphic distribution; M, Miocene; N, number; Orig, Origin. P, Pliocene; Pl, Pleistocene; R, Recent; ZL: autozooidal length; ZW: autozooidal width. Symbols in the column Orig: *ooecium formed by the distal autozooid; § ooecium formed by the distal kenozooid; ? uncertain. In the columns Suture and Dim Or the asterisk indicates the occurrence of the feature. Information is mostly compiled from the original descriptions. Measurements provided in μm. Additional information from Duvergier (Reference Duvergier1924), Buge (Reference Buge1957), Grischenko et al. (Reference Grischenko, Gordon, Ayumu, Makoto, Naotomo and Mawatari2004), Winston et al. (Reference Winston, Vieira and Woollacott2014), NMNH 1, and NMNH 2.

Figularia was introduced by Jullien (Reference Jullien1886, p. 608) who designated Lepralia figularis Johnston, Reference Johnston1847, an Atlanto-Mediterranean extant species, as the type species of the genus, and included an additional fossil species Lepralia elegantissima based on the unique drawing available (Seguenza, Reference Seguenza1880, p. 83, pl. 8, fig. 11). This latter species, depicted with oral spine bases, is more likely to be a species of Cribrilaria (see also Remarks on Cribrilaria profunda n. sp.). Oral spines are absent in the type species F. figularis (see Soule et al., Reference Soule, Soule and Chaney1995, fig. 45C), as well as in all living and fossil specimens found to date (e.g., Figs. 6, 7). The absence of oral spines has also been reported almost consistently in the diagnosis of the genus, with only a few exceptions (e.g., Gordon, Reference Gordon1984). Further diagnostic characters include a complete crossbar in the vicarious avicularia, and the presence of large, symmetrical ectooecial fenestrae and a median carina in the ooecium (see Soule et al., Reference Soule, Soule and Chaney1995; Hayward and Ryland, Reference Hayward and Ryland1998; Kukliński and Barnes, Reference Kukliński and Barnes2009; Yang et al., Reference Yang, Seo, Min, Grischenko and Gordon2018).

Figure 6. Figularia figularis (Johnston, Reference Johnston1847), Southern Tyrrhenian Sea, Rosso collection PMC. I. Pl. B.71.b, Apollo Bank sample. (1) Small fragment consisting of five autozooids, two ovicellate, and a vicarious avicularium; left ooecium is formed by the distal autoooid, right by the distal kenozooid with frontally visible costal shield; (2) close-up of the ooecium formed by the distal kenozooid. Scale bars: (1) 500 μm; (2) 200 μm.

Figure 7. Figularia figularis (Johnston, Reference Johnston1847), Scoppo, sample 24 top, early Calabrian, MNN19b-19c biozones, Rosso collection PMC I. Pl. B.71.c. (1) Fragment with few autozooids (note the teratologic autozooid); (2) close-up of the distal half of the teratologic autozooid shown in (1); (3) fragment with four, incomplete autozooids; (4) close-up of the orifice. Scale bars: (1, 3) 500 μm; (2, 4) 200 μm.
The erect colony-form has never been mentioned in the generic diagnosis before. However, Busk (Reference Busk1884, p. 132) described Figularia philomela as “free; erect or decumbent (hemescharan).” Subsequently, Hayward and Cook (1979, p. 76) found a bilaminar fragment of F. philomela interpreted as part of an erect foliaceous colony possibly arising from an encrusting phase (var. adnata of Busk, Reference Busk1884). Gordon (Reference Gordon1989, p. 15, 16) recorded the occasional occurrence of an erect bilamellar lobe, arising from the adjacent encrusting zooids, in a colony of Figularia mernae Uttley and Bullivant, Reference Uttley and Bullivant1972 from Puysegur Bank, off the South Island of New Zealand. The fan-shaped colonies of the newly discovered Figularia species from Capo Milazzo, although often fragmentary (Fig. 8), show a configuration comparable to that observed in F. mernae, with basal zooids elongated and arranged in back-to-back adjacent pairs (Fig. 8.1, 8.2, 8.6). The lack of a costate frontal shield, with no obvious evidence of breakage, in several proximal/basal zooids, suggests that simplified polymorphs, reminiscent of those in Corbulipora MacGillivray, Reference MacGillivray1895 (see Bock and Cook, Reference Bock and Cook2001) may occur. However, the raising of the erect fan-shaped portions from an encrusting phase is doubtful until encrusting colonies, or at least isolated encrusting zooids, are found.

Figure 8. Figularia spectabilis n. sp., Capo Milazzo, sample Cala Sant'Antonino center, 2015, Gelasian, PMC. B22. 5.4.2015.b, paratypes, colony morphology. (1, 2) Lateral view of two fan-shaped colony fragments with thin cylindrical proximal base; (3) side view of a narrow ribbon-like fragment; (4, 5) inclined proximal view and lateral view of fan-shaped colony fragments with slightly diverging sides; (6) proximal view of a fan-shaped colony fragment; (7, 8) inclined distal and top view of a fan-shaped colony fragment. Scale bars: (1, 7, 8) 1 mm; (2–6) 500 μm.
The ooecium in Figularia is generally described as bivalved/bifenestrate (Ostrovsky, Reference Ostrovsky2013). In F. figularis, the prominent bilobate ooecium is formed by the distal autozooid, with two costae meeting in the midline leaving a suture and/or forming a slightly raised carina; each costa bearing a large, irregularly shaped and transversely elongate fenestra (membranous area in non-cleaned specimens). The colony fragment of F. figularis from the Apollo Bank (Tyrrhenian Sea, Mediterranean) shows that ooecia formed by the distal kenozooid can co-occur in the same colony in this species (Fig. 6). Though uncommonly reported, and here recorded in F. figularis for the first time, the co-occurrence of ooecia produced by the distal autozooid and kenozooid is known in other cribrilinids, such as Cribrilina punctata (Hassall, Reference Hassall1841), “Puellina” harmeri Ristedt, 1985 (see also discussion in Rosso et al., Reference Rosso, Beuck, Vertino, Sanfilippo and Freiwald2018), Cribrilaria innominata (Couch, Reference Couch1844) (see Chimenz Gusso et al., Reference Chimenz Gusso, Nicoletti and Bondanese2014), Puellina saldanhai Harmelin, Reference Harmelin2001, and Puellina mikelae Harmelin, Reference Harmelin2006. Following Rosso et al. (Reference Rosso, Beuck, Vertino, Sanfilippo and Freiwald2018), the latter two species are here allocated to the genus Cribrilaria: Cribrilaria saldanhai (Harmelin, Reference Harmelin2001) n. comb. and Cribrilaria mikelae (Harmelin, Reference Harmelin2006) n. comb. Both ovicell variants sometimes may appear within the same colony (e.g., in C. punctata and “P.” harmeri) indicating a developmental plasticity of this character (reviewed in Ostrovsky, Reference Ostrovsky2013). A similar plasticity in ovicell formation is only known in some Calloporidae (Ostrovsky and Schäfer, Reference Ostrovsky and Schäfer2003; Ostrovsky et al., Reference Ostrovsky, Nielsen, Vávra and Yagunova2009; Ostrovsky, Reference Ostrovsky2013) that are presumed ancestors of cribrilinids.
The kenozooid producing the ooecium in F. figularis shows a crescent-shaped shield of short radial costae, each with a single pelma as in the autozooids, but also with a single intercostal pore (Fig. 6). The same structure is also evident in the fossil species from Capo Milazzo (Fig. 9). Ovicells with ooecia formed by the distal kenozooid also occur in other species currently assigned to this genus, based on examination of available SEM images and, to a lesser extent, drawings (see Table 3).
Ostrovsky (Reference Ostrovsky2013, fig. 1.28A) illustrated sectioned decalcified ovicells of F. figularis in which most of the brood cavity is situated in the proximal part of the distal zooid predominantly below the colony surface, thus corresponding to endozooidal type. Whether this position of the brood cavity was an effect of decalcification of the skeleton (and, thus, sagging of the originally raised ooecium) during preparation for sectioning is currently not clear, but this contradicts most descriptions showing hyperstomial ovicells in this species (see references above). Still, a degree of the brood cavity immersion may vary, and, for example, both hyperstomial and subimmersed ovicells are known within the genus Figularia, and hyperstomial, subimmersed, and endozooidal ovicells are described in the different species of Puellina (Ostrovsky, Reference Ostrovsky2013). Subimmersed ovicells were present in Recent colonies of F. figularis from the Mediterranean (A. Ostrovsky, personal observations).
Ostrovsky and Taylor (Reference Ostrovsky and Taylor2005) noted the occurrence of species of Figularia—F. clithridiata (Waters, Reference Waters1887), F. tahitiensis Waters, Reference Waters1923, and F. pulcherrima Tilbrook, Hayward and Gordon, Reference Tilbrook, Hayward and Gordon2001—having costate ooecia (see also Ostrovsky, Reference Ostrovsky, Wyse Jackson, Buttler and Spencer Jones2002). Winston et al. (Reference Winston, Vieira and Woollacott2014) remarked that the occurrence of costate ooecia in F. pulcherrima possibly suggests a better allocation of this species in a distinct genus. Inclusion of costae in the construction of the ooecium has also been observed in Figularia hilli (Osburn, Reference Osburn1950), with two small costae similar to those of the frontal shield added proximally to the larger ooecial halves (see Table 3).
Yang et al. (Reference Yang, Seo, Min, Grischenko and Gordon2018), while including pseudoporous ooecia in the diagnosis of Figularia, also suggested the examination of species with multiple ectooecial pseudopores in order to determine if they are genuinely congeneric. These species are here re-assigned to different genera (see also below and Table 4).
Suboral costae often differ from the other pairs. In the type species of Figularia, suboral costae merge, forming a smooth, wide shelf facing the orifice, most evident in ovicellate zooids (Fig. 6). Wide suboral costae associated with ovicellate zooids were also observed in F. rhodanica Li, Reference Li1990. In F. pelmatifera Gordon, Reference Gordon1984 the suboral pair of costae develops into two lateral, divergent, spinose processes (see Gordon, Reference Gordon1984, pl. 19, fig. E).
A certain variability occurs in the presence/absence of pelmata in the frontal shield, and in their position along the costal length. Sometimes this variability was noted (e.g., Gordon, Reference Gordon1984). Nevertheless, all Figularia species lacking pelmata (i.e., not included in formal descriptions and/or undetectable in available images) are fossil, except “F. philomela var. adnata” (Busk, Reference Busk1884), suggesting that their absence may be a preservation artefact.
The ancestrula is generally not mentioned in species descriptions to our knowledge. In the amended diagnosis, we include characters of the ancestrula for the first time, based on the ancestrula found in a colony of F. figularis from the Mediterranean illustrated in Rosso et al. (Reference Rosso, Sanfilippo, Sciuto, Serio, Catra, Alongi, Viola and Leonardi2019b, fig. 5C). The large size of both autozooids and ancestrula (0.65 × 0.67 mm) and the absence of spines are rare and remarkable among cribrilinids, which usually have small, tatiform ancestrulae, and this may have implications on the systematics/phylogeny of this genus within the family Cribrilinidae. However, observation of ancestrulae in additional species is needed to confirm whether this morphology is constant among congeners, which has been proven not to be the case in other cheilostome genera, such as e.g., Escharina Milne Edwards, Reference Milne Edwards1836 (see Berning et al., Reference Berning, Tilbrook and Rosso2008).
Several species previously assigned to Figularia were recently displaced in different genera of the families Cribrilinidae and Calloporidae (e.g., Vitrimurella, Reginella Jullien, Reference Jullien1886, Inferusia Kukliński and Barnes, Reference Kukliński and Barnes2009, Valdemunitella Canu, Reference Canu1900; see Bock and Gordon, Reference Bock and Gordon2020), and Jullienula Bassler, Reference Bassler and Moore1953 (Yang et al., Reference Yang, Seo, Min, Grischenko and Gordon2018). Here, we suggest further displacements: both Figularia? ampla Canu and Bassler, Reference Canu and Bassler1928, only tentatively included in Figularia when first described, and Emballotheca? capitifera, Canu and Bassler, Reference Canu and Bassler1929, subsequently referred to his new genus Calyptotheca by Harmer (Reference Harmer1957) and to Figularia by Di Martino and Taylor (Reference Di Martino and Taylor2018), fit better in Vitrimurella, owing to the pseudoporous zooidal gymnocyst and ooecia, and the extremely reduced costate shield. Figularia ryukyuensis Kataoka, Reference Kataoka1961 and F. jucunda Canu and Bassler, Reference Canu and Bassler1929 also need to be revised, pending examination of the type material. These species have pseudoporous ooecia formed by the distal kenozooid without a visible frontal part. Figularia duvergieri Bassler, Reference Bassler1936 has an unusual denticulate proximal orifice margin, and lacks costal pelmata and fenestrae in the ooecium. A detailed revision based on SEM images is needed to confirm generic allocation for these problematic species (Table 4). This issue has been partially addressed by López Gappa et al. (Reference López-Gappa, Pérez, Almeida, Iturra, Gordon and Vieirain press).
Figularia figularis (Johnston, Reference Johnston1847)
Figures 6, 7; Table 5
- Reference Johnston1847
Lepralia figularis Johnston, p. 314.
- Reference Prenant and Bobin1966
Figularia figularis; Prenant and Bobin, p. 604, fig. 2010 I–IV, VI.
- Reference Hayward and Ryland1998
Figularia figularis; Hayward and Ryland, p. 338, fig. 120, cum syn.
- Reference Hayward and McKinney2002
Figularia figularis; Hayward and McKinney, p. 38, fig. 16 D–E.
- Reference Berning2006
Figularia figularis; Berning, Reference Berning2006, p. 49, pl. 3, figs. 7, 10, cum syn.
- Reference Chimenz Gusso, Nicoletti and Bondanese2014
Figularia figularis; Chimenz Gusso et al., p. 167, fig. 84a–c.
Table 5. Measurements (in μm) of Figularia figularis and Figularia spectabilis n. sp. *Refers to an aberrant zooid (see text for further explanation). L: length; W: width.

Holotype
NHMUK 1847.9.16.39, English Channel, Recent.
Occurrence
Figularia figularis is widely distributed in the Atlanto-Mediterranean area since the middle Miocene (Moissette et al., Reference Moissette, Delrieu and Tsagaris1993; Berning, Reference Berning2006). This species has been commonly reported from shelf habitats, mostly from the deep shelf, often associated with deep coralligenous facies (Di Geronimo et al., Reference Di Geronimo, Giacobbe, Rosso and Sanfilippo1990; Ballesteros, Reference Ballesteros, Gibson, Atkinson and Gordon2006), and at the shelf break in both the Mediterranean (110–145 m; see Harmelin and d'Hondt, Reference Harmelin and d'Hondt1992) and the eastern Atlantic as far north as the British Isles (Hayward and Ryland, Reference Hayward and Ryland1998).
Materials
Rosso collection PMC. I. H. B.71.b, Apollo Bank sample: two specimens, Recent; Rosso-Collection PMC I. Pl. B.71.c, Scoppo: sample 24 top: two specimens, early Calabrian, MNN19b-19c biozones.
Remarks
Two fossil fragments were found, each consisting of a few zooids (Fig. 7). Zooidal morphological characters allow a reliable identification, even in the absence of ovicells and avicularia. Morphometrics fall within the ranges reported for this species. Inferred teratology in an autozooid resulted in a double-bifurcated frontal shield (Fig. 7.1, 7.2). This unusual feature also occurs in the type specimen of F. tenuicosta MacGillivray, Reference MacGillivray1895 from the middle Miocene of Victoria, Australia (Bock, Reference Bock2020). Although F. figularis exhibits a certain range of morphological variability, some historical records, mostly beyond its confirmed geographical range, proved to be different species (e.g., Brown, Reference Brown1952). The conspecificity of the colony found on a rock at Armaçao de Pêra in Portugal (Souto et al., Reference Souto, Reverter-Gil, De Blauwe and Fernández-Pulpeiro2014) needs to be verified. This colony has an unusual triangular ooecial fenestra with narrow horizontal part and could represent a different species.
Figularia spectabilis new species
Figures 8–11, Table 4

Figure 9. Figularia spectabilis n. sp., Capo Milazzo, sample Cala Sant'Antonino center, 2015, Gelasian, PMC. B22. 5.4.2015.a, holotype, ooecium. (1) Colony fragment with unique ovicellate zooid and vicarious avicularium; (2) close-up of the ovicellate zooid with ooecium formed by the distal kenozooid. Scale bars: (1) 500 μm; (2) 200 μm.

Figure 10. Figularia spectabilis n. sp., Capo Milazzo sample Cala Sant'Antonino center, 2015, Gelasian, PMC. B22. 5.4.2015.b, paratypes, autozooids. (1) Fragment of a bilaminar branch with zooids arranged in longitudinal rows and distal vicarious avicularium; (2) group of autozooids; (3) close-up of elongated autozooid with well-defined boundaries and growth lines in the gymnocyst (note the smooth texture of the costae, converging towards the midline, and the elongate pelmata); (4) wider autozooid with large wedge-shaped costae and very large drop-shaped pelmata; (5) close-up of some costae; (6) orifice; (7) orifice with closure plate or calcified operculum. Scale bars: (1, 2) 500 μm; (3, 4) 200 μm; (5–7) 100 μm.

Figure 11. Figularia spectabilis n. sp., Capo Milazzo, sample Cala Sant'Antonino center, 2015, Gelasian, vicarious avicularia. (1) Holotype PMC. B22. 5.4.2015.a; (2, 3) paratypes PMC. B22. 5.4.2015.b, same details as the holotype (note the spatulate rostrum and the thin crossbar); (3) view showing the wide rostral palate. Scale bars: 200 μm.
Holotype
PMC. B22. 5.4.2015.a: bilaminar fragment including some autozooids and the only observed ovicell. Cala Sant'Antonino, sample Cala Sant'Antonino center, 2015, Gelasian.
Paratypes
PMC. B22. 5.4.2015.b: additional 39 fragments from the same sample as the holotype, including several fan-shaped colony portions. One fragment from sample 17 (2000), Cala Sant'Antonino center.
Diagnosis
Colony erect, bilaminar with fan-shaped fronds, the tapering proximal terminations possibly consisting of heteromorphs, likely rising from an encrusting phase. Zooidal frontal shield consisting of flat costae, each with a large, elongate drop-shaped pelma placed on its peripheral half; gymnocyst wider laterally and proximally, narrower distally, with faint striations. Vicarious avicularia elongate, spatulate, with extensive rostral palate and complete crossbar. Ovicell subimmersed, presumably cleithral. Ooecium formed by the distal kenozooid with frontally visible costate part, and consisting of two very large, wing-shaped costae merging in the midline producing a longitudinal suture, with two large fenestrae exposing wide areas of endooecium; the costae of the ooecium-producing kenozooid smaller, forming a distal, crescent-shaped crown, each costa with a small pelma.
Occurrence
Exclusively known from early Pleistocene (Gelasian) deep-water sediments of Capo Milazzo (NE Sicily, Italy).
Description
Available colony fragments bilaminar, fan-shaped (the largest ~2 mm long by 3 mm wide); fragments diverging distally at variable angles from a subcylindrical proximal portion, consisting of four zooids arranged in back-to-back pairs (Fig. 8.1, 8.2, 8.4–8.8). Other fragments of similar size include only the edges of presumably ribbon-like colonies (Figs. 8.3, 10.1). Putative proximal heteromorphs, possibly arising from an encrusting phase and forming the basal stalk, lacking calcified frontal shield. Zooidal boundaries marked by grooves. Zooids large, about twice as long as wide (L/W = 1.72), gently arched distally, wedged proximally. Gymnocyst more extensively exposed proximally and laterally (Figs. 8.2, 8.3, 8.5, 10.1–10.4), locally obliterated by recrystallization (Fig. 10.5). Costate shield extensive (~75% of the frontal surface), gently convex, formed by 7–14 flat and smooth costae (maximum basal width 72–111 μm), varying from short and subtriangular proximally to long and parallel sided distally; the suboral pair often the largest (Fig. 10.1–10.5). Costae defined by grooves, connected by an uncertain number of intercostal bridges, presumably 3–4 (Fig. 10.5), with small oval to subcircular intercostal pores in between. A longitudinal suture marking the costal fusion along zooidal midline (Fig. 11.1). Each costa bearing a single, elongate, drop-shaped pelma with the rounded base placed in correspondence with the base of the costa, while the acute vertex extends up to half to two thirds of costal length. Orifice oval to round, slightly longer than wide, concave proximally, gently arched distally, outlined by a rim of calcification (Fig. 10). Oral spines absent. Avicularia vicarious, infrequent, elongate and slightly asymmetrical, varying in size; rostrum long, spatulate, directed distally and slightly inclined, facing frontally (Figs. 9.1, 11); post-mandibular area short, palate wide, crossbar complete (Fig. 11.3). Ovicell subimmersed, presumably cleithral. A single observed ooecium formed by the distal kenozooid with frontally visible costate shield of 10 costae, longer than wide, wider and slightly more prominent than the ovicellate zooid (Fig. 9). Very large ooecium consisting of two flat, wing-shaped costae converging along the midline, the fusion marked by a longitudinal suture, distally with two small tubercle-like prominences. Large rhomboidal fenestra exposing finely granular endooecium. Orifice of the ovicellate zooid slightly larger than those of autozooids, rounded rectangular. Closure plates or calcified opercula sometimes occluding orifices (Fig. 10.7).
Etymology
From the Latin spectabilis, meaning remarkable, exceptional, alluding to the distinctive architecture of the colony and ooecium.
Remarks
The morphology of the colony, zooids and ooecium distinguish Figularia spectabilis n. sp. from congeners. The flabellate to short, ribbon-like morphology of the colony, with putative heteromorphs placed basally, may suggest the occurrence of basal rhizoids for fixation to the substratum. Alternatively, the connection to an encrusting portion may develop through “sites of articulation” as in Bryobaculum carinatum Rosso, Reference Rosso2002a, occurring in the same sediment.
Discussion
Five species of cribrilinid bryozoans, three of which are new to science, namely Cribrilaria profunda n. sp., Glabrilaria cf. G. pedunculata, G. transversocarinata n. sp., Figularia figularis, and F. spectabilis n. sp., were found in Pleistocene deep-water sediments from north-eastern Sicily.
Figularia figularis was already recorded from the area by Seguenza (Reference Seguenza1880) and Neviani (Reference Neviani1900), while C. profunda n. sp. was possibly recorded as Lepralia planicosta (see Remarks above), while the remaining three species, including G. cf. G. pedunculata, represent new records.
Cribrilinids are generally rare in Plio-Pleistocene associations from deep-water environments in Sicily and Calabria, as well as in their enclaves in shallow waters, such as past submarine cave habitats, from which a single species, Cribrilaria venusta (Canu and Bassler, Reference Canu and Bassler1925), and undetermined cribrilinid taxa were previously reported (Di Geronimo et al., Reference Di Geronimo, D'Atri, La Perna, Rosso, Sanfilippo and Violanti1997, Reference Di Geronimo, Messina, Rosso, Sanfilippo, Sciuto, Vertino, Freiwald and Roberts2005; Rosso, Reference Rosso2005; Rosso et al., Reference Rosso, Sanfilippo, Ruggieri, Maniscalco and Vertino2015). Thus, this study raises the total number of cribrilinids from these paleoenvironments to six species in three genera. Shallower shelf paleoenvironments from the same regions, mostly Pleistocene but as old as Miocene, yielded seven species of cribrilinids: Cribrilaria radiata (Moll, Reference Moll1803), C. hincksi (Friedl, Reference Friedl1917), C. innominata (Couch, Reference Couch1844), Puellina gattyae (Landsborough, Reference Landsborough1852), Distansescharella seguenzai Cipolla, Reference Cipolla1921, Gephyrotes moissettei Di Martino and Rosso, Reference Di Martino and Rosso2015, and “Cribrilina punctata” (Hassall, Reference Hassall1841), the latter species probably being a Collarina (Barrier et al., Reference Barrier, Casale, Costa, Di Geronimo, Oliveri and Rosso1987b; Harmelin et al., Reference Harmelin, Boronat, Moissette and Rosso1989; Di Geronimo et al., Reference Di Geronimo, Costa, La Perna, Randazzo, Rosso and Sanfilippo1994; Rosso and Sanfilippo, Reference Rosso and Sanfilippo2005; Di Martino and Rosso, Reference Di Martino and Rosso2015). As for other taxa authored by Seguenza (Reference Seguenza1880), the loss of the type material makes it difficult to confirm the status of some cribrilinid species, such as Lepralia thiara, L. mitrata, and L. mitrata v. radians, in addition to the previously mentioned L. elegantissima and L. planicosta. Analogously, the real identity of some other species (briefly described and lacking illustrations) in Waters (Reference Waters1878), De Stefani (Reference De Stefani1884), Hincks (Reference Hincks1884), and Neviani (Reference Neviani1900, and references therein) is doubtful.
Focusing only on deep-water assemblages, cribrilinids are present with three species in both the Gelasian associations from Capo Milazzo and the Calabrian (MNN19b-19c biozones) of Scoppo. These figures are comparable to those found in present-day deep-water associations from the Mediterranean and Atlantic (Bahama Bank), in which cribrilinids usually occur with 2–3 species (Rosso et al., Reference Rosso, Beuck, Vertino, Sanfilippo and Freiwald2018). However, the Gelasian of Capo Milazzo includes at least 46 cheilostome species, and the cribrilinid relative percentage is ~6%, which is lower than the 10–18% found in present-day assemblages (Rosso and Sciuto, Reference Rosso and Sciuto2019). No comparison can be made for the Calabrian of Scoppo whose bryozoans are still under investigation.
Discovery of a new species of Figularia, F. spectabilis n. sp., led to the emendation of the genus diagnosis and the re-examination of the 32 species and one variety currently assigned to the genus, based on drawings and photographic material available from the literature. This preliminary survey allows us to confidently reassign two species based on published scanning electron micrographs of the type material. The newly proposed combinations are Vitrimurella capitifera (Canu and Bassler, Reference Canu and Bassler1929) n. comb. and Hayamiellina quaylei (Powell, Reference Powell1967a) n. comb., as also suggested by Kukliński et al. (Reference Kukliński, Grischenko and Jewett2015). Thirteen species remain doubtful and their assignment to more suitable genera requires examination of the type material (Table 4).
At present, 18 species, including Figularia spectabilis n. sp., match the diagnosis of the genus. This figure will likely change further after a more detailed revision of some fossil species and species left in open nomenclature (see Berning, Reference Berning2006 for F. haueri and F. figularis; Di Martino et al., Reference Di Martino, Taylor and Portell2017 and Cook et al., Reference Cook, Bock, Hayward, Gordon, Cook, Bock, Gordon and Weaver2018 for two different Figularia spp.) as well as cryptic species/species complexes (e.g., F. clithridiata and F. fissa). Based on our literature review, the diversity of Figularia is reduced by about one-half, from 33 (including F. spectabilis n. sp.) to 18 species, with a revision in the stratigraphic range, but only little variation in the geographic distribution of the genus. The genus possibly appeared in the Cenozoic of Europe and Australia, and commonly occurred in sediments in the European-Mediterranean area during the Miocene. Of the 12 species of Figularia living today, 10 species are found in the Pacific and Australasian region. Only two species, F. figularis and F. dimorpha, fall outside this area, being recorded in the Atlanto-Mediterranean and southwestern Atlantic regions, respectively.
A twofold future investigation is sought. This includes an examination of the type material of all the species in the genus to confirm their status, prioritizing those that appear to remain problematic (see Table 4; issue partially addressed by López Gappa et al., Reference López-Gappa, Pérez, Almeida, Iturra, Gordon and Vieirain press), and an accurate re-examination of all species records to refine both the temporal and spatial distribution of the genus and reconstruct its diversification history, as well as disentangle species complexes.
Acknowledgments
M.H. Dick (University of Hokkaido, Japan), P.E. Bock (National Museum of Victoria, Melbourne, Australia), and N. Vávra (University of Vienna, Austria) are thanked for their kind help with the bibliography. A. Viola (University of Catania) assisted with scanning electron microscopy. L. Vieira (Universidade Federal de Pernambuco, Brasil), D.P. Gordon (NIWA, Wellington, New Zealand), and B. Berning (Oberösterreichisches Landesmuseum, Linz, Austria) provided constructive comments that greatly improved the originally submitted manuscript. This research was supported by the University of Catania “Piano della Ricerca 2016/2018 (code n. 227221132118)” and “PiaCeRi - Piano Incentivi per la Ricerca di Ateneo 2020-22 linea di intervento 2,” while sampling of the studied material was carried out in the frame of previous projects. E. Di Martino received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation program (grant agreement No 724324 to L.H. Liow). A. Ostrovsky thanks the Russian Science Foundation (grant 18-14-00086) for financial support of the studies on cheilostome brood chambers. This is Catania Paleontological Research Group: contribution n. 465.



















