Introduction
Anthropogenic activities such as worldwide trade or travel led to an increase of invasive species around the world, which is a major driver for shifting species compositions (Colautti et al., Reference Colautti, Bailey, Van Overdijk, Amundsen and MacIsaac2006; Pejchar & Mooney, Reference Pejchar and Mooney2009) and loss of ecosystem functions (Cardinale et al., Reference Cardinale, Duffy and Gonzalez2012). Even though ecosystems are well organized and aligned structures, they are still dynamic and reorganize themselves continuously. If a non-indigenous species enters a habitat, it might face competition with native or other invasive species for space and food. In general, it is difficult to measure the impact of an invasive species on ecosystem functions in a new habitat due to the complexity of interacting factors in an ecosystem (Kumschick et al., Reference Kumschick, Gaertner and Vilà2015). Even though the magnitude of the impact is hard to measure, there are several studies attributing changes to the presence of introduced species (Doody et al., Reference Doody, Rhind, Green, Castellano, McHenry and Clulow2017; Atalah & Sanchez-Jerez, Reference Atalah and Sanchez-Jerez2020; Livingstone et al., Reference Livingstone, Isaac and Cadotte2020). Albins (Reference Albins2013), for example, compared the influence of an invasive predator fish (Lionfish, Pterois volitans) on the local diversity of a prey community at Bahamian coral reef, to the influence of a native predator fish (coney grouper, Cephalopholis fulva). He illustrated that the presence of the invasive species had an impact on prey communities, regardless of whether the native piscivore was present or not. Nevertheless, it remains difficult to measure the long-term impact of the invader on the conquered ecosystem, as changes in prey community, including herbivores, which are keeping seaweeds under control, or cleaner fish that control ectoparasite density on other fish species, may lead to a complete reconstruction of the coral reef with unpredictable consequences (Albins, Reference Albins2013).
Comparing the number of introduced species with established invasive ones, it seems by far more common that new species are not able to establish themselves in a new environment. Local species are often more resilient, the amount of introduced individuals and the adaptability of new species to habitats have a decisive impact and lead in few cases to stable, invasive populations (Carlton & Geller, Reference Carlton and Geller1993; Kolar & Lodge, Reference Kolar and Lodge2001). In rare cases, introduced species are able to reproduce quickly, with tremendous consequences for local ecosystems. For example, the introduction of brown snakes (Boiga irregularis) to Guam – where they forage on local birds and rodents, but do not face any predator – led to the extinction of many native bird species (Wiles et al., Reference Wiles, Bart, Beck and Aguon2003).
Among the multitude of ecological factors that are covered in invasion research, a neglected but important topic are neozoan parasites (Poulin, Reference Poulin2017). On the host–parasite level, the new host can be seen as the newly conquered ecosystem, with the same scenarios being possible as described above for free-living species. Accordingly, if a new parasite species conquers a new region, it is in need of suitable hosts. If such a host is already occupied by other parasites, it either needs to find other suitable hosts, outcompete the existing parasite or co-occur within the same host, otherwise it is not able to survive and establish a population. However, if parasites do co-exist in one host and are closely related, it is also possible that they produce hybrid offspring (King et al., Reference King, Stelkens, Webster, Smith and Brockhurst2015). Several studies have demonstrated that hybridization is not only possible, but that that hybrid parasites might have a better host exploitation, faster maturation time and a better resistance against the host's immune system (Oey et al., Reference Oey, Zakrzewski and Gravermann2019).
Natural hybridization is a mechanism that is commonly examined in evolutionary science and considered as one of the major drivers and sources for genetic variance (Arnold, Reference Arnold2004; Barton, Reference Barton2008; Harrison et al., Reference Harrison, Berumen, Saenz-Agudelo, Salas, Williamson and Jones2017). In some instances, hybrid offspring can develop in both host species of their parental generation, which provides them with a better host range. This was shown, for example, for hybrid offspring of Schistosoma bovis, a parasite of cattle, and Schistosoma haematobium, a common human parasite, collected from children in Senegal (Webster et al., Reference Webster, Diaw, Seye, Webster and Rollinson2013). Schelkle et al. (Reference Schelkle, Faria, Johnson, van Oosterhout and Cable2012) suggest that hybridized monogeneans may exhibit a higher capability to escape the host immune system. In contrast, it is also possible that hybridization between parasites can limit the adaptations that one species develops to a host and, therefore, decreases their infectivity (Dybdahl et al., Reference Dybdahl, Jokela, Delph, Koskella and Lively2008). However, hybrids may face subsequent reproductive challenges, as some may be unable to produce fertile offspring (Al-Ahmad et al., Reference Al-Ahmad, Galili and Gressel2006; Thomsen et al., Reference Thomsen, Schauser, Bertelsen, Vejlsted, Grøndahl and Christensen2011).
The well-studied swim bladder nematode Anguillicola crassus is one example of a parasite that has managed to outcompete an already established invasive parasite, Anguillicola novaezelandiae, with the same habitat requirements: the swim bladder of European eels (Anguilla anguilla). In the 1970s, the nematode A. novaezelandiae, originating from the Shortfin eel Anguilla australis, was introduced to Lake Bracciano, Italy, where the parasite was able to establish a stable population in the native European eel (A. anguilla) population (Paggi et al., Reference Paggi, Orecchia, Minervini and Mattiucci1982). However, because the lake is not connected to other waterbodies, the parasite population remained in this particular lake and did not spread further. After the introduction of the closely related invasive species A. crassus, originating from the Japanese eel (Anguilla japonica) in the early 1980s, both species were reported to co-occur in Lake Bracciano, even though mixed infections in eels have never been reported (Moravec et al., Reference Moravec, Di Cave, Orecchia and Paggi1994). Nevertheless, a few years later, A. novaezelandiae seems to have gone extinct, and A. crassus is the only species reported from eels from Lake Bracciano in Italy (Münderle, Reference Münderle2005). Later, Grabner et al. (Reference Grabner, Dangel and Sures2012) demonstrated that mixed infestations of both nematode species in one eel produce hybrid offspring under laboratory conditions.
The aim of this study is to build on the previous results from Grabner et al. (Reference Grabner, Dangel and Sures2012), which are based on a single infested eel. We combine infestation hybridization experiments with molecular validation of hybridization to investigate hybridization events more explicitly in the F1 (1st filial) generation of A. crassus and A. novaezelandiae in European eels. However, in order to validate if the genetic advantage of A. crassus might be an explanation for the disappearance of A. novaezelandiae from Lake Bracciano, a multi-generation study with several eels has to be performed.
Materials and methods
Animal source
European eels (A. anguilla) were purchased from an eel farm (Albe Fischfarm, Haren/Rütenbrock, Germany), where A. crassus infections have not been previously recorded (Dangel et al., Reference Dangel, Keppel and Sures2013; Hohenadler et al., Reference Hohenadler, Honka, Emde, Klimpel and Sures2018). The general absence of the parasite was verified by the dissection of ten randomly chosen eels, which were checked by light microscopy for infestation of the swim bladder.
Larvae (L2) of A. crassus were collected from European eels from the River Rhine caught by fishermen. L2 of A. novaezelandiae were obtained from a lab culture, which originated from A. australis from New Zealand (see also Grabner et al., Reference Grabner, Dangel and Sures2012; Dangel & Sures, Reference Dangel and Sures2013). Life cycles were established according to Haenen et al. (Reference Haenen, Van Wijngaarden and Borgsteede1994).
To prevent the accidental release of A. novaezelandiae or potential hybrid larvae into the waste water system, all used tank water was collected and boiled before being discharged into the sewage system.
Infestation experiments
F0 generation
For the hybridization experiment, four eels were inoculated with ten larvae (L3) of A. crassus and A. novaezelandiae each by a stomach tube (1.5 mm diameter; B. Braun Melsungen AG, Germany) according to Sures & Knopf (Reference Sures and Knopf2004). After inoculation, eels were kept for 150 days in aerated 80 l tanks with a PVC tube as environmental enrichment. Twice a week, they were fed ad libitum with eel pellets (DAN-EX 2848, BioMar A/S, Brande, Denmark) and one-third of the water was changed the day after feeding. After 150 days post inoculation (dpi), eels were dissected, adult nematodes were counted and sexes were distinguished by light microscopy.
F1 generation
Each gravid female from the previous hybridization experiment was carefully washed to remove eggs attached to the outer cuticle. Developed eggs containing F1 L2 were removed from the uterus. One batch of eggs was stored in 70% ethanol for further molecular analysis and another batch was transferred to tap water to initiate hatching of L2 that were fed to copepods (Macrocyclops albidus). Developed F1 L3 stages were removed from copepods after 14 days, and 20 eels were inoculated with these as described above. Each eel was inoculated with 11–27 L3 individuals originating from a single female nematode. The further procedure was performed as described above for the F0 (parental) generation, including checking gravid females for embryonated eggs.
Molecular analysis
Small pieces of the pharynx or cuticle were cut out of adult individuals, and washed multiple times in Milli-Q water, to remove contaminations of the host tissue. DNA was extracted with a salt precipitation protocol as described in Grabner et al. (Reference Grabner, Weigand and Leese2015). To verify species identity of the parental generation, molecular barcoding was performed using species-specific primer targeting cox I according to Grabner et al. (Reference Grabner, Dangel and Sures2012). Primer sets for each species were run separately for every individual sample. The polymerase chain reaction (PCR) mix contained 10 μl OneTaq® 2X Master Mix (New England Biolabs, Frankfurt am Main, Germany), 0.5 μM of each primer and 1 μl of sample DNA, and was topped up to 20 μl with PCR-grade water. The PCR was run on a peqStar Labcycler at 95°C for 5 min, 35 cycles of 95°C, 58°C and 72°C each for 45 s, and a final elongation at 72°C for 5 min. PCR products were checked by standard agarose gel electrophoresis (1.5% agarose, 85 V, 100–1000 bp ladder). Bands for A. crassus are expected at 303 bp, and at 404 bp for A. novaezelandiae.
Analysis of microsatellite markers was used to identify a possible hybrid origin of the F1 generation. The markers AcrCT04 and AcrCA102 (Wielgoss et al., Reference Wielgoss, Sanetra, Meyer and Wirth2007) were used as described in Grabner et al. (Reference Grabner, Dangel and Sures2012). PCR was conducted as described above, with the following conditions: 94°C for 5 min, 35 cycles of 94°C, 55.9°C and 72°C each for 45 s and a final elongation at 72°C for 10 min.
PCR products were further analysed with a Fragment Analyzer™ (Agilent Technologies, Waldbronn, Germany) using a 33 cm capillary and the dsDNA 905 Reagent Kit (Agilent Technologies). DNA concentrations were quantified by Fragment Analyzer™ Automated CE System PROSize® 3.0. Marker fragment sizes were evaluated based on PCR products amplified with the ArcCT04 and ArcCA102 of all F0 adult worms. The resulting fragment sizes were assigned to the respective Anguillicola species. Because the microsatellite markers yield fragments of 100–260 bp (ArcCT04) and 297–332 bp (ArcCA102) (Wielgoss et al., Reference Wielgoss, Sanetra, Meyer and Wirth2007), signals <100 bp and >400 bp, as well as signals with a relative intensity of <5%, were not considered further. In addition, fragments between 136 and 139 bp were excluded, as they appear for both species.
To account for the uncertainty of 3–5 bp of the microsatellite measurement in the resulting fragments, closely spaced bands were merged as follows, resulting in fragment sizes that were exclusively found in one or the other species and named accordingly (AC: A. crassus; AN: A. novaezelandiae): AC1 = 115–117 bp; AC2 = 136–139 bp; AC3 = 146–149 bp; AN1 = 119–124 bp; AN2 = 129–133 bp; AN3 = 141–142 bp.
From the F1 generation, a total of 30 eggs and 48 adult individuals originating from A. crassus mothers and 50 eggs and 88 adult individuals originating from A. novaezelandiae mothers were individually examined.
Results
F0 generation
All four inoculated eels were infested with various numbers of Anguillicola spp. individuals, with recovery rates ranging between 15% and 60%. Initial screening of the nematodes revealed that eel no. I was infested with females only. Since no offspring is possible without male individuals, these nematodes were not considered for further investigation. The infracommunities of the other eels were composed as follows: eel no. II: 6 ♀, 6 ♂; eel no. III: 6 ♀, 4 ♂; eel no. IV: 4 ♀, 4 ♂. All females were gravid, apart from two individuals – one in eel no. II and one in eel no. IV.
Species were determined by species-specific cox I primers and microsatellite analysis of the parental generation revealed three distinguishable alleles for both A. crassus (AC1-3) and A. novaezelandiae (AN1-3) (table 1). The results of species identification by cox I primers matched the results of species-specific microsatellite alleles consistently. Only one individual (IIIW6) showed unambiguous alleles of A. novaezelandiae, but could not be clearly distinguished by cox I primers as bands for both A. novaezelandiae and for A. crassus were visible. Two individuals (IVW2 and IVW3) showed no band in the cox I PCR, but as their microsatellite alleles showed a clear A. novaezelandiae pattern, they were considered as such.
Table 1. Molecular analysis of microsatellite markers (AcrCT04) of adult A. crassus and A. novaezelandiae of all examined eels (I–IV). Fragments were merged as follows: AC1 = 115–117 bp; AC2 = 136–139 bp; AC3 = 146–149 bp; AN1 = 120–124 bp; AN2 = 129–133 bp; AN3 = 141–142 bp.

a Species determination targeting cox I.
For six individuals (IIM4, IIIW1, IIIM3, IIIW5, IVM3, IVM4), no distinct microsatellite pattern was visible. The detected fragments were lying outside the range of the microsatellite alleles located for the two species, which are, therefore, considered as unspecific fragments. This remained the case even after repeating the measurement.
F1 generation
Recovery rates of nematodes originating from A. crassus mothers ranged between 4% and 67%, with 3.0 ± 2.2 ♀ and 3.1 ± 3.2 ♂. Recovery rates of nematodes originating from A. novaezelandiae mothers ranged between 11% and 65%, with 3.6 ± 2.8 ♀ and 1.7 ± 1.3 ♂. Most of the females had either no eggs or poorly developed/unembryonated eggs, except for three females originating from an A. crassus mother, which showed normally developed and embryonated eggs.
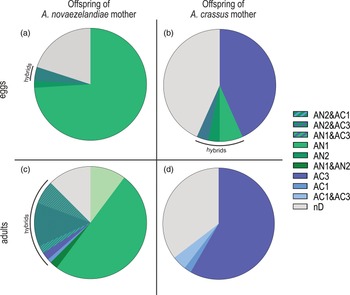
The ratio of hybrids and non-hybrids differs between eggs (containing L2) and developed adults of F1 generation (fig. 1). Eggs that originated from A. crassus females revealed 13% offspring with alleles of both species and 43% offspring with A. crassus alleles only, whereas eggs that originated from A. novaezelandiae females revealed 4% offspring with alleles of both species and 76% offspring with only A. novaezelandiae alleles. Hybridization was not detected in any of the adult offspring originating from A. crassus females. Adult offspring of A. novaezelandiae females revealed a percentage of 25% with alleles of both species, whereas 63% showed only alleles of A. novaezelandiae. The number of individuals without a distinct pattern for offspring originating from an A. crassus mother differs between 43% (eggs) and 35% (adults), and for offspring originating from an A. novaezelandiae mother, between 20% (eggs) and 13% (adults).

Fig. 1. Ratio of alleles of F1 generation. Shades of blue/purple represent A. crassus alleles; shades of green represent A. novaezelandiae alleles. (a) Eggs (with L2) from A. novaezelandiae mother (n = 50); (b) eggs (with L2) from A. crassus mother (n = 30); (c) adults from A. novaezelandiae mother (n = 88); (d) adults from A. crassus mother (n = 48). AC1 = 115–117 bp; AC3 = 144–150 bp; AN1 = 119–126 bp; AN2 = 128–133 bp. nD, no distinct pattern of fragments and, therefore, no species allocation possible.
Discussion
In the present study, we provide additional details on the relevance of hybridization between the two eel swim bladder nematodes A. crassus and A. novaezelandiae. Previously, Grabner et al. (Reference Grabner, Dangel and Sures2012) suggested that A. crassus may have genetic advantages over A. novaezelandiae, as their findings indicated that hybridization appears to be possible only between A. novaezelandiae females and A. crassus males. Since they described this pattern based on nematodes obtained from one single eel only, it had remained uncertain whether this finding is reproducible. To verify the hypotheses of genetic advantages, the present study provides evidence for the viability of hybrid offspring and indicates that only hybrid offspring of A. novaezelandiae females can develop to F1 adults. We distinguish between hybrid larvae, which were released by the mother nematode but not developed further, and those that developed to the adult stage after passage through the copepod and experimental infection of an eel. Our results confirm the findings by Grabner et al. (Reference Grabner, Dangel and Sures2012) with respect to possible hybridization between both species of Anguillicola, and give further information about hybrid development.
In the present study, the length of the fragments amplified with the AcrCT04 primers varied between 120 bp and 142 bp, while previous data showed a uniform pattern for A. novaezelandiae of a single allele of 109 bp obtained by the AcrCT04 primers (Grabner et al., Reference Grabner, Dangel and Sures2012). This is due to the fact that a new field isolate of A. novaezelandiae was used for the laboratory cycle in the present study, showing a different allelic pattern. Interestingly, alleles of A. novaezelandiae were found only in the egg stages obtained from A. crassus mothers. Those eggs obtained from A. crassus mothers that were passed through a copepod and were used to infest an eel showed exclusively A. crassus alleles in the developing F1 adults. This indicates that hybrid eggs and larvae originating from the A. crassus female/A. novaezelandiae male crossing only develop to the larval stages. Even though the length difference between AC1 (115–117 bp) and AN1 (120–124 bp) may be at the limit of measuring accuracy of the method, it was consistently the case in all measurements performed that samples of both species never exceeded this limit. Therefore, we consider the size assignment of the alleles as valid.
Nevertheless, we cannot exclude the possibility that those hybrid nematodes develop to adult stages, as the examined sample size is still too small to give complete proof. Among individuals that derived from an A. novaezelandiae mother, the number of hybrids increased from 4% in the egg stage to 25% in adult nematodes, which is a strong indication that the development proceeds with greater success in this hybrid crossing. Recovery rates of hybrid offspring originating from both species are not known, as we cannot detect if an infectious larva is a hybrid beforehand without dissecting it; however, the further development into adult stages can be seen as a good predictor. In addition, the number of offspring without any distinct allelic pattern is higher in individuals originating from A. crassus mothers (35–43%), compared to those from A. novaezelandiae mothers (13–20%). This could be due to a different binding efficiency of the AcrCT04 primers for the two species, resulting in a lower density of bands for A. crassus.
The finding of the present study that A. novaezelandiae female/A. crassus male crossings exist and can even develop to the adult stage adds to the information provided by Grabner et al. (Reference Grabner, Dangel and Sures2012), who analysed only egg stages and, thus, could only speculate about further development. Applying our results to the situation in Lake Bracciano in the late 1980s, the interpretation depends on the fate of the F1 adults, which we still cannot predict with certainty. Basically, there are two possibilities – either hybrid offspring is viable and fertile, or hybridization leads to a dead end of reproduction. If hybrids are fertile, it might even be a disadvantage for A. crassus to produce hybrid offspring with A. novaezelandiae, as the former has by far the better adaptation to the eel's immune response (Knopf et al., Reference Knopf, Naser, Van Der Heijden and Taraschewski2000; Keppel et al., Reference Keppel, Dangel and Sures2014), and there is the possibility that hybrid offspring will lack some of those adaptations. The life cycle of A. crassus is also more efficient compared to A. novaezelandiae, as the larvae are released over a longer period of time. Accordingly, these larvae are capable of infesting the intermediate host for a longer period of time as well (Dangel et al., Reference Dangel, Keppel and Sures2013). Therefore, it may be worse for hybrid offspring of A. crassus to lose this efficiency – although this is only speculation according to current knowledge, since no valid data on hybrid offspring performance are available. The effect of the hybrids on the populations of the two Anguillicola species would also depend on the potential differential reproductive success of each of the two species with the hybrids. However, we can only speculate about the further development and fertility of the F1 generation. In other species, male hybrids in particular are often facing sterility, as has been found for Drosophila, mice and other animals (Haldane, Reference Haldane1922; Price & Bouvier, Reference Price and Bouvier2002; Sun et al., Reference Sun, Ting and Wu2004; Thomsen et al., Reference Thomsen, Schauser, Bertelsen, Vejlsted, Grøndahl and Christensen2011; Kagawa & Takimoto, Reference Kagawa and Takimoto2018; Widmayer et al., Reference Widmayer, Handel and Aylor2020); however, there are also a variety of known studies showing that hybrids can indeed be fertile (Wallis & Beardmore, Reference Wallis and Beardmore1980; Close & Bell, Reference Close and Bell1997; Volf et al., Reference Volf, Benkova, Myskova, Sadlova, Campino and Ravel2007). If hybrid offspring are not fertile, this is to some extent a disadvantage for both species, as some of their reproduction effort leads to a dead end. Yet, it seems reasonable that A. crassus was able to combine its ecological advantage of a more efficient life cycle (Dangel et al., Reference Dangel, Keppel and Sures2013), underlined by theoretical modelling of the population growth rate of the two species (Dangel et al., Reference Dangel, Keppel, Le, Grabner and Sures2015), with some genetic advantage to contribute to the extinction of A. novaezelandiae in Lake Bracciano. The latter had to face an additional fitness impairment as it lost some reproductive output to non-viable or non-fertile hybrids, which it was not able to cope with. Nevertheless, existing in constant competition with another species is an energy-consuming process, such that in the long run it was more beneficial to eliminate a competitor and accept possible minor disadvantages – for example, a slightly worse adaptation to the immune response of the host.
Conclusively, this research contributes to a better understanding of what happened in the Lake Bracciano in the late 1980s and early 1990s – hybridization between the two species might have decreased the reproductive fitness of both, but due a more efficient life cycle and population growth rate, A. crassus could eventually make up for this disadvantage, while A. novaezelandiae has gone extinct.
Future experimental studies should focus on the viability and fertility of the F2 (2nd filial) generation to further clarify the fate of hybrid individuals in a population. Furthermore, the gene flow between the two Anguillicola species should be measured using a high number of genomic markers, using double-digest restriction-site-associated DNA, which has been shown to be efficient in detecting hybridization in previous studies (e.g. Xu & Hausdorf, Reference Xu and Hausdorf2021; Paulus et al., Reference Paulus, Brix and Siebert2022).
Acknowledgements
We thank Dr Arne Beermann and Dominik Buchner, Aquatic Ecosystem Research, University of Duisburg-Essen, for their assistance with microsatellite analysis, and Prof Dr Christoph Koch, Ernst-Abbe University of Applied Science, Jena, for his comments on the manuscript.
Financial support
The authors declare that no financial support was received.
Conflict of interest
None.
Ethical standards
All experimental protocols were approved by the Ethics Council (Landesamt für Natur, Umwelt und Verbraucherschutz, Nordrhein-Westfalen, Germany, permit number 84–02.05.40.16.017) and were carried out in accordance with the relevant guidelines and regulations.