Hypocalcemia is still a frequent occurrence in dairy cows with a wide variation of prevalence between herds and individual cows (Perruchoud et al., Reference Perruchoud, Maeschli, Bachmann, Walkenhorst, Mevissen and P Zanolari2017). With the onset of milk production after parturition the cow loses 1.8–2.5 g of calcium with each liter of colostrum. This deficit must be quickly compensated to maintain calcium homeostasis (Khol et al., Reference Khol, Moser, Miklis, Dirisamer and Wittek2020). If the calcium loss is not compensated quickly enough, the serum calcium concentration decreases, and subclinical or clinical parturient paresis is the consequence (Degaris and Lean, Reference Degaris and Lean2008). In this case, the calcium deficit must be compensated immediately to avoid secondary damages to muscle and skeleton. This is usually achieved by intravenous calcium infusion and supported with glucose infusion. Oral calcium supplements can also be used (Perruchoud et al., Reference Perruchoud, Maeschli, Bachmann, Walkenhorst, Mevissen and P Zanolari2017).
During the dry period, the cow needs little calcium, consequently the metabolism is set to calcium storage. After parturition calcium is secreted in colostrum and then milk and the organism must switch the calcium metabolization within a short time (Degaris and Lean, Reference Degaris and Lean2008). However, the receptors and mechanism need a certain time for adaption. Consequently, a deficiency occurs temporarily (Lean et al., Reference Lean, DeGaris, McNeil and Block2006). Numerous prophylactic concepts have been proposed and applied either at group or cow level to minimize hypocalcemia (Perruchoud et al., Reference Perruchoud, Maeschli, Bachmann, Walkenhorst, Mevissen and P Zanolari2017). Examples for measures at group level are feeding of low calcium rations to transition cows, adding calcium binding compounds to the ration or inducing a metabolic acidosis by the feeding of anionic salts leading to activation of calcium absorption mechanisms from bone (increased osteoclast activity: Khol et al., Reference Khol, Moser, Miklis, Dirisamer and Wittek2020). A frequently applied measure at cow level is the injection of 10 million IU of vitamin D3 one week before the expected date of calving (Khol et al., Reference Khol, Moser, Miklis, Dirisamer and Wittek2020). This increases the total Ca concentration on the first 3 d after calving but shortens the gestation length and decreases milk production (Venjakob et al., Reference Venjakob, Bauerfeind, Staufenbiel, Heuwieser, Borchardt, Stangl, Hirche, Kononov and Wilkens2024). Therefore, it is not recommended for genereal use to prevent hypocalcemia (Venjakob et al., Reference Venjakob, Bauerfeind, Staufenbiel, Heuwieser, Borchardt, Stangl, Hirche, Kononov and Wilkens2024).
Selection of the most suitable prophylactic measures is dependent on prevalence, management and feeding condition of the herds. Small- and medium-sized dairy farms (average number of 34 cows per farm: BMLRT, 2023) are typical in Austria, therefore, grouping of cows which are at a similar stage of lactation is practically impossible. This makes groupwise prophylactic measures impractical. The consequence is that under Austrian dairy farm conditions the prophylactic measures must be applied at cow level, which is both cost and labor intensive. Therefore, the assessment of individual cows according to their risk for hypocalcemia would be very beneficial, by enabling individually customized prophylactic measures.
Currently there appear to be no available parameters with sufficient predictive value to predict the risk for hypocalcemia before it occurs during the dry period. Animals at risk can be estimated by age, milk yield, treatment history and feeding management, but more precise predictions are not possible. The partially bone associated enzyme alkaline phosphatase (ALP) has been investigated as a parameter to predict the risk of periparturient hypocalcemia in cattle (Eckermann, Reference Eckermann2007). Cows with clinical paresis had significantly decreased ALP activities in serum and an increased fractional elimination of calcium in comparison to healthy control animals in the transition period. However, no calculation of the predictive value has been done.
The aim of this study was to assess several antepartum parameters for diagnostic value in predicting hypocalcemia postpartum. The underlying hypothesis was that determining alkaline phosphatase (ALP), ionized calcium (iCa), total serum calcium (Ca) and net acid/base excretion (NABE) two weeks antepartum would allow the identification of individual cows that are at increased risk of developing postpartum hypocalcemia.
Materials and methods
The research was performed in two parts using identical methods. They took place on different farms in different areas and at different times, as one part was conducted in 2017 (part A) and the other one from 2020 to 2021 (part B).
Study part A was approved by the Ethics and Animal Welfare Committee of the University of Veterinary Medicine, Vienna in accordance with the university´s guidelines for Good Scientific Practice (GSP) and authorized by the Austrian Federal Ministry of Education, Science and Research (GZ 68.205/0231-WF/V/3b2016) in accordance with current legislation. Study part B was discussed and approved by the institutional Ethics and Animal Welfare Committee in accordance with GSP guidelines and national legislation.
In part A, 101 Simmental cows in 31 herds in the Austrian federal state of Upper Austria were included from February 2017 to September 2017 (Table 1). Farms having reported numerous cases of clinical hypocalcemia previous to the study took part in this investigation. The incidence of hypocalcemia in these farms was between 20 and 30%. All cows which calved in the selected herds over the study period were included. The mean age of the animals with a physiological Ca content in the blood postpartum was 5.7 years (3–11 years), and that of the cows with clinical hypocalcemia was 6.5 years (2–11 years). In part B, 100 cows (23 Holstein-Friesian, 77 Simmental) in 13 herds from the Austrian federal state of Lower Austria were examined from September 2020 to March 2021. Only cows that calved in the period from September 2020 to March 2021 were considered for the experiment. The mean age of the animals with a normal physiological Ca content in the blood postpartum was 4.4 years (2–12 years), and that of the cows with clinical hypocalcemia was 6.2 years (3–11 years).
Table 1. Cow characteristics and total serum Ca antepartum and postpartum

HF, Holstein-Friesian; SIM, Simmental; Apart from breed, values are median with max/min in parentheses.
In both studies, serum and urine samples were collected from each cow at enrolment two weeks before the calculated date of calving and on the day of calving. Blood samples were collected from the vena caudalis mediana using a vacutainer system (VACUETTE®, Greiner Bio-One, Kremsmünster Austria). Urine was collected using a urinary catheter; the urine tubes were completely filled and closed immediately. All samples were immediately cooled to 4°C and stored for a maximum of two hours before the blood samples were centrifuged for five minutes at 1500 g. The ALP activity and the total Ca concentration were measured in the serum samples using the Cobas C111 Analyzer (Roche Austria GmbH, Vienna). The urine was stored at −21°C till analyzed. The fractionated net excretion of acid/base (NABE) was detected titimetrically in the urine samples using the method of KUTAS (Reference Kutas1965).
In addition in part B only, a device to measure the iCa on farm was available. The iCa was measured with the point of care measurement device LAQUAtwin Ca-11C (Horiba, Kyoto, Japan) immediately after blood sampling at the farm. Due to technical problems with the device, iCa could be sampled in 80 cows only.
Statisical analysis
The data were analyzed using Microsoft Excel 2010 (Microsoft Corporation, Redmond, USA) and SPSS Statistics (IBM Corporation, Amok, USA). The total calcium concentration in the blood serum of each cow, taken within 12 h after calving, was used as the reference value for that cow for all statistical calculations. All variables were evaluated for correlation by Spearman's rank correlation coefficient. The sensitivity and specificity to predict hypocalcemia were further analyzed using the ROC (receiver operating characteristic) analysis and AUC (area under the curve). In this analysis, the total calcium concentration postpartum was used to identify hypocalcemic cows, with <2.00 mmol/l defined as hypocalcemia (Goff, Reference Goff2008). Using the Youden index calculation threshold values could be optimized and adjusted. Statistical significance was declared at P < 0.05 or better.
Results
The median (and maximum and minimum) total serum calcium values in part A cows were 2.57 (1.45–3.22) mmol/l antepartum and 2.11 (1.12–2.82) mmol/l postpartum. Equivalent values for part B were 2.47 (2.05–2.74) mmol/l and 2.02 (1.13–2.52) mmol/l. Calculation of the associations between the ALP activity, the concentration of Ca, iCa (only part B) and NABE measured 14 d antepartum to postpartum Ca concentration are presented in Table 2.
Table 2. Data relating to the ability of antepartum alkaline phosphatase (ALP), total calcium (Ca), ionized calcium (iCa) and net acid/base excretion (NABE) to predict hypocalcemia postpartum
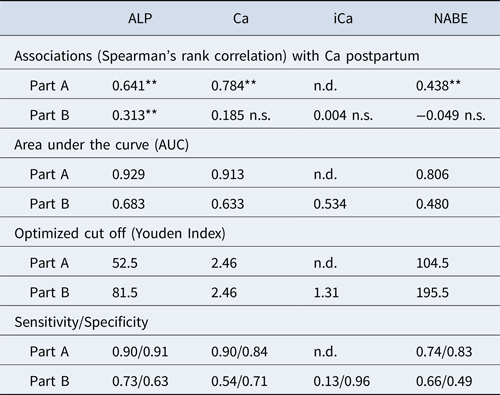
**: P < 0.01 n.s., not significant n.d., not determined.
AUC represents the sensitivity and specificity of ALP, Ca, NABE and iCa as predictors.
Sensitiivty and specificity relate to Youden index analysis
The statistical evaluation showed that in part A the correlation of ALP a.p. was twice as high (0.64) as in part B (0.31) (Table 2). Furthermore, a substantial association (0.78) for Ca antepartum with Ca postpartum was found in part A, but only a non-significant correlation could be demonstrated for the same parameters in part B. The correlation for NABE in part A was 0.44, and no significant correlation could be demonstrated in part B. The iCa could measured in part B only, but no significant correlations could be found.
The AUC indicates the sensitivity and specificity to predict hypocalcemia. A high sensitivity and specificity are indicated by a higher value. The diagnostic values to predict postpartum hypocalcemia characterized by AUCs in part A are higher than in part B for each of the measured parameters (Table 3, Figs. 1 and 2).
Table 3. Area under the curve (AUC) represents the sensitivity and specificity of ALP, Ca, NABE and iCa antepartum in part A and part B to predict hypocalcemia postpartum

a Alkaline phosphatase antepartum.
b Total serum calcium antepartum.
c Net acid/base excretion antepartum.
d Ionized serum calcium ante partum.

Figure 1. In red the ROC curves of the Alkaline Phosphatase (ALP) part A, with an AUC of 0.929 and an Youden Index of 52.5 U/l.

Figure 2. In red the ROC curves of the Alkaline Phosphatase (ALP) part B, with an AUC of 0.683 and an Youden Index of 81.5 U/l.
Calculation of the optimal cut off (Youden Index) for total Ca ante partum to predict hypocalcemia resulted in the same cut off for both parts, but sensitivity and specificity were much higher for part A (Table 4). On the other hand, the cut off for NABE ante partum to predict hypocalcemia was nearly twice as high in part B as in part A.
Table 4. Optimized cut off (Youden Index) for ALP, Ca, NABE and iCa antepartum and the total serum calcium concentration postpartum in the different parts

a Alkaline phosphatase antepartum.
b Total serum calcium antepartum.
c Net acid/base excretion antepartum.
d Ionized serum calcium antepartum.
For each parameter also the sensitivity (SEN) and specificity (SPE) are shown in parentheses (SEN/SPE).
Discussion
We aimed to evaluate the potential to predict the risk of postpartum hypocalcemia in cows by determining ALP, Ca, iCa and NABE approximately two weeks ante partum. There was a major difference between the predictive value of ALP activity between the two parts of the study. The correlation between ALP antepartum and Ca postpartum in part A was 0.641 and the AUC was 0.929, showing a moderate correlation with a relatively high predictive value (Fig. 1). In contrast in part B, the correlation between ALP anrepartum and Ca postpartum was 0.313 and the AUC from the ROC was only 0.683 (Fig. 2). ALP is mainly produced in bones but also in the liver and can, therefore, be used as indicator for bone metabolism and liver function (Carlson, Reference Carlson1996). The bone specific ALP isoenzymes but also bone markers like C-terminal telopeptide of type I collagen have been shown to be more sensitive than total ALP (Staric and Hodnik, Reference Staric and Hodnik2021). However, in routine laboratory tests the less specific total ALP is measured only. As total ALP activity was also evaluated in both parts of the study it might be possible that the liver function had an impact on ALP activity. However, since the samples were taken about 2 weeks before parturition at a time in which the energy demand is still on a low level it seems unlikely that metabolism had a major effect on liver function and, thereby, the total ALP concentration. Furthermore, it has been described that the genetic locus of the different fractions of ALP is located at different chromosomes (Crofton, Reference Crofton1982). It may, therefore, be possible that genetics also have a certain impact, contributing to the distinct differences found in the two parts of the study, as different populations of cows and breeds were enrolled. Whether this might have contributed to the differences in predictive value of ALP remains unknown. Staric and Hodnik (Reference Staric and Hodnik2021) reported that the bone specific ALP and C-terminal telopeptide were significantly associated to postpartum hypocalcemia during winter but not during summer. However, we could not confirm this: part A was conducted during summer and revealed higher correlations than part B, which was conducted in winter and spring. From the present data it was impossible to conclude if any seasonal influence may have affected the predictive value, nevertheless, exposure to the sun light has an impact on intestinal Ca absorption increases due to increased vitamin D production and may influence prevalence and indirectly the predictive value of the parameters (Horst et al., Reference Horst, Goff and Reinhard2005; Staric and Hodnik, Reference Staric and Hodnik2021).
A major, and possible confounding, difference between the two parts of the study was the selection of the herds. In part A herds with farmer-reported frequent cases of hypocalcaemia were asked to take part in the study, in contrast to part B, for which the herds were randomly selected. This selection process might have contributed to the differences in the risk prediction. Further analyses of larger datasets from herds with low and high hypocalcaemia risk are necessary to support this assumption.
Another difference between parts A and B are the geographic locations resulting in variation of feeding rations. The mineral composition of the soil and the feed can influence the dietary cation–anion difference and so the acidotic and alkaline character of feeding (Connelly et al., Reference Connelly, Rodney Harris, Kuehnl, Andrade, Sonnewend Andrade, Henschel, Block, Lean and Hernandez2024). This can be detected by determination of the NABE (Kutas, Reference Kutas1965). A substantial individual fluctuation in the NABE was found in both parts of our study indicating that there are differences between individual herds but not necessarily between regions. The AUC in Part A was higher (0.806) than in Part B (0.480: Fig. 2). Therefore, the interpretation of this parameter should be considered with caution. In the present study the NABE was not correlated with hypocalcemia, which is in contrast to the literature, demanding further investigations (Hörügel and Fürll, Reference Hörügel and Fürll1998). However, the detection by determination of the NABE in urine is typically applied at group level and not at individual cow level.
There was no significant correlation between total Ca antepartum and postpartum in Part B (0.185), in contrast to part A, where the correlation was high (0.784). The AUC was higher in Part A (0.913) compared to Part B (0.633: Fig. 1). Older literature describes a correlation between low calcium intake in the dry period and the mobilization of calcium postpartum (Van de Braak et al., Reference Van de Braak, Van't Klooster and Malesteint1986). This could be replicated only in Part A of this investigation. The different results between Part A and Part B are not explainable.
In cattle, nearly half of total Ca in the plasma is bound whilst the other half is ionized and only this portion (iCa) is available for the organism (Constable et al., Reference Constable, Trefz and Stämpfli2019; Yongpeng et al., Reference Yongpeng, Marcos and Jeroen2022). As a handheld device for measuring the iCa has recently become available, the total serum Ca concentration can be assessed immediately. This can be useful for the cowside diagnosis of hypocalcemia, but in the present study no significant correlation between the iCa antepartum and the total Ca postpartum was found, which is in accordance with the literature (Karapinar et al., Reference Karapinar, Tumer, Constable and Buczinski2024). That study also found no correlation between Ca and iCa at the same point in time. A question that remains open concerns which other factors are involved in the clinical signs of hypocalcemia, and how much of the calcium is ionized. If more factors are involved, the definition of clinical hypocalcemia (Goff, Reference Goff2008) should be adapted. Unfortunately, iCa could not be evaluated in part A, since the device was not on the market at that time. Another limitation of the study was that the group of animals included was on average relatively young (Table 1). The prevalence of hypocalcemia increases with each lactation by about 9% (Lean et al., Reference Lean, DeGaris, McNeil and Block2006). In older cows, the mechanisms for calcium homeostasis become less effective and this prolongs the duration of hypocalcemia (Hernández-Castellano et al., Reference Hernández-Castellano, Hernandez and Bruckmaier2020). Further investigations on more animals with clinical hypocalcemia are needed, and it would then be useful to include measurement of 25(OH) vitamin D3, as recent studies show promising results for the prediction of disease around calving (Wisnieski et al., Reference Wisnieski, Brown, Holcombe, Gandy and Sordillo2020).
In conclusion, our aim was to identify antepartum laboratory parameters allowing a risk prediction of postpartum hypocalcemia in individual animals. While the ALP and the total calcium serum concentration showed promising results in herds with a substantial prevalence of hypocalcemia this could not be replicated in herds which were randomly selected. The urinary NABE and the serum ionized calcium antepartum also showed only moderate-to-weak association with the risk for developing hypocalcemia. Therefore, none of the parameters investigated in the present study can be considered a reliable parameter for prediction of the hypocalcemia risk in cows.








