Over the past decade, type 2 diabetes has become an important global health issue owing to its increasing prevalence( 1 ). Diabetes also threatens the health of people in Taiwan; diabetes ranked fifth among the leading causes of death in 2016( 2 ). Oxidative stress is related to the pathogenesis of type 2 diabetes. An increase in reactive oxygen species (ROS) in diabetes might activate flux through the polyol pathway, increase advanced glycation end-products, interfere with insulin signalling pathways, lead to insulin resistance and hyperglycaemia and induce complications of diabetes( Reference Bournat and Brown 3 – Reference Nowotny, Jung and Höhn 6 ). Thus, antioxidant supplementation has been considered an advantageous adjuvant therapy to reduce oxidative stress for patients with type 2 diabetes.
Ubiquinone (an oxidised form of coenzyme Q10) is a lipid-soluble nutrient component that participates in the mitochondrial respiratory chain of ATP synthesis( Reference Siemieniuk and Skrzydlewska 7 – Reference Bentinger, Tekle and Dallner 9 ). Ubiquinone in food or dietary supplements is in an oxidised form, and after oral intake it might be transformed to a reduced form (ubiquinol) to participate in ROS scavenging in the human body( Reference Bentinger, Tekle and Dallner 9 ). Most of the ubiquinone supplements used in clinical applications are lipid-soluble and are administered in an oxidised capsule form; these supplements were successfully used as an antioxidant adjuvant therapy for patients with coronary artery disease and hepatocellular carcinoma after surgery( Reference Lee, Huang and Chen 10 – Reference Liu, Huang and Cheng 12 ). A novel liquid ubiquinol (hydro-soluble and a reduced form of coenzyme Q10) dietary supplement was recently developed( Reference Mae, Sakamoto and Morikawa 13 ). Solubilised formulations of ubiquinone have superior bioavailability and significantly enhanced plasma coenzyme Q10 responses( Reference Bhagavan and Chopra 14 – Reference Miles, Horn and Miles 16 ). Some observational studies have shown that diabetes patients have higher oxidative stress and lower level of coenzyme Q10 compared with the healthy population( Reference Watts, Playford and Croft 17 – Reference Shen and Pierce 19 ). However, clinical data regarding the application of ubiquinol supplementation to diabetes patients remain limited and inconsistent( Reference Hodgson, Watts and Playford 20 , Reference Suksomboon, Poolsup and Juanak 21 ). Thus, the purpose of this study was to examine the effect of liquid ubiquinol supplementation (100 mg/d) on glucose parameters, lipid profiles and antioxidant capacity in type 2 diabetes patients.
Methods
Study design
This clinical study was conducted as a double-blind, randomised, parallel, placebo-controlled trial. Adult patients with type 2 diabetes were defined as those diagnosed with a glyco Hb (HbA1c) value ≥6·5 %, a fasting glucose level ≥7·0 mmol/l or a 2-h plasma glucose level ≥11·1 mmol/l during an oral glucose tolerance test or those who used anti-hyperglycaemic drugs. We excluded patients with liver or renal disease, pregnant women, patient with hypoglycaemia (fasting glucose <3·3 mmol/l) or hypertriglyceridaemia (TAG≥5·65 mmol/l) and those currently using vitamin supplements or warfarin therapy. The study was approved by the Institutional Review Board of Chung Shan Medical University Hospital, Taiwan, and the clinical trial was registered at Clinical Trials.gov (NCT02622672). This trial started recruiting subjects in January 2016, and data acquisition for the last subject was completed in March 2017. Each subject provided written informed consent to participate in the study.
Experimental groups
A total of fifty type 2 diabetes patients were recruited to this study and randomly assigned to the placebo (water, glycerol and lecithin, n 25) or liquid ubiquinol (QuinoMitQ10® Fluid; MSE Pharmazeutika GmbH, 100 mg/d, n 25) group. Placebo and liquid ubiquinol had the same colour and taste. Before the study, the investigators instructed the subjects regarding the use of liquid ubiquinol supplements at a dose of 33 mg t.i.d. One drop (0·14 ml) of supplement provides 8·3 mg of ubiquinol; we instructed subjects to take four drops before breakfast, lunch and dinner (a total of twelve drops daily). We asked the subjects to return the supplied bottle of supplement every 4 weeks, and then we weighed the bottle to verify their supplement use, thereby monitoring compliance. The intervention was administered for 12 weeks. Three subjects were lost to follow-up during the intervention (did not return); as a result, forty-seven subjects completed the study (placebo group, n 23; liquid ubiquinol group, n 24). The flow chart of the participants illustrating the number of subjects who completed the study in each group is shown in Fig. 1.
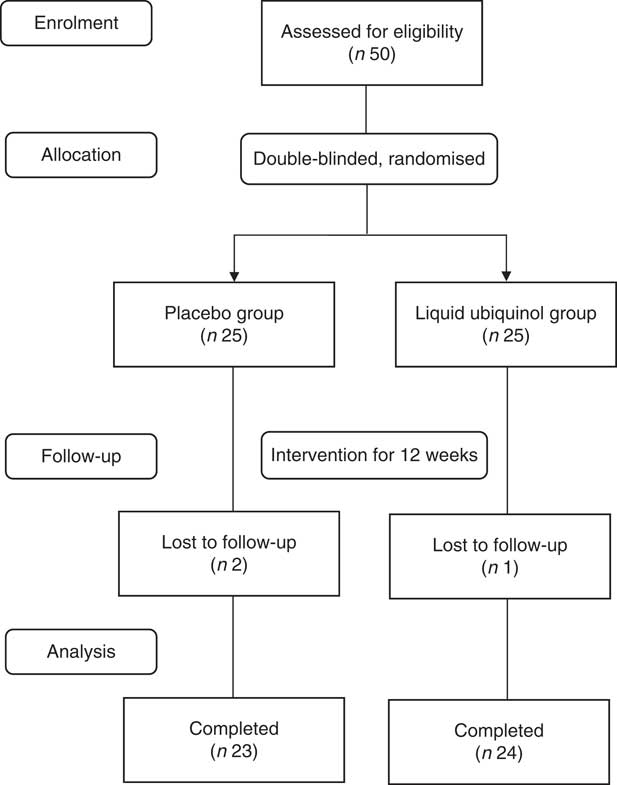
Fig. 1 Participant flow diagram.
Anthropometric measurement and dietary records
Data regarding the characteristics of each subject were acquired using questionnaires and medical records. The subjects’ blood pressures and anthropometric data, such as body weight, height and waist circumference, were measured, and the BMI (kg/m2) was calculated. Dietary intakes during the study were assessed using 24-h recall dietary records. The dietary records were analysed using the Nutritionist Professional software package (E-Kitchen Business Corp.).
Blood collection and haematological measurements
The fasting blood specimens were collected at weeks 0, 4, 8 and 12, using vacutainer tubes containing sodium fluoride, EDTA and without anticoagulant (Becton Dickinson). The samples were centrifuged at 3000 rpm and 4°C for 15 min, and the plasma, serum and erythrocytes were separated. Serum fasting glucose, blood urea N, creatinine, glutamic oxaloacetic transaminase, glutamic pyruvate transaminase, uric acid, total cholesterol (TC), TAG, LDL-cholesterol and HDL-cholesterol were measured using an automated biochemical analyser (Hitachi 7070 & 7600; Hitachi High-Technologies Corporation).
Blood glucose measurements
The HbA1c value was measured at weeks 0 and 12, using an automated HbA1c analyser (Arkray, Inc.). Serum insulin and C-peptide levels were measured by an automatic chemiluminescence analyser (Siemens). The homoeostatic model assessment of insulin resistance (HOMA-IR), HOMA-β and quantitative insulin sensitivity check index (QUICKI) were calculated using the following formulas: HOMA-IR=fasting glucose (mmol/l)×insulin (μU/ml)/22·5; HOMA-β=20×insulin (μU/ml)/(fasting glucose (mmol/l)−3·5); QUICKI=1/(log insulin (μU/ml)+log fasting glucose (mg/dl)). The anti-glycaemic medication effect scores (MES) were calculated according to the overall utilisation of anti-glycaemic agents of each subject at baseline and after 12 weeks of supplementation( Reference Mayer, Jeffreys and Olsen 22 , Reference Nathan, Buse and Davidson 23 ).
Oxidative stress markers, antioxidant enzyme activity and coenzyme Q10 measurements
Serum-oxidised LDL-cholesterol (Ox-LDL-C) was measured at weeks 0 and 12 using a commercially available ELISA kit (Mercodia). Plasma malondialdehyde (MDA) was measured as described previously( Reference Botsoglou 24 ). Erythrocyte were diluted 25× with sodium phosphate buffer for superoxide dismutase (SOD) and glutathione peroxidase (GPx) measurements, and 250× sodium phosphate buffer was used to dilute erythrocyte for the measurement of catalase (CAT) activity. The methods for measuring CAT, SOD and GPx in erythrocyte were previously described( Reference Paglia and Valentine 25 – Reference Aebi 27 ); the protein content of erythrocyte was determined using a commercially available bicinchoninic acid kit (Thermo) and the CAT, SOD and GPx activity levels are expressed as unit/mg of protein. All the analyses were performed in duplicate, and the variations of repeated determinations were within 5 % for the same sample. The level of plasma coenzyme Q10 was measured by HPLC( Reference Littarru, Mosca and Fattorini 28 ).
Statistical analysis
For the sample size calculation, we expected that the change in the levels of fasting glucose (primary outcome) would be 1·0 (sd 1·5) mm after liquid ubiquinol supplementation; therefore, the desired power was set to 0·8 for the detection of a true effect and to an α value equal to 0·05, with a minimum of twenty subjects in each intervention group. All the statistical analyses were performed using the SigmaPlot software (version 12.0; Systat). The Kolmogorov–Smirnov test was used to examine the normal distribution of variables. Student’s t tests (a parametric test) or Mann–Whitney rank sum tests (a non-parametric test) were used to compare the mean values of continuous variables between the placebo and liquid ubiquinol groups. One-way repeated measure ANOVA (a parametric test) or Friedman repeated analysis of variances on ranks (a non-parametric test) were used to compare the values at baseline (week 0), and at weeks 4, 8 and 12 within the group and post hoc tests were used to further examine the significant differences within the group. Wilcoxon’s signed-ranked tests (a non-parametric test) were used to compare the MES of anti-glycaemic agents at weeks 0 and 12 within the group. For categorical response variables, differences between the two groups were assessed by the χ 2 test (a parametric test) or Fisher’s exact test (a non-parametric test). McNemar’s test (a non-parametric test) was used to compare the proportion of anti-glycaemic agents after supplementation within the group. Pearson’s product moment correlations were used to examine the correlations between plasma coenzyme Q10 and glucose parameters after supplementation. Tests were two-sided and statistical significance was set to P<0·05. The means and standard deviations and medians are presented for all data.
Results
The baseline characteristics of the subjects are shown in Table 1. The mean age of the subjects was 61 years, and the sex proportion, blood pressure, anthropometry parameters, haematology and dietary intake were not significantly different between the two groups at baseline.
Table 1 Characteristics of the subjects (Mean values and standard deviations and medians)
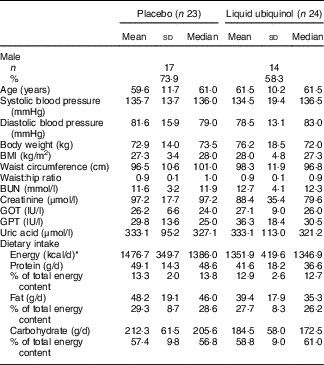
BUN, blood urea N; GOT, glutamic oxaloacetic transaminase; GPT, glutamic pyruvate transaminase.
*To convert energy in kcal to kJ, multiply by 4·184.
The levels of glucose homoeostasis parameters and lipid profiles after supplementation are shown in Fig 2. After 12 weeks of supplementation, the HbA1c value was decreased significantly in the liquid ubiquinol group compared with baseline (Fig 2(a), P=0·03). Subjects in the liquid ubiquinol group had a slightly lower level of fasting glucose than the placebo group at week 4 (Fig 2(a), P=0·06). Regarding lipid profiles, the subjects in the placebo group had a significantly lower HDL-cholesterol level at week 12 compared with baseline (P<0·01) and a slightly lower level than those in the liquid ubiquinol group (P=0·07).
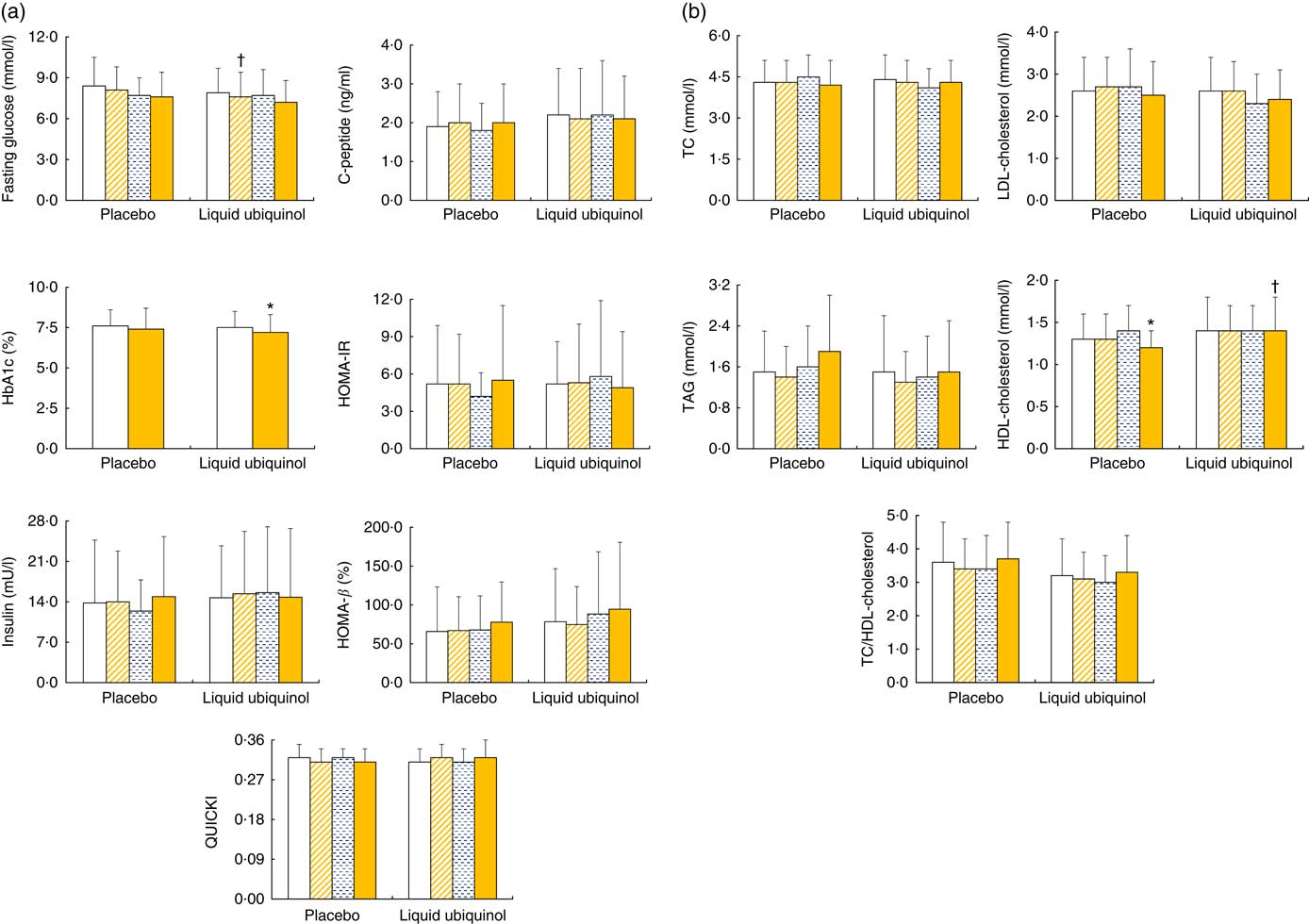
Fig. 2 Glucose levels (a) and lipid profiles (b) in diabetes patients after supplementation. Values are means and standard deviations and medians. ![]() , Week 0;
, Week 0; ![]() , week 4;
, week 4; ![]() , week 8;
, week 8; ![]() , week 12; HbA1c, glyco Hb; HOMA-IR, homoeostatic model assessment of insulin resistance; QUICKI, quantitative insulin sensitivity check index; TC, total cholesterol. HbA1c was measured at weeks 0 and 12. * Values were compared with week 0 (HDL-cholesterol, P<0·01; HbA1c, P=0·03). † Values were compared between the two groups (fasting glucose, P=0·06; HDL-cholesterol, P=0·07).
, week 12; HbA1c, glyco Hb; HOMA-IR, homoeostatic model assessment of insulin resistance; QUICKI, quantitative insulin sensitivity check index; TC, total cholesterol. HbA1c was measured at weeks 0 and 12. * Values were compared with week 0 (HDL-cholesterol, P<0·01; HbA1c, P=0·03). † Values were compared between the two groups (fasting glucose, P=0·06; HDL-cholesterol, P=0·07).
In addition, we calculated the proportion of subjects using anti-glycaemic agent and anti-glycaemic agent MES, and the data are shown in Fig. 3. The proportion of using an anti-glycaemic agent (thiazolidinediones) was significantly decreased after 12 weeks of liquid ubiquinol supplementation (Fig. 3(a), decreased by 25 to 8·3 %, P=0·04). Subjects in the liquid ubiquinol group had significantly lower median values for MES than at baseline (Fig. 3(b), decreased by 0·84 to 0·65 points, P=0·06) and significantly lower median values for MES than those in the placebo group (Fig. 3(b), 0·65 v. 1·3 points, P=0·03).
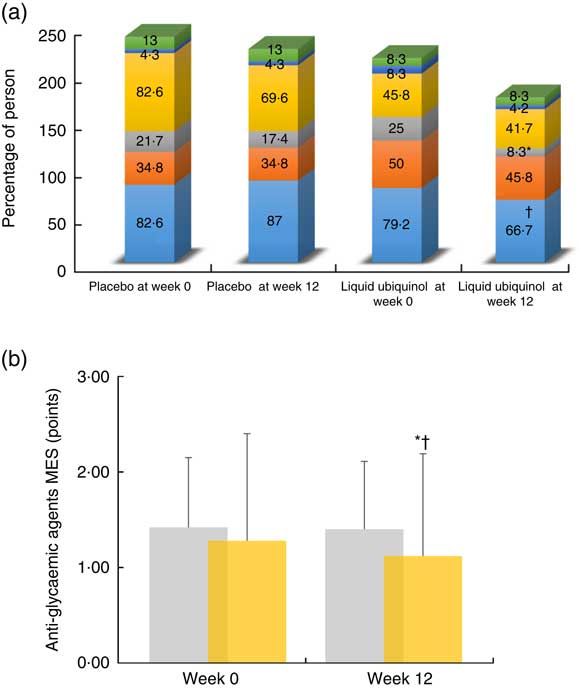
Fig. 3 Proportion of anti-glycaemic agent users (a) and anti-glycaemic agent medication effect scores (MES, b) after supplementation. a: ![]() , Biguanides;
, Biguanides; ![]() , sulfonylurea;
, sulfonylurea; ![]() , thiazolidinediones;
, thiazolidinediones; ![]() , DPP-4 inhibitors;
, DPP-4 inhibitors; ![]() , a-glucosidase inhibitors;
, a-glucosidase inhibitors; ![]() , insulin;
, insulin; ![]() , placebo;
, placebo; ![]() , liquid ubiquinol. * Values were compared with week 0 ((a): thiazolidinediones, P= 0·04; (b): MES, P=0·06). † Values were compared between the two groups ((a): biguanides, P=0·07; (b): MES, P=0·03).
, liquid ubiquinol. * Values were compared with week 0 ((a): thiazolidinediones, P= 0·04; (b): MES, P=0·06). † Values were compared between the two groups ((a): biguanides, P=0·07; (b): MES, P=0·03).
Fig. 4 shows the levels of plasma coenzyme Q10, oxidative stress and antioxidant enzyme activities after supplementation. After supplementation, the level of plasma coenzyme Q10 (Fig 4(a), P<0·01) and the antioxidant enzyme (Fig 4(c), CAT and GPx) activities were significantly increased in the liquid ubiquinol group compared with baseline (Fig 4(c), CAT, P<0·01; GPx, P=0·03). Subjects in the liquid ubiquinol group had a significantly higher SOD activity than those in the placebo group at week 8 (Fig 4(c), P=0·01). However, the MDA and Ox-LDL-C levels (Fig 4(b)) did not differ significantly after supplementation.
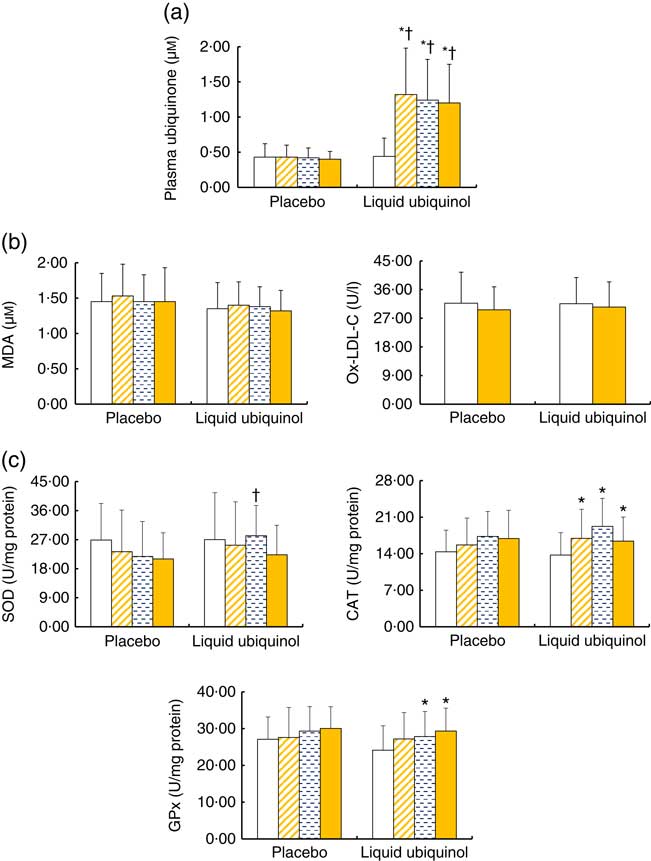
Fig. 4 Levels of plasma coenzyme Q10 (a), oxidative stress (b) and antioxidative enzyme activities (c) in diabetes patients after supplementation. Values are means and standard deviations and medians. ![]() , Week 0;
, Week 0; ![]() , week 4;
, week 4; ![]() , week 8;
, week 8; ![]() , week 12; MDA, malondialdehyde; Ox-LDL-C, oxidized LDL-cholesterol; SOD, superoxide dismutase; CAT, catalase; GPx, glutathione peroxidase. Ox-LDL-C was measured at weeks 0 and 12. * Values were compared within the group (plasma ubiquinone, P<0·01; CAT, P<0·01; GPx, P=0·03). † Values were compared between the two groups (plasma ubiquinone, P<0·01; SOD, P=0·01).
, week 12; MDA, malondialdehyde; Ox-LDL-C, oxidized LDL-cholesterol; SOD, superoxide dismutase; CAT, catalase; GPx, glutathione peroxidase. Ox-LDL-C was measured at weeks 0 and 12. * Values were compared within the group (plasma ubiquinone, P<0·01; CAT, P<0·01; GPx, P=0·03). † Values were compared between the two groups (plasma ubiquinone, P<0·01; SOD, P=0·01).
The correlations between the plasma coenzyme Q10 level and glucose parameters after supplementation are shown in Table 2. The plasma coenzyme Q10 level was correlated significantly with the insulin level (r −0·20, P=0·05), HOMA-IR (r −0·19, P=0·07), QUICKI (r −0·32, P=0·03) and anti-hyperglycaemic MES (r −0·19, P=0·03) after 12 weeks of supplementation.
Table 2 Correlations between plasma coenzyme Q10 and glucose parameters after supplementation
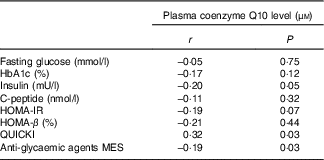
HbA1c, glyco Hb; HOMA-IR, homoeostatic model assessment of insulin resistance; QUICKI, quantitative insulin sensitivity check index; MES, medication effect scores.
Discussion
Since 1966, ubiquinone supplementation has been investigated as a clinical application for diabetes patients. Shigeta et al.( Reference Shigeta, LIzumi and Abe 29 ) was the first researcher to use ubiquinone (120 mg/d) for diabetes patients and found that 67 % of diabetes patients exhibited significantly decreased glucose levels after supplementation. Hodgson et al.( Reference Hodgson, Watts and Playford 20 ) and Kolahdouz Mohammadi et al.( Reference Kolahdouz Mohammadi, Hosseinzadeh-Attar and Eshraghian 30 ) used dosage of ubiquinone supplementation (200 mg/d) in patients with type 2 diabetes, and the results showed a significant lowering effect on HbA1c after 12 weeks of supplementation. On the basis of these findings( Reference Hodgson, Watts and Playford 20 , Reference Shigeta, LIzumi and Abe 29 , Reference Kolahdouz Mohammadi, Hosseinzadeh-Attar and Eshraghian 30 ), we hypothesised that ubiquinone supplementation at a dose of 200 mg/d might benefit glucose control in diabetes. Because ubiquinone is a lipid and an oxidised form of coenzyme Q10, it should be transformed into ubiquinol to participate in human metabolism; therefore, a liquid ubiquinol supplement was recently developed. Many studies found that the hydro-soluble ubiquinol supplement has a higher bioequivalence than lipid-soluble ubiquinone and no side effects( Reference Bhagavan and Chopra 14 – Reference Miles, Horn and Miles 16 ). Thus, we used 100 mg/d of liquid ubiquinol (a half dose of lipid-soluble ubiquinone) for type 2 diabetes patients. In the present study, we found that the HbA1c level was significantly reduced in 6·7 % of the subjects after 12 weeks of supplementation (Fig. 2(a)). Although we failed to detect a significant improvement in the fasting glucose, insulin and C-peptide levels, the plasma coenzyme Q10 level was significantly correlated with insulin, HOMA-IR and QUICKI values (Table 2). The HOMA-IR and HOMA-β indexes represent the secretory function of insulin and the function of β-cells, respectively( Reference Bonora, Targher and Alberiche 31 , Reference Matthews, Hosker and Rudenski 32 ). Although these values did not reach statistical significance, the HOMA-IR index was reduced by 12 % (from 4·3 to 3·8 %) in the liquid ubiquinol group compared with the placebo group (from 4·1 to 4·0 %); meanwhile, the HOMA-β index increased by 30 % (from 53·8 to 70·1 %) in the liquid ubiquinol group. In a recent study conducted by Amin et al.( Reference Amin, Asaad and Salam 33 ), the authors proposed as a possible mechanism of action in which coenzyme Q10 might improve insulin sensitivity through modulation of the insulin receptor and glucose transporters (GLUT4). In addition to the direct effects of ubiquinol on glucose parameters, we also noted that the anti-glycaemic medication proportions of thiazolidinediones (pioglitazone) and biguanides (metformin) were reduced in the liquid ubiquinol group after 12 weeks of intervention in the present study (Fig. 3(a)), and the anti-glycaemic MES was also reduced after liquid ubiquinol supplementation (Fig. 3(b)). On the basis of these results, we hypothesise that liquid ubiquinol supplementation might be an efficacious adjuvant therapy for type 2 diabetes patients who are also being treated with anti-glycaemic medications.
Type 2 diabetic patients are at an increased risk of oxidative stress, and this oxidative stress is related to poor glycaemic control( Reference El-ghoroury, Raslan and Badawy 18 ). Hodgson et al.( Reference Hodgson, Watts and Playford 20 ) found that ubiquinone supplementation can improve glucose control in patients with type 2 diabetes and hyperlipidaemia, but they did not detect a significant effect of reducing oxidative stress. In the present study, we found that type 2 diabetes patients exhibited a significant increase in antioxidant enzyme activities after liquid ubiquinol supplementation (Fig. 4(c)), and the activities of CAT and GPx significantly increased by 19·4 and 21·6 %, respectively; moreover, SOD activity was significantly higher in the liquid ubiquinol group than in the placebo group at week 4 (Fig. 4(c), P=0·01). Although we failed to identify significant lowering effects of liquid ubiquinol on oxidative stress markers (Fig. 4(b), MDA and Ox-LDL-C), the changes in the MDA level tended to be lower in the liquid ubiquinol group than in the placebo group after 12 weeks of supplementation (data not shown, −0·13 (sd 0·45) v. 0·10 (sd 0·36) μm, P=0·07, data not shown). As a result, we hypothesise that liquid ubiquinol might produce an increase in the antioxidant capacity of type 2 diabetes patients, which could be related to improve insulin sensitivity( Reference Amin, Asaad and Salam 33 , Reference Rains and Jain 34 ), particularly in those who suffer from a higher level of oxidative stress.
Patients with type 2 diabetes commonly present with dyslipidaemia( Reference Mooradian 35 ). The features of dyslipidaemia in diabetes include higher TAG and LDL-cholesterol level, and lower HDL-cholesterol level( Reference Mooradian 35 , Reference Barter 36 ). Lipid changes in diabetes might be attributed to insulin resistance, which leads to an increased flux of plasma free fatty acids( Reference Mooradian 35 ). In this study, liquid ubiquinol supplementation did not alter the levels of lipid profiles (TC, TAG and LDL-cholesterol) in these subjects from baseline to week 12 (Fig. 2(b)). However, we observed a significantly reduced HDL-cholesterol level in the placebo group (Fig. 2(b), P<0·01), which was slightly lower than that in the liquid ubiquinol group (Fig. 2(b), P=0·07) after 12 weeks of supplementation. A previous study indicated that hydro-soluble ubiquinone (120 mg/d) might increase HDL- cholesterol level in the hypertensive patients with coronary artery disease( Reference Singh, Niaz and Rastogi 37 ). Mohr et al.( Reference Mohr, Bowry and Stocker 38 ) proposed that oral ubiquinone supplementation might increase the resistance of LDL-cholesterol to peroxidation through its antioxidant capacity. Although we did not observe an increase in HDL-cholesterol level after liquid ubiquinol supplementation, we suggest that liquid ubiquinol at a dose of 100 mg/d could maintain HDL-cholesterol level (>1·3 mmol/l), possess protective antioxidant properties and improve diabetic control.
This investigation constitutes the first clinical study of liquid ubiquinol supplementation in type 2 diabetes patients and provides direct evidence clarifying the relationship among the plasma coenzyme Q10 level and glucose, lipid profiles and antioxidant ability. However, longer intervention studies with larger sample sizes should be performed to confirm the longer-term effect of liquid ubiquinol supplementation in type 2 diabetes patients. Moreover, an optimal dose of liquid ubiquinol should be defined for lowering fasting glucose and lipid levels. In conclusion, oral intake of 100 mg/d liquid ubiquinol might benefit type 2 diabetes patients by increasing antioxidant enzyme activity levels, reducing HbA1c levels and maintaining HDL-cholesterol levels.
Acknowledgements
The authors thank the pharmacist, Yi-Shan Cheng, for providing information on the pharmacokinetics of liquid ubiquinol and the medicine records. The authors express the sincere appreciation to the subjects for their participation.
This study was supported by a grant from the Reformhaus Taiwan Co., Ltd. (CS2-15095).
The authors contributions were as follows: C.-H. Y. and Y.-J. C. performed the study and subjects inclusion. B.-J. L. and Y.-C. L. helped to perform the study and with the data analyses. P.-T. L. conceived of the study, participated in its design and coordinated the study. C.-H. Y. and P.-T. L. drafted the manuscript. All authors read and approved the final manuscript.
The authors declare that there are no conflicts of interest.








