Worldwide, obesity and overweight are highly prevalent risk factors for a number of chronic conditions, including diabetes, CVD and cancer( 1 ) Despite its lower incidence in Japan (as compared with other countries), obesity still raises public-health concerns here, especially towards male adults, children and adolescents( Reference Matsuura, Nunoi and Miyake 2 , Reference Kubo 3 ). Therefore, there has been much interest in exploring possible determinants of weight gain. Among various factors suggested to play important roles in weight control, the energy density (ED) of foods has received considerable attention( 1 , Reference Poppitt and Prentice 4 – Reference Drewnowski, Almiron-Roig and Marmonier 7 ). ED is defined as the available dietary energy in a given unit of weight (kJ/g (kcal/g))( Reference Yao and Roberts 6 , Reference Drewnowski, Almiron-Roig and Marmonier 7 ). It has been reported that an increase in the ED of a diet, if not compensated by a reduction in the total amount of food consumed, would lead to a passive increase in energy intake, because people who maintain a constant body weight (BW) are used to eating a relatively constant weight of food( Reference Poppitt and Prentice 4 , Reference Bell, Castellanos and Pelkman 8 ).
Most controlled trials report a relationship between dietary ED and weight gain in the overweight and/or obese population, with manipulation of the ED among groups( Reference Saris, Astrup and Prentice 9 – Reference Due, Larsen and Mu 15 ); however, these interventions were of relatively short duration. Observational studies, which can reflect the influence of the usual diet over a longer period of time, would corroborate or supplement the results of interventional studies. Nevertheless, observational studies among free-living people have been few and inconclusive( Reference Iqbal, Helge and Heitmann 16 – Reference Romaguera, Angquist and Du 21 ). In addition, available studies have been mostly based on data from the USA and Europe. Therefore, data are needed regarding non-Western populations about the relationships between ED and BW changes. The objective of this study was to examine the relationships between dietary ED and weight gain during about 10 years of follow-up in a cohort of the Japanese adult population.
Methods
Study population
Subjects for this study were cohort members from the Takayama Study, a population-based cohort study conducted in Takayama City, Gifu, Japan. In brief, the Takayama Study is an ongoing prospective cohort study that aims to investigate associations between lifestyle factors and chronic diseases. In 1992, baseline data were collected from 31 152 persons aged over 35 years, which yielded a participation rate of 85·3 % of a total of 36 990 residents invited. A detailed description of the study design and methods used can be found elsewhere( Reference Shimizu 22 ).
Dietary and non-dietary measurements
At baseline, diet, lifestyle and health-related variables were collected using a self-administered questionnaire. These included questions on demographic characteristics (age, BW, height, sex), education level, smoking, diet, physical activity and medical and reproductive histories (menopausal status and use of hormone replacement therapy). Diet, including alcoholic beverages, was assessed with a validated 169-item semi-quantitative FFQ. For each food item or dish, subjects were asked about consumption frequency and usual unit or portion size during the previous 1 year. Based on these data, daily intake of nutrients and food groups was calculated. ED was calculated for each subject by dividing daily energy intake (kJ (kcal)) by the total weight of food consumed (g) based on food only (excluding all beverages). We chose this method for the calculation of ED because (1) it is reportedly the most commonly used and (2) individuals do not eat less food to compensate for energy added by beverages( Reference Karl and Roberts 23 ). A detailed description of the FFQ, its reliability and validity( Reference Shimizu 24 ) and the method used for calculating nutrient intakes has been published previously( Reference Shimizu, Ohwaki and Kurisu 25 ).
BMI was calculated as BW (kg) divided by the square of body height (m2). Physical activity was assessed by asking the average hours per week spent performing various kinds of activities during the previous year. The time per week spent at each intensity of activity was multiplied by its corresponding energy expenditure requirements, expressed as a metabolic equivalents (MET), and summed up to yield a score (MET-h/week). The details including its validity are described elsewhere( Reference Suzuki, Kawakami and Shimizu 26 ).
Follow-up survey
In July 2002, we conducted a follow-up survey to assess BW in the population. The study subjects for the follow-up survey were restricted to those who were 70 years of age or younger at baseline (n 26 546). Among these, between 1 September 1992 and 1 July 2002, 1505 participants were deceased, fifty-one participants were physically unable to complete the questionnaire and 2598 participants had moved. Of the remaining 22 392 individuals to whom we sent the questionnaire, 14 975 (66·9 %) (6674 men and 8301 women) responded. The questionnaire included questions on BW, smoking status, medical and reproductive histories. BMI did not differ between respondents and non-respondents, as described elsewhere( Reference Nagata, Nakamura and Fujii 27 ). Age-adjusted means of ED did not differ between respondents and non-respondents in men (4·85 v. 4·85 kJ/g (1·16 v. 1·16 kcal/g)). Female respondents were more likely to have had lower ED than non-respondents (4·69 v. 4·73 kJ/g (1·12 v. 1·13 kcal/g)). Height was considered stable among the examined adult population; therefore, we chose to report the outcome variable as changes in BW (g), rather than changes in BMI. The outcome variable was defined as the difference in BW between baseline and follow-up.
For the present analysis, subjects who reported having cancer (sixty-two men and 212 women), diabetes (372 men and 169 women), ischaemic heart disease (229 men and 236 women) and stroke (fifty men and twenty women) at the baseline were excluded, because these conditions usually require dietary restrictions. In addition, 407 subjects (183 men and 224 women) were excluded because of incomplete responses to the questions concerning height and BW. The response rate after the above-mentioned exclusions was 66·7 %. Hence, the analytic population at baseline consisted of 13 218 subjects (5778 men and 7440 women). This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the ethical board of Gifu University Graduate School of Medicine. Written informed consent was obtained from all subjects.
Statistical analysis
The association between dietary ED and change in BW was investigated using multiple regression analyses (PROC GLM) and other SAS statistical procedures. The means of weight change according to quartile (Q) of ED were calculated. All analyses were stratified by sex. Covariates included in the models were the following: age, BW, height, physical activity score, alcohol consumption (ml/d), energy intake (kcal/d) and years of education (<11 years, 12–14 years >15 years) and menopausal status (premenopausal, postmenopausal and women only) at baseline and change in smoking status during the follow-up (never to never, never to current/former, former to former, current to former, former to current, current to current and unknown). These variables are known or suggested potential confounders from previous studies( Reference Ledikwe, Blanck and Khan 28 , Reference Aburto, Cantoral and Hernández-Barrera 29 ). Furthermore, potential confounding effects of dietary intakes of total fat (g/d), vegetables (g/d), glycaemic load and coffee (cups/d) were included in the model. The glycaemic load is the calculated product of the carbohydrate content and glycaemic index, as described previously( Reference Oba, Nagata and Nakamura 30 ) Tests for linear trend across quartiles of dietary ED were performed by treating the median of ED for each quartile as continuous variables.
Results
Tables 1 and 2 describe the characteristics of the population according to quartiles of dietary ED in men and women at baseline (1992). At the baseline, differences in BW, BMI and physical activity score among those with ED in Q1 relative to Q4 were not statistically significant in either male or female populations (P>0·05). ED, age, alcohol and caffeine intake and glycaemic load were higher and vegetable intake was lower among those with ED in Q4 relative to Q1 in both men and women (P=<0·05). Interestingly, total energy and fat intakes were higher among those with ED in Q4 relative to Q1 in women, but not in men.
Table 1 Characteristics according to quartiles (Q) of dietary energy density (ED) in the male population at baseline (1992) (Mean values and standard deviations)

BW, body weight; MET, metabolic equivalents.
* P values for difference of means or χ 2 test for Q1 v. Q4.
† Dietary ED medians for Q1, Q2, Q3 and Q4 were 0·98, 1·11, 1·22 and 1·34, respectively.
Table 2 Characteristics according to quartiles (Q) of dietary energy density (ED) in the female population at baseline (1992) (Mean values and standard deviations)

BW, body weight; MET, metabolic equivalents.
* P values for difference of means or chi-square test for Q1 v. Q4.
† Dietary ED medians for Q1, Q2, Q3 and Q4 were 0·95, 1·07, 1·17 and 1·30, respectively.
The prospective variation of weight gain associated with dietary ED in men and women is shown in Tables 3 and 4, respectively. The mean BW changes in 9 years and 10 months were 17 (se 4221) and −210 (se 3889) g for men and women, respectively. The present data are in accordance with previous epidemiological studies, which report a low incidence of obesity and mostly stable (but also decreasing) BMI in the Japanese population( Reference Oba, Nagata and Nakamura 30 , Reference Murakami, Sasaki and Takahashi 31 ). Our FFQ was designed to measure an individual’s relative intake of nutrients rather than absolute values. Some of the values for dietary nutrients and food groups may have been overestimated by our questionnaire. The mean values estimated from the FFQ were generally higher than those estimated from twelve daily diet records.
Table 3 Weight change in the male population by categories of dietary energy density (ED) (Mean values with their standard errors)

Q, quartile; BW, body weight.
* P linear trend across quartiles.
† Adjusted for age, BW, height, physical activity score, alcohol consumption, energy intake, years of education and change of smoking status.
‡ Additional adjustment for dietary intake of total fat, vegetables intake, glycaemic load and coffee intake.
Table 4 Weight change in the female population by categories of dietary energy density (ED) (Mean values with their standard errors)
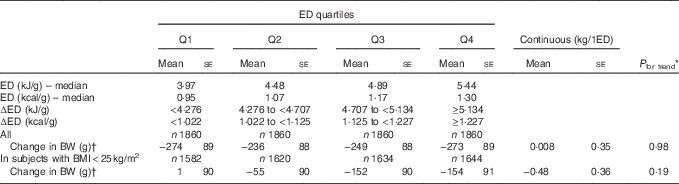
Q, quartile; BW, body weight.
* P linear trend across quartiles.
† Adjusted for age, BW, height, physical activity score, alcohol consumption, energy intake, years of education, menopausal status and change of smoking status.
Table 3 shows that male participants with greater dietary ED at baseline experienced significantly greater weight gain (P for trend=0·01), after controlling for the covariates. Even when total energy was not included as a covariate, the result was not altered: the weight change for 4 kJ/g (1 kcal/g) ED was 1·09 (se 0·43) kg (P for trend=0·01). Besides age, weight, height and alcohol intake at baseline and smoking cessation at follow-up were found to be significant confounders. Additional adjustment for dietary intake of total fat, vegetables, glycaemic load and coffee somewhat strengthened the association between ED and weight change in all men. The mean weight differences were −295 (se 131), −334 (se 110), −68 (se 112) and 196 (se 124) g from the lowest to the highest quartiles, respectively. The weight change for 4 kJ/g (1 kcal/g) ED was 1·51 (se 0·55) kg (P for trend=0·006) in the entire group. However, these dietary factors, as well as the major macronutrients proteins and carbohydrates, did not show statistically significant relationships with weight change. A somewhat stronger association between ED and weight gain was observed in individuals who ingested an above-median amount of carbohydrates (the weight changes for 4 kJ/g (1 kcal/g) ED were 1·41 (se 0·63) and 0·77 (se 0·59) kg for high- and low-carbohydrate intake, respectively). However, the interaction term was not statistically significant (P=0·65). Likewise, fat and protein intake were not modifiers of the relationship between ED and weight change. The correlation between dietary fibre and vegetable intake was high (r 0·91) and adjustment for dietary fibre instead of vegetables did not alter the results. Physical activity was assessed at follow-up. Additional adjustment for physical activity at follow-up or use of the mean of physical activity scores at baseline and follow-up did not alter the results.
Exclusion of 953 (16·5 %) men with BMI≥25 kg/m2 somewhat strengthened the association between ED and weight change; the weight change for 4 kJ/g (1 kcal/g) ED was 1·69 kg (P for trend=0·003). Exclusion of men with BMI≥23 kg/m2 instead of BMI≥25 kg/m2 did not alter the results substantially: the weight change for 4 kJ/g (1 kcal/g) ED was 1·88 (se 0·70) kg (P for trend=0·007).
There was no significant association between ED and weight change in either the total women population or in normal-weight women only (Table 4). Additional adjustment for dietary factors or physical activity at follow-up did not alter the results.
Discussion
In the present study, we observed a significant association between high-ED diet and BW gain among Japanese males, but not females. So far, to our knowledge, four studies have reported an association between ED and subsequent weight change in free-living populations( Reference Iqbal, Helge and Heitmann 16 , Reference Vergnaud, Estaquio and Czernichow 18 – Reference Savage, Marini and Birch 20 ). None of these studies included the Japanese or other Asians. Two of them observed a significantly positive association in overweight (≥25 kg/m2) subjects but not in normal-weight subjects( Reference Iqbal, Helge and Heitmann 16 , Reference Vergnaud, Estaquio and Czernichow 18 ). In the former study, of 1762 Danish men and women, a significantly positive association was observed in obese women only, but the calculation of dietary ED included beverages and water, which differed from other studies( Reference Iqbal, Helge and Heitmann 16 ). In the latter study, of 2707 French men and women, the mean weight gain over 6 years was 2·41 v. 1·45 kg for the highest as compared with the lowest tertile of ED (median ED 1·58 v. 1·16), respectively, in overweight men and women( Reference Vergnaud, Estaquio and Czernichow 18 ). Another study, of 89 432 subjects from five European countries, also reported that ED was not significantly associated with weight change in subjects with baseline BMI<25 kg/m2 (change of weight was 0·029 kg/year for 4 kJ/g (1 kcal/g) ED), but the association was non-significantly inverse in those with BMI≥25 kg/m2 ( Reference Du, van der and Ginder 19 ). The remaining study, of 192 non-Hispanic White women in the USA, observed a greater positive association in the entire group: the mean weight gain over 6 years were 6·4 v. 2·5 kg for high (≥7·74 kJ/g (≥1·85 kcal/g)) as compared with low ED (≤6·3 kJ/g (≤1·5 kcal/g)) diet, but the association did not vary by BMI classification( Reference Savage, Marini and Birch 20 ).
The present study demonstrated a significantly positive trend for the association between ED and weight change in a Japanese male population, which is generally lean. Furthermore, this association was strengthened by restricting the population to normal-weight subjects only. The fact that dietary ED can be related to weight gain even in a lean population raises further concerns about the risks for overweight and obesity. We observed that total energy intake at baseline was unrelated to weight gain. We hypothesise that a high-ED diet may cause an increase in energy intake, which, in turn, leads to weight gain. Unfortunately, however, we could not evaluate the change in energy intake as well as ED after the baseline.
There has been a suggestion that normal-weight subjects are more physically active and therefore likely to be able to manage energy-dense diets more easily than overweight subjects. However, in our study, physical activity at the baseline, as well as at follow-up, was included as covariates. Although our results did not support that ED was unrelated to weight change in normal-weight men, there is a possibility that ethnicity rather than body size may modify the association between ED and weight change( Reference Howarth, Murphy and Wilkens 32 ).
We failed to observe a positive association between ED and weight change in women. The mechanism linking higher ED and a large gain in weight was speculated through an increase in total energy intake. In women, high-ED diet at baseline could not be linked to high-energy intake at follow-up because concern about weight gain may have influenced their diet. However, a study to determine the relationship between ED and subsequent energy intake would have to measure changes in diet repeatedly. Women are more concerned about weight and could under-report information about weight and high-ED food consumption( Reference Martí-Henneberg, Capdevila and Arija 33 ). Reports suggest that such under-reporting is more common among female, obese and overweight subjects( Reference Macdiarmid and Blundell 34 ), which may have led to the observed null association.
This study has the following known limitations. First, self-report of weight and diet may have had an influence on the results; nevertheless, our validity study showed that the correlation coefficients between self-reported and measured height and weight among a subsample was high; r 0·85 for height and r 0·97 for weight in men, and corresponding values were 0·93 and 0·97, respectively, in women. In addition, our measurement of FFQ has been well validated for nutrients and food groups. Second, selective misclassification/mismeasurement of ED or weight may have occurred and this may be especially relevant to the results among women. However, considering that BMI and ED at baseline were similar between respondents and non-respondents, it is unlikely that men with high ED and high weight gain or men with low ED and low weight gain were selectively included into the study. Third, although much information was collected at follow-up, including changes in smoking status, for instance, we could not assess diet including ED as well as total energy intake at the follow-up. Fourth, the response rate was modest in this study and we could not obtain the BW status of subjects who had died or moved away during the follow-up period. On the other hand, strengths of the present study include its longitudinal design, a large number of subjects and a long follow-up period.
In conclusion, the present prospective study in Japanese men and women suggests that high-ED diets are positively associated with BW gain in the male population, including normal-weight subjects, but not in the female population. Current dietary recommendations in Japan do not refer to ED. Further studies including prospective and interventional studies are needed to clarify the potential effects of ED on weight modification in the Japanese population. In addition, because studies on ED and weight gain are limited to Western populations, the present study ought to be repeated in other Asian populations.
Acknowledgements
The authors thank all the participants in the Takayama study.
This study was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Science, Sports, Culture and Technology of Japan and by a travel grant from the Federal District Research Foundation (Fundação de Amparo à Pesquisa do Distrito Federal FAP-DF) of Brazil to K. M. S.
K. M. S., K. W. and C. N. contributed to the study interpretation and to the writing of the manuscript. K. W. contributed to the statistical analysis. J. L. L. Z. provided input into the final draft of the manuscript. C. N. designed the study, conducted the statistical analysis and contributed to the hypothesis formulation.
The authors declare that there are no conflicts of interest.







