Giardia intestinalis (synonyms: G. lamblia, G. duodenalis) is a flagellated protozoan parasite, whose species complex comprises a series of largely host-adapted assemblages. Infection with this pathogen can be asymptomatic but it usually manifests as an acute or chronic disease. Symptoms include chronic diarrhoea, bloating, sulphurous belching (historically referred to as the ‘purple burps’), nausea and significant weight loss [Reference Jakubowski1]. From a public health perspective, this pathogen receives little attention compared to other protozoan parasites, yet it is likely to be underreported and has the potential to cause large outbreaks [Reference Morch2, Reference Daly3]. Furthermore, long-term sequelae associated with this infection can be serious [Reference Wensaas4].
From 2000, the annual number of Giardia reports to Health Protection Scotland (HPS) has decreased over time from 281 reports to 185 (2012) (Fig. 1). In order to ascertain the infectious assemblage, we performed a snapshot genotyping study. Recent data suggests assemblages A and B, are actually different species but clinical outcomes may be influenced by both species and the host–parasite interaction [Reference Jerlstrom-Hultqvist, Ankarklev and Svard5]. To that end, we also assessed the influence of self-reported previous medical history on symptomatology and outcomes.

Fig. 1. Laboratory reports of Giardia intestinalis to HPS, 2000–2012.
In Scotland, daily reports of National Health Service (NHS) laboratory isolations of pathogens are uploaded to the Electronic Communication of Surveillance in Scotland (ECOSS) database in HPS. For the purposes of this study, we contacted several NHS boards (Ayrshire & Arran, Borders, Greater Glasgow & Clyde, Lanarkshire) and asked the local microbiologists to forward all stool samples in 2011 and 2012, which were confirmed as microscopy-positive for G. intestinalis. Scotland has 14 NHS boards and the four study NHS boards serve 2·2 million inhabitants; about 44% of the Scottish population. We also asked that stool samples from patients who were microscopy-negative but had chronic diarrhoea, were referred to the Scottish Parasite Diagnostic Reference Laboratory (SPDRL). Further inclusion criteria were stools which were negative for microbiology from patients with ‘gastrointestinal disturbances’ who were deemed to be at high risk, i.e. (a) had close contact with a known Giardia positive, (b) foreign travel history and (c) recent contact with recreational/outdoor waters.
DNA was extracted from anonymized, consented samples using the QIAamp DNA Stool Mini kit (Qiagen, USA) and subjected to a semi-nested polymerase chain reaction (PCR) assay targeting the β-Giardin gene [Reference Caccio, Giacomo and Pozio6]. PCR-positive samples were sequenced (Applied Biosystems 3500XL, Life Technologies, USA) to identify assemblages. Bi-directional sequences were aligned using EMBI website tools to obtain a consensus which was manually edited following the sequence chromatogram. The consensus sequence was used to search the GenBank database for similarities using the CBI Blastn tool.
Both demographic and potential exposure data on anonymized cases were reviewed, and were entered into a SPSS database. The statistical significances of associations between numerical variables were investigated by using χ 2 tests. All analyses were performed using SPSS v. 21 (SPSS Inc., USA) with a significance level of 5%.
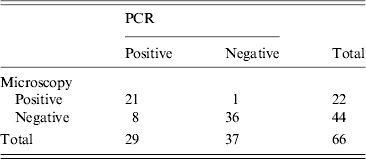
Sixty-nine isolates were forwarded on to SPDRL for confirmation by microscopy and PCR. Of these, 66 isolates were tested by both methodologies and therefore, three were excluded from further analysis. Of the 66 isolates, 36 were negative by both diagnostic procedures and were therefore excluded from further analysis.
In order to compare traditional microscopy with PCR in identifying Giardia-positive isolates, we assessed the sensitivity and specificity of the latter test. Sensitivity was 95·4% [95% confidence interval (CI) 78·2–99·1], while specificity was 81·8% (95% CI 68·0–90·5) (Table 1).
Table 1. Sensitivity/specificity of microscopy compared with molecular analysis
The mean age (±s.d.) of infection in those confirmed positive for Giardia was 31·7 ± 3·6 years (range 1–63 years). Of the 30 positive cases, 18 (60%) were male. These data are broadly comparable with the national mean age of 39·0 ± 1·4 years (range 1–97 years) and gender (56% of isolates were from males). Of the 30 cases in our study, 22 stated that they had travelled abroad or outside Scotland in the preceding 6 weeks before their illness. The most commonly stated continents were Africa (n = 8), Europe (n = 8) and Asia (n = 8).
The most frequently reported symptoms included chronic diarrhoea (80%), tiredness (42%), nausea (40%), abdominal pain (27%) and belching (23%). Fever (two cases) and vomiting (one case) were rarely reported. Of the 30 confirmed cases, five required admission to hospital. Mean length of stay in hospital was 5 ± 1 days (range 1–8 days). Twenty cases were prescribed oral antibiotics including metronidazole (n = 15), tinidazole (n = 1) and streptoquine (n = 1). Of the remaining ten cases, five were given no treatment, two were told to orally rehydrate and two cases were prescribed analgesia. One individual received loperamide. Only fever was statistically associated with hospitalization (P = 0·028).
In this snapshot study, assemblage A was the most frequently isolated (72%) followed by assemblage B (14%). Mixed A/B assemblages accounted for 10% of cases (Fig. 2). An unusual assemblage was isolated from a patient who had returned from Ghana, which contained a number of nucleotide substitutions not present in either assemblages A or B. Due to the small numbers of assemblages other than A, there was no statistical association between assemblage and categorical variables.

Fig. 2. Frequency of Giardia assemblages in Scottish cases (n = 29).
This descriptive epidemiology study underlines the importance of molecular technologies in genotyping Giardia isolates since the technique is much more sensitive than traditional microscopy. It further emphasizes that infection with Giardia species is likely to be under-ascertained in Scotland and its recent association with complications such as irritable bowel syndrome [Reference Wensaas4] merits further research into a pathogen which is considered to be low priority by many public health organizations [Reference Robertson7]. While sporadic infections may predominate, there is the potential for large water-borne outbreaks to occur, even in countries which have a robust municipal drinking-water infrastructure [Reference Steen and Damsgaard8].
In this study, the majority of those infected were adult males, which is an unusual finding. However, the male preponderance was also typical for the national datasets in both 2011 and 2012. Most epidemiological studies appraising Giardia have shown that adult females are more likely to be affected by this pathogen [Reference Hanevik9, Reference Breathnach, McHugh and Butcher10]. Foreign travel appears to be an important predictor of infection where it has been postulated that recreational water contact, cyst-contaminated drinking water (or ice) and food may be risk factors for acquisition of infection [Reference Breathnach, McHugh and Butcher10]. While the Indian subcontinent has historically been associated with Giardia infection, travellers returning from Africa and Europe were equally affected in this study.
In order to ascertain whether the reduction in Giardia cases was associated with a change in infectious assemblage in Scotland, we performed genotyping to see whether the reduction was at least partly attributable to change in assemblage in the population. A comprehensive worldwide analysis of Giardia assemblages in humans has historically shown that assemblage B tends to predominate [Reference Caccio and Ryan11]. Such data were corroborated by a study performed in London between 1999 and 2005, which showed that assemblage B accounted for 73% of cases [Reference Breathnach, McHugh and Butcher10].
In our study, we found that the majority of cases were infected with assemblage A, which has been suggested to be associated with milder infections [Reference Breathnach, McHugh and Butcher10]. This may partly account for the apparent decrease in Giardia cases in Scotland, since those infected may be less likely to consult their general practitioner. At odds with this finding is that in our study, 20% of cases were admitted to hospital for further treatment. Once again this reiterates that clinical outcomes are influenced not only by species but also by the host–parasite interaction [Reference Jerlstrom-Hultqvist, Ankarklev and Svard5]. It further exemplifies that public health priorities should reflect the potential seriousness of infection with this pathogen and development of genotyping assays is one measure by which this can be achieved.
ACKNOWLEDGEMENTS
The authors thank Sandra Mitchell, Monklands Hospital, Lanarkshire for her assistance with data collection and the staff at microbiology laboratories within Ayrshire & Arran, Lanarkshire, Borders, and Greater Glasgow Health boards for kindly providing samples to include in this study.
DECLARATION OF INTEREST
None.





