Introduction
Cervical cancer is the second most common tumour and certainly the most common genital cancer of women worldwide [Reference Shwe1]. More than 85% of the cervical cancer global burden occurs in developing countries, where it accounts for 13% of all female cancers.
The incidence of cervical cancer in Serbia is one of the highest in Europe, with age-standardised rate of 23.8/100.000 according to the WHO [Reference Bruni2]. According to the Cancer Registry of Serbia, the incidence rates were 21.6–27.1/100.000 from 2003 to 2009 [3], and the data collected in the year 2013 shows the age-standardised rate of 20.3/100.000 [4].
Human papillomavirus (HPV)-related diseases have taken an increased importance over the years and are now a major concern for public health. There is a strong evidence that HPV has causal role for the development of a subset of squamous cell carcinoma (SCC) in the anogenital area (vulva, vagina, cervix, anus and penis) and in the head–neck area (pharynx, larynx and oral cavity) [Reference Syrjänen5]. Persistent infection with one or more of approximately 20 highly oncogenic HPV or high-risk-HPV (HR-HPV) is necessary but not a sufficient aetiological agent for cervical neoplasia [Reference Kim6]. More than 80% of HPV infections lead to spontaneous regression and elimination of the virus within 2 years of initial contact. In minority of cases, persistent infection is established and 25% of HR-HPV-infected women develop cervical intraepithelial neoplasia in the first degree (CIN I), with further progression (CIN II/III). Cervical cancer would develop in 10% of all patients and in 1% of HR-HPV-positive women over a number of years.
HPV infection is an early step in the cervical carcinogenesis pathway, by the mechanism of viral oncogenes integration into the host cell which is leading to the progression of dysplasia. One tumour suppressor protein, p16INK4A, which is the product of CDKN2A gene is found to be overexpressed in cervical cancers as a result of functional inactivation of RB oncogene by the HPV E7 protein [Reference Mai7]. Expression of p16INK4A appears to correlate with the degree of cervical neoplasia. Because of that, p16INK4A could be used as a predictive biomarker that can identify women with cervical dysplasia who may be at risk of developing higher grades of CIN or carcinoma.
Malignant transformation of cervical cells and tumour development are of multifactorial origin and are the results of HPV genotype, host cofactors including genetic and immunological status, and environmental and behavioural cofactors including high parity, oral contraceptives, tobacco smoking, infection with other sexually transmitted diseases (STD), early sexual intercourse, promiscuity, poor socio-economic conditions, dietary and nutritional factors, etc. [Reference Castellsagué, Bosch and Muňoz8]. Smoking, multiparity and oral contraceptive use have definitively been shown to be associated with CIN3 and cervical cancer [Reference Appleby9, Reference Plummer10]. Chemical tobacco-related carcinogens have direct mitogenic effect causing DNA damage and it has been shown that smoking may reduce the number of Langerhans cells and other markers of immune response [Reference Castellsagué, Bosch and Muňoz8].
High parity has been found to be associated with an increased risk for high-grade squamous intraepithelial lesion (HSIL) and cervical cancer [Reference Castellsagué, Bosch and Muňoz8, Reference Bosch11]. It has been proposed that the developing or healing cervix as a consequence of deliveries, cervical trauma or any other STD infection represents a high-risk environment, which can aid HPV to reach the basal layer of cervical epithelium and establish a persistent infection. Hormonal changes in pregnancy modulate the immune response to HPV and induce the persistence of the virus.
A systematic review of hormonal contraceptive use has reported that the risk of in situ cervical cancer was increased even for women with <5 years hormonal contraceptive use, but the risk of invasive cervical cancer increased only after 5 years of use [Reference Tao12].
Sexual habits such as young age at first intercourse and an elevated number of sexual partners are increased risk factors for sexually transmitted infection. Male HPV infection significantly contributes to infection and subsequent cervical disease in women. In areas with a high incidence of cervical cancer, men's sexual behaviour is in itself a risk factor and is critical in cervical cancer prevention [Reference Capra13, Reference Giuliano14]. There are several mechanisms by which sexually transmitted co-infections may act, by direct genotoxicity or by the induction of cervical inflammation leading to genotoxic damage through the release of oxidative metabolites [Reference Verteramo15].
Although persistent infection may progress to CIN or invasive cancer, neoplastic changes typically take years to occur. Detectable HPV infection and high-grade lesions are most often detected by screening about a decade after the acquisition of infection, and invasive cancer is usually diagnosed in women who are 40 years or older [Reference Gargano16].
Obesity or overweight is a risk factor for many chronic diseases, including cancer, and numerous studies showed that body mass index (BMI) was associated with the incidence of various cancers in women [Reference Prompakay17]. There are also studies reporting a positive association between BMI and an abnormal Pap smear, which indicates that the higher the BMI, the greater are the risks of an abnormal Pap smear [Reference Maruthur18, Reference Lee19].
Three HPV vaccines have been licenced and are available in many countries throughout the world: the bivalent (16/18), the quadrivalent (6/11/16/18) and the nonavalent (6/11/16/18/31/33/45/52/58). With the implementation of an effective vaccination programme, most HPV-associated disorders (genital warts and cervical, vaginal, vulvar, penil, anal and oropharingeal cancers) in males and females can be prevented. The vaccines can also indirectly protect unvaccinated individuals through herd immunity. However, the significant reduction in precancerous lesions and HPV-related tumours can be reached only if vaccination coverage reaches more than 80%. The nonavalent vaccine has the potential to offer protection against approximately 90% of cervical cancers, up from 70% offered by the quadrivalent [Reference Capra20, Reference Yang and Bracken21].
The aim of the present study was to assess the impact of environmental and behavioural cofactors on the development of cervical disorders in HR-HPV-positive women in Serbia. This kind of investigation has been conducted for the first time on the population of Serbia.
Methods
Patients and methods
This was a cross-sectional study comprising 541 women referred to the Clinic of Gynecology and Obstetrics ‘Narodni Front’ Belgrade, Serbia for abnormal cervical screening results from 2014 to 2016. Written informed consent was obtained from all women enrolled in the study. The protocol of the study was reviewed and approved by the Ethics Committee, Faculty of Medicine, University of Belgrade, decision number 29/XI-2.
All women were examined for cytology and HPV status. Endocervical swabs and smears from visible changes on the ectocervix were taken and tested at the Virology Laboratory, Institute of Microbiology and Immunology, Faculty of Medicine, University of Belgrade, in order to determine the presence and type of HPV infection. All patients included in the study have filled in a detailed questionnaire, which contained their basic data, socio-demographic data, information on lifestyle and sexual habits and other data of importance for the acquisition and progress of HPV infection.
Based on a Pap cytological finding on the cervix and according to the Bethesda classification (2001), patients were classified into four groups: women with normal cervical cytology as control group (PAP-II); women whose cytology was defined as atypical cells of unknown origin (ASCUS); patients with low-grade squamous intraepithelial lesion (LSIL) or CIN I, corresponding to the slight changes in the cervical epithelium or koilocytosis (cells with perinuclear enlightenment indicative of HPV infection) and women with HSIL corresponding to moderate and/or severe cervical dysplasia (CIN II/III).
HPV detection and typing
In order to determine the presence and type of HPV infection, endocervical swabs and/or swabs from visible lesions in the ectocervix were taken using cervical cytobrush techniques (Cervex-Brush® Combi, Rovers, The Netherlands). The swabs were immersed in tubes containing 1 ml of transport medium in order to maintain the humidity of the sample. For viral testing, further processing involved ‘squeezing of the cytobrush’ in a special medium (liquid-based cytology samples, CytoScreen, preserve, Cyt). After that the samples were centrifuged at 2000–3000 rpm. Commercial DNA extraction kit (QIAamp DNA Mini Kit, QIAGEN Inc., USA) was used to isolate total DNA from the resulting sediment, according to the manufacturer's protocol.
The amplification of HPV DNA L1 and E1 gene was performed with two sets of primers, MY09/MY11 and GP1/GP2, respectively. PCR was performed in a 25 µl volume reaction mix containing QiagenTaq PCR Master Mix (QIAGEN Inc.), 1 µmol of each HPV primer, 0.5 µmol of each β-globin primer and 5 µl of extracted DNA [Reference Gravitt22, Reference Azzimonti23]. PCR protocol for the detection of L1 gene comprised initial denaturation at 95 °C for 5 min, followed by 40 cycles of 30 s at 94 °C, 30 s at 58 °C, 1 min at 72 °C and a final elongation of 20 min at 72 °C [Reference Gravitt22]. The protocol for the detection of E1 gene comprised initial denaturation at 85 °C for 5 min, followed by 40 cycles of 1 min at 94 °C, 1 min at 50 °C, 90 s at 72 °C and a final elongation of 10 min at 72 °C [Reference Azzimonti23]. The presence of specific 450 bp band for L1 gene and 450 bp band for E1 gene, detected by agarose gel electrophoresis with ethidium bromide staining, was considered a positive result. Any specimen negative both for HPV amplicon and β-globin amplicon was considered unsatisfactory.
HPV genotyping was performed by direct DNA sequencing. HPV-positive samples were purified by QIAGEN MinElute PCR Purification Kit (QIAGEN Inc.) according to the manufacturer's protocol. The purified PCR products were subsequently sequenced with Big Dye Terminator 3.1. Cycle Sequencing Kit (PE Applied Biosystems, USA) using the same set of primers. Sequencing reactions were analysed on the ABI Prism 310 Genetic Analyzer. The obtained nucleotide sequences were analysed using Sequence Analysis 5.1 software. HPV genotypes were determined by comparison with HPV reference strains and documented virus sequences available in the GeneBank database using BLAST tool (http://www.ncbi.nlm.nih.gov/BLAST/). The nucleotide sequence was assigned to HPV type if it corresponded more than 95% to a known HPV genotype.
Questionnaire
The interviewer-based questionnaire was administered to all the participants involved in the study. It queried basic information about the patient, socio-demographic and behavioural data, patient's reproductive history, sexual habits, morbidity from other STDs and other information of significance for HPV-related cervical disorders.
Statistical analyses
Statistical analysis was performed using χ 2 or Fisher's exact test for nominal variables and the unpaired Student t test for continuous variables. Continuous variables were expressed as the mean ± standard deviation, or median (range) or proportions. The analysis of differences among more than two groups was performed with the one-way analysis of variance test or non-parametric Kruskal–Wallis test.
Single-factor analysis was performed for each possible risk cofactor for HR-HPV and analysed by the logistic regression model.
Logistic regression was used to generate odds ratios (ORs) and corresponding 95% confidence intervals (CIs) as measures of association of demographic, anamnestic and clinical variables with diagnoses of different cytological status compared with controls.
P < 0.05 was considered to indicate a statistically significant result. All statistical analyses were performed using SPSS version 19.0 (IBM Corp.)
Results
A total of 541 women with abnormal cervical screening results have been processed on this study, out of which 105 were HPV-positive (19.4%) and 84 (15.5%) were HR-HPV-positive. The final investigated group included 84 women infected with HR-HPV who were classified into four subgroups according to their cytological status on the cervix (Fig. 1).
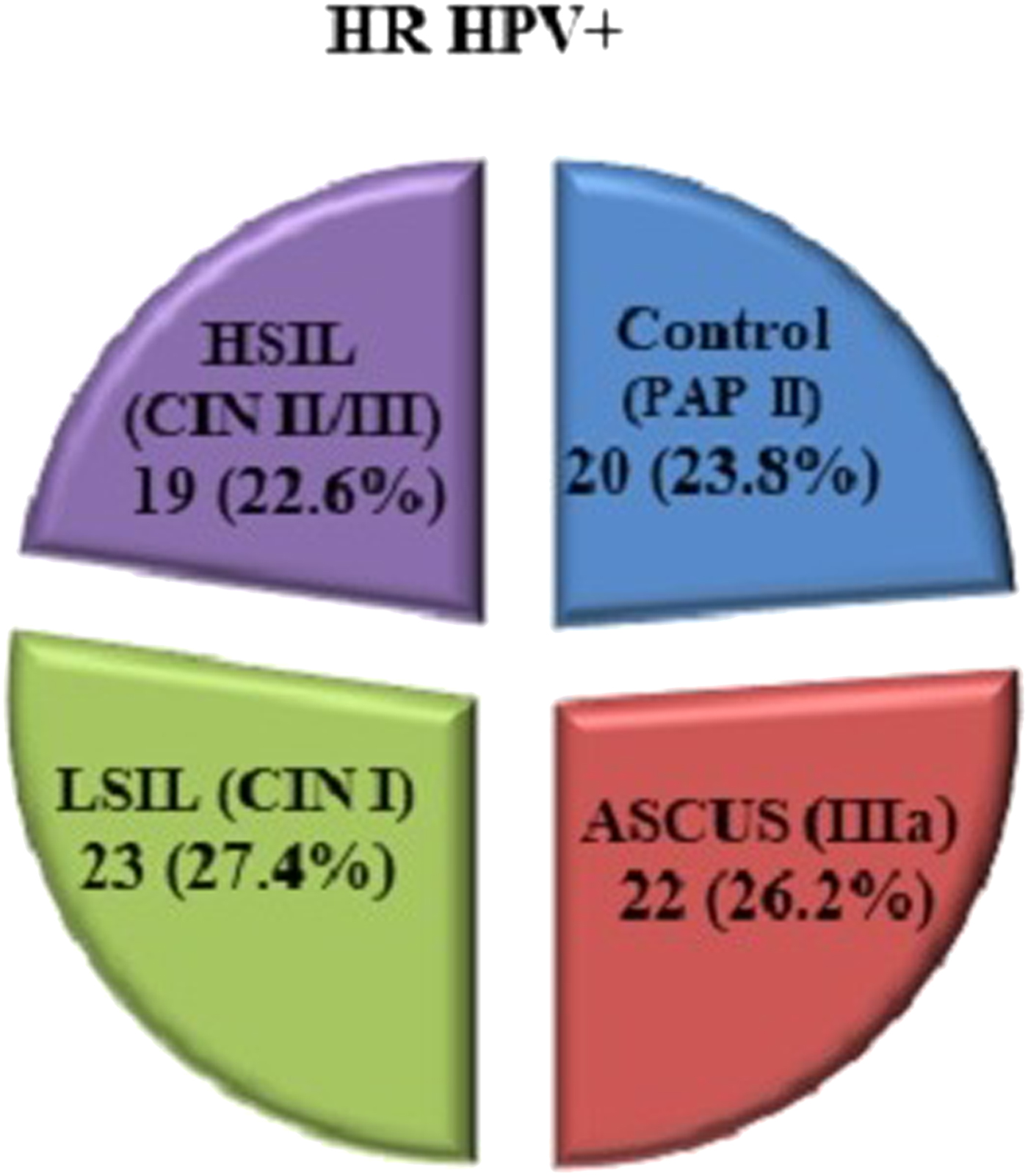
Fig. 1. Distribution of the HR-HPV-positive women according to the cytological findings.
Distribution of HR-HPV genotypes according to cytological status
Twelve HR-HPV genotypes were identified in the investigated group. Their total frequencies and distribution according to cytological status on the cervix were shown in Table 1. The most frequent genotype in all groups according to cytological status was 16 (42.9%), followed by 45 (15.5%) and 31 (10.7%). The frequency of genotype 16 was not significant compared with the frequency of all other genotypes together. However, the frequency of HPV 16 dominated in the HSIL group (89.5%), while it was notably lower in other groups (10–50%), which was statistically significant (P < 0.001).
Table 1. Distribution of HR-HPV genotypes according to cytological status

Population characteristics
The youngest woman in the study was 20 and the oldest was 73 years old. Women in the control group were on average 34.5 ± 8.2 years old and the women with ASCUS 37.1 ± 9.0 years old, which is not significantly different from the group of women with LSIL changes that were on average 37.4 ± 9.5 years old. Women with HSIL changes were significantly older than women from other groups, 46.7 ± 12.2 on average (Table 2).
Table 2. Demographic characteristics of the analysed groups

* Significant P < 0.05, χ 2, Fisher's exact test, Student t test, mean ± s.d., n (%).
A significant number of women come from urban areas, primarily from Belgrade. In terms of this demographic feature, all compared groups were similar. The distribution of patients by level of education showed that women with HSIL usually were with secondary education and in significantly smaller number of cases with higher education. The similarity of the examined subgroups of patients exists also in distributions by marital and employment status. Noticeable is a slightly higher incidence of women with HSIL findings that were unemployed, but this difference did not prove as statistically significant (Table 2).
The distribution of risk factors for moderate/severe dysplasia of the respective groups is shown in Table 3.
Table 3. Analysis of risk factors for the moderate/severe dysplasia in investigated groups
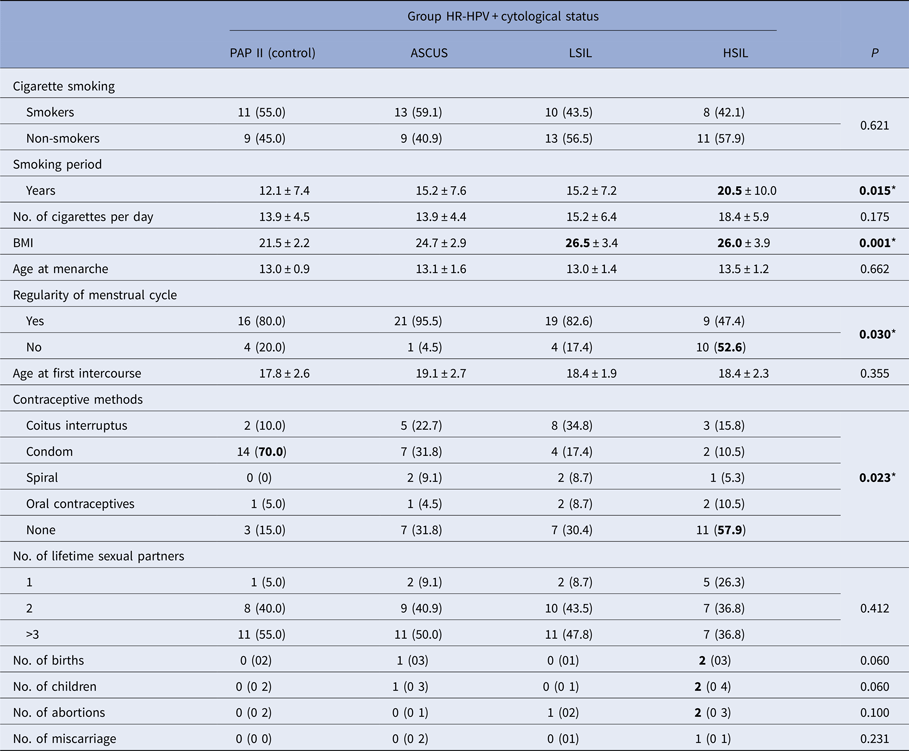
* Significant P < 0.05, Kruskal–Wallis test, χ 2 test, mean ± s.d., n (%), median (min–max).
There was a similar representation of smokers in all analysed groups, 42−59%. Average smoking period ranged from 12 to 20 years and the longest smoking periods were found in the group with HSIL (P = 0015). The average number of cigarettes per day ranged from 14 to 18, which was not significantly different between the groups.
Average nutritional status of patients expressed as a BMI was the lowest in the control group and the group with ASCUS. In patients with LSIL and HSIL, BMI was significantly higher (P = 0001) and it was in the overweight category (BMI > 25).
The highest frequency of irregular menstrual cycles was determined in patients with HSIL cervical score, which was significantly different compared with all other groups.
In the control group, the use of condoms as a method of contraception was reported in 70% of cases, which is significantly higher compared with all other groups (P = 0023). An important characteristic of the group of patients with HSIL is the absence of use of any kind of contraception method compared with other groups, and when contraception methods were reported, the use of condoms was very rare.
All respondents, regardless of cytology, had their menarche around 13 years of age and the first sexual intercourse occurred on average, at the age of 18 or 19. The larger number of sexual partners was frequent in almost all groups (50–55%), only in the group with HSIL was slightly lower (40%). In neither case, the differences between groups were not significant.
According to the number of births, number of children, number of abortions and miscarriages, almost all compared groups were similar. Patients with the most serious form of cervical dysplasia (HSIL) had on average a largest number of deliveries and children (median = 2). A higher number of births in this group was only slightly above the designated threshold of statistical significance (P = 0.06 > 0.05) and can be considered relevant for a confidence threshold of P < 0.10 (Table 3).
Other risk factors for cervical dysplasia refer to the data about interventions on the cervix, previous infections and their treatment, other diseases of the patient as well as information about gynecological diseases in the family and are presented in Table 4.
Table 4. Analysis of other risk factors for the moderate/severe dysplasia in investigated groups
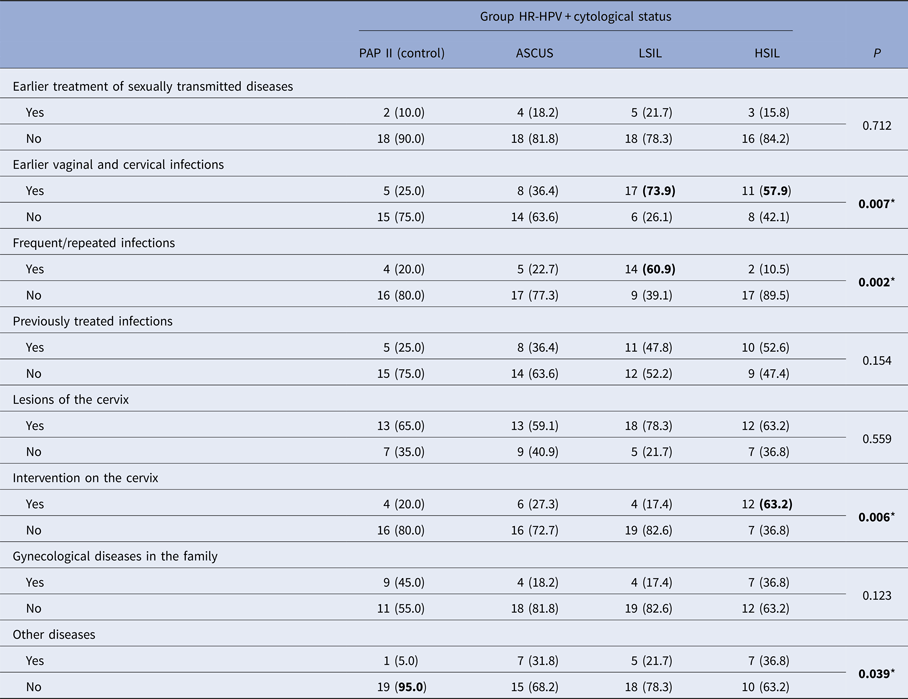
* Significant P < 0.05, χ 2 test, n (%).
Morbidity of STD in the control group was found in the lowest number of cases (10%). In other groups, the prevalence of STD was 16–22%, so the groups compared did not differ significantly.
It has been found that the previous vaginal and cervical infections were present in significantly large number of cases in women who had LSIL cytological findings (73.9%), and then in those who had HSIL (57.9%). The highest incidence of recurrent infection was observed in the findings of LSIL, significantly higher compared with all other groups. The largest number of previously treated infections was observed in the LSIL and HSIL groups, which corresponded to the largest number of previous vaginal infections or infections of the cervix in these groups.
Ureaplasma urealyticum was the most prevalently reported previous infection in three of four investigated subgroups with the following frequencies: 60% in PAP-II, 62.5% in ASCUS and 58.8% in LSIL. In the PAP-II group, this infection was associated with Mycoplasma hominis. In the LSIL group, a significant percentage (11.8%) of women reported previous Trichomonas vaginalis infection. Chlamydia trachomatis was only reported in the HSIL group with the high prevalence (81.8%).
Compared groups showed no significant difference in the distribution of cervical lesions. In the majority of cases (59–78%), regardless of cytology, all women had at least one confirmed lesion on the cervix during life time. A significantly higher frequency of interventions on the cervix occurred in patients with HSIL, which is a statistically significant difference compared with the other groups.
The occurrence of gynecological diseases in the family of patients was noted primarily in the control group and then in HSIL group, but there was no statistically significant difference.
In the group with PAP II cytological findings, the presence of other diseases was noted in 5% of cases, and in other groups in 22–37%, which was statistically significant.
Figure 2. shows the ORs and corresponding 95% CIs as measures of association of observed variables in comparison with different cytological statuses. Only those variables that were significant as risk factors in the disruption of normal cytological status were shown, or whose OR and 95% CI values were found significant (P < 0.05).
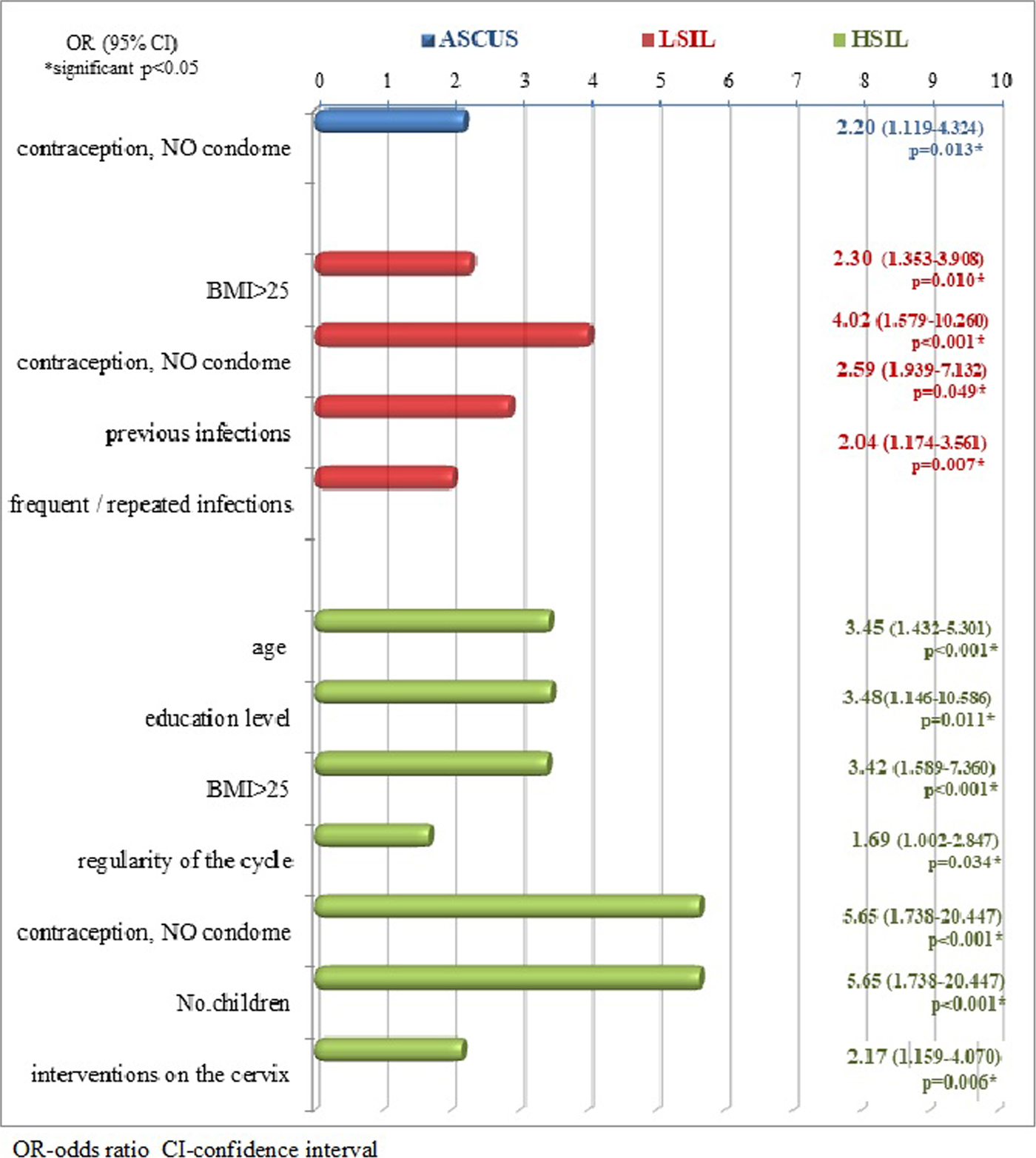
Fig. 2. Risk factors in different cytological findings in relation to the control group.
Discussion
Infection with HR-HPV types has been established as a necessary but not a sufficient aetiological factor in the development of cervical disorders and invasive cervical cancer. It is known that many factors other than HR-HPV infection itself are related to viral persistence, progression of cervical disorders and carcinogenesis. This study aimed to show which environmental and behavioural cofactors in HR-HPV-positive women in Serbia were relevant for the development of cervical disorders.
The global world prevalence of HPV infection was reported to be 11–12% in women without cervical abnormalities [Reference Forman24]. There is however a considerable regional variation with highest prevalence in sub-Saharan Africa (24%), Eastern Europe (21%) and Latin America (16%). Since Serbia belongs to the high-prevalence region of Eastern Europe and this study included women with normal and abnormal cervical cytological status, the HPV prevalence of 19.4% was in accordance with previously reported data. The study also showed that 80% of HPV-positive women harboured HR-HPV genotypes.
The genotypes isolated most frequently throughout the world, both in patients with cervical carcinoma and in healthy controls, were 16, 18, 45, 31 and 58 [Reference Syrjänen5], or according to other authors 16, 18, 52, 31 and 58 [Reference Forman24]. This research showed HPV 16, 45 and 31 as most frequent, while 18, 52 and 58 were present in lower frequencies. It confirmed the known highly oncogenic potential of type 16 since this type was significantly associated with HSIL. The previous research from Serbia reported type 16 to be the most prevalent HPV type in both cervical SCC and adenocarcinoma [Reference Stamenkovic25].
This study found that women with HSIL were significantly older than women in the other groups (46.7 ± 12.2 on average) and that was in accordance with the fact that HR-HPV types require more time to progress to cervical disease. Other authors also found that women 46–55 years were in a greater risk for HSIL and noted the tendency for developing HSIL in older women, 43–56 years old [Reference Shwe1, Reference Tao12].
Several other cofactors which were found to be of significance by this study such as BMI, gravidity, parity, smoking and persistence of HPV and other genital infections could also be age-related. It was also shown that in the HSIL group, certain hormonal disbalance, presented as an irregularity of the menstrual cycle, may be a relative risk factor (OR = 1.69). This can also be related with the age of the patients and their entering the menopause, considering that in younger women irregularity of the menstrual cycle has been indicated only in few cases.
The level of education has been proven to be associated with cervical disease by many authors [Reference Tao12, Reference Gargano16, Reference Wong, Loke and Chan26] and it was also confirmed by this research. The distribution of patients by level of education showed that women with HSIL were mostly with lower educational level and this feature proved to increase the chances for HSIL changes for over three times. It is possible that lower educational level could be a marker for a combination of characteristics such as inadequate screening, an early age of first sexual intercourse and first pregnancy, high parity, sexual behaviour and possible co-infection with other infectious agents in addition to HPV infection [Reference Louie27].
Overweight defined by BMI > 25 is a cofactor that increases the chance of LSIL and HSIL changes 2–3 times in patients with HR-HPV infection [Reference Maruthur18, Reference Lee19]. In patients with LSIL and HSIL, average nutritional status of patients expressed as a BMI was significantly higher and it was on average in the overweight category. One explanation is that overweight can cause increased serum sex hormone levels, which have been linked to a higher risk of cancer of the female reproductive tract. A number of studies reported that overweight women participate less often in screening programmes and thus increase the chances of cervical changes of a high degree [Reference Prompakay17].
Condom use has been considered as a protective factor for the development of cervical dysplasia in HR-HPV infection [Reference Shew28]. This research showed that the factor of great influence in any irregular cytological finding was the absence of condom use. This form of protection was applied in a significantly small number of cases in all groups except the control where the frequency of use was 95%. Due to the lack of condom use, the chance for the development of HSIL increased over five times (OR = 5.65, 95% CI 1.738–20.447), for the occurrence of LSIL four times (OR = 4.02, 1.579–10.260) and for the occurrence of ASCUS about two times (OR = 2.02, 95% CI 1119–4324) in the case of HR-HPV infection. The finding of the protective role of condom use is also confirmed by the results of other studies [Reference Kim6, Reference Cornelis29], which indicated that the use of condoms prevented continuous transmission between sexual partners of HPV and other STD agents. It can be concluded that condom use promoted regression of dysplasia and clearance of HR-HPV and research has shown that intervention with condom use for at least 3 months can promote CIN regression and HPV clearance allowing effective virus elimination by the immune system [Reference Cornelis29]. Another explanation of positive role of condom use is that continuous exposure to semen in women infected with HR-HPV also increases the risk of precancerous changes [Reference Kim6].
This research has shown the unequivocal connection between the development of cervical changes in the case of co-infections on the cervix with other infectious agents that favour HPV persistence and the occurrence of adverse changes. This has been confirmed by numerous studies and the mechanisms of pathophysiological changes that occur are multiple [Reference Tao12, Reference Verteramo15, Reference Castle and Giuliano30]. The mechanisms are direct genotoxicity, induction of cervical inflammation leading to genotoxic damage through oxidative metabolites and induction of HPV persistence [Reference Castle and Giuliano30]. This was confirmed in the present study because morbidity of STD in the control group was found in the lowest number of cases (10%). In the LSIL and HSIL groups, the earlier cervical and vaginal infections dominated. Earlier infections of the cervix that were not treated, and that have been repeated, increased the chance of LSIL 2–3 times. High level of U. urealyticum seems to be a cofactor in the development of HPV-related cervical dysplasia. The presence of U. urealyticum may play a role both in initiating cellular anomalies and in viral persistence. In vitro investigation reported that Mycoplasma infections cause chromosomal changes and cell transformation through gradual progressive chromosomal loss and translocations [Reference Verteramo15]. The fact that women with HSIL were very frequently previously infected with C. trachomatis can be explained by literature data. It is known that C. trachomatis infection may increase susceptibility to HPV causing microabrasions or alterations of epithelial cells thus facilitating the entry of virions. Also, C. trachomatis infection can reduce the host ability to resolve HPV infection by the modulation of cervical immune response [Reference Verteramo15, Reference Quinónez-Calvache31]. Thus, it can be concluded that genital co-infections significantly increase the progression of HR-HPV-induced cytological changes.
Studies showed that high parity in HR-HPV-positive women has been found to be associated with an increased risk for HSIL with increasing number of pregnancies [Reference Castellsagué and Muñoz32]. Women with seven or more full-term pregnancies had a fourfold increase in the risk of developing SCC as compared with nulliparous women and the risk of HSIL significantly increased with increasing number of live births. Hormonal, traumatic and immunological hypotheses are plausible biological mechanisms. High parity maintains the transformation zone on the exocervix for many years, facilitating the direct exposure to HPV and other sexually transmitted co-factors [Reference Kim6, Reference Gargano16]. Hormonal changes in pregnancy modulate the immune response to HPV and induce the persistence of the virus. Hormonal-related mechanisms influence the progression from pre-malignant to malignant cervical lesions by promoting the integration of HPV-DNA into the host genome, which results in the deregulation of E6 and E7 expression. This research has shown that higher number of deliveries increases the chance for HSIL over five times. According to the number of births, children, abortions and miscarriages, almost all compared groups were similar but patients with the most serious form of cervical dysplasia (HSIL) had on average a largest number of deliveries and children (median = 2). This was significantly higher in comparison with all other groups, but at a given sample of patients could not be demonstrated as a statistically significant feature.
Various interventions on the cervix similarly contribute to the emergence and progression of changes and are also associated with already existing changes in the cervix. Intervention on the cervix with a significantly higher frequency occurred in patients with HSIL, which is a statistically significant difference compared with other groups increasing the chances of developing HSIL to about two times.
The carcinogenic effect of smoking by-products as direct mitogenic factor causing DNA damage is already well proven. Immunological alterations have also been proven in terms of reduction of the number of Langerhans cells in the cervix and reduction in NK cells activity [Reference Hellberg33, Reference Simen-Kapeu34]. One of the studies also showed that the prevalence of HR-HPV infection is higher among smokers and is dose-dependent [Reference Vaccarella35]. The smoking effect persists over several years after smoking cessation, and smokers or former smokers have a threefold higher risk of developing severe cervical changes than non-smokers [Reference Sarian36]. Even though there was a similar representation of smokers in all analysed groups and the average smoking period ranged from 12 to 20, the longest smoking periods were found in the group with HSIL. The average number of cigarettes per day ranged from 14 to 18, which was not a significant difference between the groups.
The larger number of sexual partners was frequent in almost all groups (50–55%). Only in the group with HSIL, it was slightly lower (40%) perhaps because of their marital status, since most of these women have been married for many years. So it is very likely that the greater number of sexual partners was important for obtaining a HPV infection more than it was for the development of already present changes on the cervix.
Even though previous studies [Reference Castellsagué and Muñoz32, Reference La Vecchia and Boccia37] confirm the association of oral contraceptives with cervical carcinogenesis primarily when the period of use was over 5 years, this investigation did not show the same result. The limitation of this study, in regard of oral contraceptives usage, was that it comprised large number of never users, few short-term users and few former users with extended time since last use.
Relatively moderate size of investigated group of HR-HPV-positive women also represented the limitation. The small sample size limited the ability to conclude more on the influence of specific HPV types on the development of cervical disorders. Besides, the study group was derived from selective population since it comprised women who were already selected in primary health care based on suspected cervical changes. Therefore, the HPV prevalence in the study could not represent the prevalence in general population.
In conclusion, HR-HPV-positive women in Serbia were primarily from the urban areas and cofactors contributing to the development of their cervical disease were older age, overweight, lower educational level, previous recurrent and untreated vaginal and cervical infections, interventions on the cervix, high parity and long-term smoking habit. On the other hand, condom use was found to have a protective role in the development of cervical changes. Information about these cofactors might be very important for the development of more efficient cancer prevention programmes and promotion of anti-HPV vaccination.
Acknowledgements
For technical assistance in performing the study, the authors thank Laboratory Technicians Gabrijela Pavlovic and Marija Zivotic from the Virology Department, Institute of Microbiology and Immunology, Faculty of Medicine, University of Belgrade.
Financial support
This study was supported by the Ministry of Education, Science and Technological Development, Republic of Serbia, Research Grant No. 175073.
Ethical standards
This study was conducted according to the approval of Ethics Committee of Faculty of Medicine, University of Belgrade, decision number 29/XI-2.









