Introduction
In Malaysia, rice (Oryza sativa L.) is believed to have been cultivated since the 10th century in the state of Kedah (Hamid Reference Hamid2010). However, structured rice cultivation began in 1664, patterned after production practices in Thailand (Hamid Reference Hamid2010). In 2019, Malaysia had a total rice production area of 684,416 ha, of which 425,613 ha was under the government-scheme for irrigated cultivation granaries (Department of Agriculture Malaysia 2019). A rice granary is a vast arable land with a centralized canal irrigation system under the management of federal agriculture and considered by the national Agriculture Policy of Malaysia as a major rice-producing area. Twelve rice granary areas have been developed; 10 of these are in Peninsular Malaysia; the other 2 are located in East Malaysia (Sabah and Sarawak states). The rice granaries are Muda Agricultural Development Authority (MADA), Kemubu Agricultural Development Authority (KADA), Integrated Agricultural Development Area (IADA) Kerian, IADA Barat Laut Selangor, IADA Pulau Pinang, IADA Seberang Perak, IADA Ketara, IADA Kemasin Semerak, IADA Pekan, IADA Rompin, IADA Kota Belud and IADA Batang Lupar.
Cultivated rice can be categorized into three major subspecies: (1) tropical and subtropical Asia long-grained indica rice; (2) short/medium-grained japonica rice in the temperate regions of northern China and Japan; and (3) medium-grained javanica rice in the Philippines, the Madagascar mountain ranges, and Indonesia (Muthayya et al. Reference Muthayya, Sugimoto, Montgomery and Maberly2014). Historically, the Malaysian rice landraces comprised a mixture of indica and japonica subspecies before being replaced by “elite” rice cultivars (Song et al. Reference Song, Chuah, Tam and Olsen2014). These elite cultivars, derived from the exotic germplasm of the Indica line, were introduced during the industrialized farming era of Malaysia (Song et al. Reference Song, Chuah, Tam and Olsen2014).
Rice is a primary staple food that has been produced for hundreds of years in Malaysia, but rice production is yet to meet the national demand; 20% to 24% is imported from nearby countries (FAO 2018). This is due to several challenges faced by the Malaysian rice industry, namely water scarcity, extreme weather, loss of agricultural land, unfavorable soil conditions, poor crop management practices, insect pests, diseases, and weeds. Undeniably, weed infestation is one of the significant contributors to rice yield losses, not only in Malaysia, but also in most rice-growing nations. Savary et al. (Reference Savary, Willocquet, Elazegui, Castilla and Teng2000) ranked weeds above pathogens and insects as the most devastating pest in many tropical Asian rice fields; nearly 95% rice yield loss occurs globally under severe weed competition (Rabbani et al. Reference Rabbani, Bajwa and Javaid2011). Of several weeds infesting rice fields, weedy rice (Oryza sativa f. spontanea or O. sativa complex) has been recognized as the major rice weed in Asia and can cause 5% to 100% yield loss (Azmi and Abdullah Reference Azmi and Abdullah1998; Azmi and Karim Reference Azmi and Karim2008; Ruzmi et al. Reference Ruzmi, Ahmad-Hamdani and Bakar2017). Taxonomically, weedy rice belongs to the genus Oryza. The genus Oryza comprises various species and subspecies, including wild rice (Oryza rufipogon Griff.), cultivated rice, and weedy rice (Shrestha et al. Reference Shrestha, Stallworth and Tseng2019). In contrast to wild rice, weedy rice is believed to have evolved from (1) outcrossing between the wild type and rice cultivars with subsequent genetic exchange between the outcrosses and wild type or the rice cultivars or among various outcrosses (Kanapeckas et al. Reference Kanapeckas, Vigueira, Ortiz, Gettler, Burgos, Fischer and Lawton-Rauh2016; Londo and Schaal Reference Londo and Schaal2007; Qiu et al. Reference Qiu, Zhu, Fu, Ye, Wang, Mao, Lin, Chen, Zhang, Guo and Qiang2014); (2) de-domestication events of cultivars (Kanapeckas et al. Reference Kanapeckas, Vigueira, Ortiz, Gettler, Burgos, Fischer and Lawton-Rauh2016; Li et al. Reference Li, Li, Jia, Caicedo and Olsen2017; Olsen et al. Reference Olsen, Caicedo and Jia2007; Sun et al. Reference Sun, Ma, Tang, Zhao, Zhang, Wang, Song, Li, Liu and Xu2019); and (3) direct establishment from wild rice populations (Huang et al. Reference Huang, Young, Reagon, Hyma, Olsen, Jia and Caicedo2017; Shrestha et al. Reference Shrestha, Stallworth and Tseng2019).
Despite many initiatives on weedy rice management, weedy rice has remained the leading weed problem in rice for many years in Asia, the Americas, and Africa (Chauhan Reference Chauhan2013; Delouche et al. Reference Delouche, Burgos, Gealy, Zorilla-San, Labrada and Larinde2007; Mortimer et al. Reference Mortimer, Pandey and Piggin2000; Second Reference Second1991; Wahab and Suhaimi Reference Wahab and Suhaimi1991). The adoption of herbicide-resistant (HR) rice varieties initially controlled weedy rice well, but occasional survivors, or escapes, occur due to various reasons (unintentional or otherwise) and limitations in the supply, technical support, and farm conditions. The remaining weedy rice can outcross with the crop, eventually resulting in HR weedy rice outcrosses and populations (Burgos et al. Reference Burgos, Norsworthy, Scott and Smith2008; Busconi et al. Reference Busconi, Rossi, Lorenzoni, Baldi and Fogher2012; Espinoza-Esquivel and Arrieta-Espinoza Reference Espinoza-Esquivel and Arrieta-Espinoza2007; Hoyos et al. Reference Hoyos, Plaza and Caicedo2019; Kaloumenos et al. Reference Kaloumenos, Capote, Aguado and Eleftherohorinos2013; Roso et al. Reference Roso, Merotto, Delatorre and Menezes2010; Sudianto et al. Reference Sudianto, Beng-Kah, Ting-Xiang, Saldain, Scott and Burgos2013). There is renewed interest among researchers to understand and solve the HR weedy rice problem in a sustainable manner, or at least mitigate the evolution of resistance in weedy rice populations.
Scientists have defined herbicide resistance as the inherited ability of plants to survive the application of herbicide at a dose that normally would control the wild/susceptible populations (Vencill et al. Reference Vencill2002; Weed Science Society of America 1998). In the context of herbicide resistance in weeds, the herbicide-susceptible weed population becomes a population that could survive herbicide application (Moss Reference Moss2017). By February 2021, a total of 263 HR weed species have been reported worldwide; 52 of them are weeds in rice, including weedy rice (Heap Reference Heap2021). HR weedy rice has been documented in seven countries, namely the United States, Brazil, Costa Rica, Italy, Greece, Colombia, and Malaysia (Heap Reference Heap2021). All of these countries have been adopting HR rice technology (Burgos et al. Reference Burgos, Norsworthy, Scott and Smith2008; Busconi et al. Reference Busconi, Rossi, Lorenzoni, Baldi and Fogher2012; Espinoza-Esquivel and Arrieta-Espinoza Reference Espinoza-Esquivel and Arrieta-Espinoza2007; Hoyos et al. Reference Hoyos, Plaza and Caicedo2019; Kaloumenos et al. Reference Kaloumenos, Capote, Aguado and Eleftherohorinos2013; Roso et al. Reference Roso, Merotto, Delatorre and Menezes2010; Sudianto et al. Reference Sudianto, Beng-Kah, Ting-Xiang, Saldain, Scott and Burgos2013).
In Malaysia, imidazolinone-resistant (IMI-R) rice varieties (MR220CL1 and MR220CL2) in the Clearfield® Production System (CPS) (BASF Malaysia Sdn. Bhd., 40706 Shah Alam, Selangor, Malaysia) were introduced in late 2010 and since then have received positive response from farmers due to the potential of these varieties to yield five to eight times more than conventional varieties, boosting rice yields up to 9,700 kg ha−1 (Azmi et al. Reference Azmi, Azlan, Yim, George and Chew2012b; Mispan et al. Reference Mispan, Bzoor, Mahmod, MD-Akhir and Zulrushdi2019; Ruzmi et al. Reference Ruzmi, Ahmad-Hamdani and Bakar2017; Svizzero Reference Svizzero2020). However, rice growers’ continuous dependence on and inappropriate application of the IMI herbicide OnDuty™ (BASF Malaysia Sdn. Bhd.) in the CPS package has tarnished the success story of CPS. Reports of IMI-R weedy rice in CPS are mounting (Ahmad-Hamdani et al. Reference Ahmad-Hamdani, Juraimi and Mazlan2015; Dilipkumar et al. Reference Dilipkumar, Burgos, Chuah and Ismail2018; Ruzmi et al. Reference Ruzmi, Ahmad-Hamdani and Bakar2017, Reference Ruzmi, Ahmad-Hamdani and Mazlan2020) and are expected to continue to rise in the near future.
Resistance of weedy rice to IMI herbicides may evolve via gene flow from CPS rice accompanied by repeated selection with IMI herbicides, favoring increased resistant allele frequency in populations (Rajguru et al. Reference Rajguru, Burgos, Shivrain and Stewart2005; Sudianto et al. Reference Sudianto, Beng-Kah, Ting-Xiang, Saldain, Scott and Burgos2013). To a lesser extent, resistance to IMI herbicides could also arise from selection of rare de novo mutation(s) among populations, as evidenced by the findings of Sales et al. (Reference Sales, Shivrain, Burgos and Kuk2008). There is also concern about the persistence and accumulation of herbicide residue in soil as a result of continuous use of CPS. This residual activity can cause carryover injury to rotational crops (rice or other crops) that are susceptible to IMI herbicides (de Lima Fruet et al. Reference de Lima Fruet, Merotto and da Rosa Ulguim2020). Thus, the sustainability of CPS rice technology is impeded by the evolution of HR weedy rice populations and possible buildup of herbicide residue in soil. The rate of resistance evolution is influenced by biological, ecological, agronomic, and environmental factors (Moss et al. Reference Moss, Ulber and den Hoed2019). Understanding the resistance problem better and developing comprehensive management strategies to delay the evolution and spread of resistant weeds has taken almost 60 yr of research across many disciplines in plant science (Baucom Reference Baucom2019). The quest to understand the nuances of herbicide resistance in the hope of finding novel tools for weed management is a continuing challenge.
Biology and History of Weedy Rice in Malaysia
Generally, weedy rice can be defined as the unwanted plant types of the genus Oryza (Delouche et al. Reference Delouche, Burgos, Gealy, Zorilla-San, Labrada and Larinde2007; Suh Reference Suh2008) as it pertains to interference with rice production and reduction of rice grain quality. The most widespread weedy rice is of the same species as cultivated rice, Oryza sativa, and hence is most similar to the crop (Delouche et al. Reference Delouche, Burgos, Gealy, Zorilla-San, Labrada and Larinde2007). Oryza spp. are primarily self-pollinated with low levels of hybridization (Shivrain et al. Reference Shivrain, Burgos, Anders, Rajguru, Moore and Sales2007), which could account for the wide diversity of weedy rice populations worldwide (Delouche et al. Reference Delouche, Burgos, Gealy, Zorilla-San, Labrada and Larinde2007).
Weedy rice types are most distinguishable by their spikelet characteristics. Malaysian weedy rice spikelets may be awned or awnless with various hull colorations, including strawhull, intermediate strawhull, brownhull, and blackhull (Sudianto et al. Reference Sudianto, Neik, Tam, Chuah, Idris, Olsen and Song2016). The weedy rice hull has parallel trichomes, providing a better anchorage of seeds on soil surface to facilitate seed burial and prevent being washed away by heavy rain (Abraham and Jose Reference Abraham and Jose2015). Morphologically, the Malaysian weedy rice populations of today can be divided into four groups (Sudianto et al. Reference Sudianto, Neik, Tam, Chuah, Idris, Olsen and Song2016). Group 1 is comprised of the majority of awned, blackhull, and brownhull types. These plant types appeared to have descended from wild rice, lending support to the population genetics data. The majority of strawhull types fall under Group 2, which supports the second possible origin of weedy rice types: these are feral plants, or variants from Malaysian elite indica rice cultivars, which are high shattering. The third cluster is primarily composed of brownhull morphotypes. The fourth cluster contains a mixture of other weedy morphotypes, lending credence to the natural outcrossing between crop and weed and among weedy populations of various types, resulting in an admixture of plant traits (Sudianto et al. Reference Sudianto, Neik, Tam, Chuah, Idris, Olsen and Song2016). The complexity of the genesis of Malaysian weedy rice remains to be fully understood (Ruzmi et al. Reference Ruzmi, Ahmad-Hamdani and Bakar2017).
Weedy rice grain generally has a red or brown, rough pericarp, although a white pericarp is also found among some African and Asian weedy rice strains where weedy red rice is the predominant type (Delouche et al. Reference Delouche, Burgos, Gealy, Zorilla-San, Labrada and Larinde2007; Suh Reference Suh2008). Regardless of awn presence, the majority of Malaysian weedy rice types across the granaries (40% to 100%) have a red pericarp. The white-kernel rice types represent 3% to 60% of the populations. Most of the weedy rice kernels are either long/slender or round/short (Sudianto et al. Reference Sudianto, Neik, Tam, Chuah, Idris, Olsen and Song2016). The majority (77%) of weedy rice types are tall (˜100 to more than 150 cm), while only a small group (23%) are of the same height as cultivated rice (85 to 110 cm) (Dilipkumar et al. Reference Dilipkumar, Ahmad-Hamdani, Rahim, Chuah and Burgos2021). Malaysian weedy rice ecotypes mature early, with almost 80% maturing less than 100 d after emergence (Dilipkumar et al. Reference Dilipkumar, Ahmad-Hamdani, Rahim, Chuah and Burgos2021). Taller weedy rice plants have the advantage of aboveground competition over the shorter rice cultivars (Akasaka et al. Reference Akasaka, Ushiki, Iwata, Ishikawa and Ishii2009; Diarra et al. Reference Diarra, Smith and Talbert1985; Rathore et al. Reference Rathore, Singh, Kumar and Chauhan2016; Ratnasekera et al. Reference Ratnasekera, Perera, He, Senanayake, Wijesekara, Yang and Lu2014; Shivrain et al. Reference Shivrain, Burgos, Scott, Gbur, Estorninos and McClelland2010; Zhang et al. Reference Zhang, Dai, Wu, Song and Qiang2012). Weedy rice being taller than the rice crop contributes to a significant rice yield loss, especially when lodging occurs. Another important weedy trait that contributes to the persistence of weedy rice is high grain shattering (Azmi et al. Reference Azmi, Azlan, Yim, George and Chew2012b; Chauhan Reference Chauhan2013; Shivrain et al. Reference Shivrain, Burgos, Scott, Gbur, Estorninos and McClelland2010). Gripping the mature weedy rice panicle gently by hand can release more than 60% of the seeds (Zhu et al. Reference Zhu, Ellstrand and Lu2012). Because many types mature early and shatter, a large proportion of seeds would have already dropped to the ground at rice harvest. Wind gusts can also shatter weedy rice grains, giving rise to the local Malay name for weedy rice: padi angin. Padi generally translates to “paddy” or “rice,” and angin means “wind” (Ruzmi et al. Reference Ruzmi, Ahmad-Hamdani and Bakar2017).
The increased infestation of weedy rice in Southeast Asia, including Malaysia, began after wide-scale adoption of the direct-seeding method. Direct seeding was introduced in the late 1980s; thereafter, weedy rice became a major problem wherever direct seeding was practiced across rice granaries in Malaysia (Azmi and Abdullah Reference Azmi and Abdullah1998). Weedy rice infestation was first reported in the Northwest Selangor Project rice fields in 1988 (Wahab and Suhaimi Reference Wahab and Suhaimi1991) followed by the Muda area, Kedah State in 1990 (Mohammed Zuki and Kamarudin Reference Mohammed Zuki and Kamarudin1994). In 1995, weedy rice was reported in Besut in Terengganu State, and in 1996, weedy rice was found in the rice granaries in Seberang Perai of Pulau Pinang State and Kerian in Perak State (Azmi et al. Reference Azmi, Abdullah, Mislamah and Baki2000). In 2004, weedy rice infestation in the Muda area had spread in every district, with at least 10% cover (Azmi and Karim Reference Azmi and Karim2008). Weedy rice variants are the new, prevalent, and most complex weed species in the rice granaries of Peninsular Malaysia (Azmi and Baki Reference Azmi and Baki2007; Ruzmi et al. Reference Ruzmi, Ahmad-Hamdani and Bakar2017), with at least 50% infestation levels in the east and west coast granaries and up to 20% in the northern granaries (Mispan et al. Reference Mispan, Bzoor, Mahmod, MD-Akhir and Zulrushdi2019). In late 2018, a comprehensive survey on geographic distribution of resistant weedy rice populations across all 12 Peninsular Malaysia rice granaries was conducted. From the CPS IMI-herbicide screening, it was discovered that 79.4% of the surveyed populations have evolved resistance to the IMI–herbicide premix (MS Ahmad-Hamdani, unpublished data).
Planting high-shattering rice varieties, direct seeding, and the use of large combine harvester that shatters rice grains are considered the major contributing factors to the spread and dominance of weedy rice in Malaysia (Suh Reference Suh2008). Researchers have proposed several hypotheses to explain the introduction and proliferation of weedy rice in Malaysia. One of these is that weedy rice in Malaysia arose from hybridization between wild rice and the rice cultivars and between the different varieties of cultivated rice. Wild rice exists in Malaysia. Outcrossing between cultivated rice and wild rice is believed to have resulted in weedy rice variants that are morphologically similar to wild rice among weedy rice populations in the Muda area (Abdullah et al. Reference Abdullah, Vaughan, Watanabe and Okuno1996; Azmi and Karim Reference Azmi and Karim2008). This hypothesis is supported by the fact that in several rice-planting zones in Muda, weedy rice and wild rice have been observed to coexist in the same sites (Azmi and Karim Reference Azmi and Karim2008). Thus, outcrossing among wild, weedy, and cultivated rice seems the most plausible origin of weedy rice in Malaysia (Azmi and Karim Reference Azmi and Karim2008). However, the flowering time of wild rice and weedy rice does not normally synchronize, casting some doubt on this hypothesis (Azmi and Karim Reference Azmi and Karim2008). Others proposed that weedy rice has evolved through natural mutations (Lu et al. Reference Lu, Song and Chen2003) and through simultaneous interbreeding and mutations among the Oryza spp. (Azmi and Karim Reference Azmi and Karim2008). Yet another hypothesis is the “impractical but unavoidable” rice seeding establishment, causing reversion of some varietal traits to the weedy allele and producing phenotypes that would thrive better under certain stress conditions (Azmi and Karim Reference Azmi and Karim2008). This topic has been reviewed by Ruzmi et al. (Reference Ruzmi, Ahmad-Hamdani and Bakar2017). Indeed, some weedy rice types arose from de-domestication (Kanapeckas et al. Reference Kanapeckas, Vigueira, Ortiz, Gettler, Burgos, Fischer and Lawton-Rauh2016; Sun et al. Reference Sun, Ma, Tang, Zhao, Zhang, Wang, Song, Li, Liu and Xu2019). The incidental de-domestication of weedy rice has thus resulted in high physical and physiological similarities between weedy rice and cultivated rice, rendering chemical control difficult. Today, such arguments about weedy rice origins can be settled using molecular and population genetics studies.
A population genetics study of Malaysian weedy rice using simple sequence repeat (SSR) markers revealed three possible origins: (1) traditional Malaysian rice cultivars or landraces (a mixture of japonica and indica varieties), (2) Malaysian elite rice cultivars that are genetically different from the traditional landraces, and (3) the wild rice O. rufipogon. (Song et al. Reference Song, Chuah, Tam and Olsen2014). This finding is congruent with the origin of weedy rice in Thailand, where the weedy rice populations form two distinct groups based on SSR marker analysis (Pusadee et al. Reference Pusadee, Schaal, Rerkasem and Jamjod2013). The first group includes populations that are genetically similar to the companion cultivated rice variety, while the second group consists of populations that are admixtures of cultivated rice variety and O. rufipogon (Pusadee et al. Reference Pusadee, Schaal, Rerkasem and Jamjod2013). The South Asian weedy rice has high genetic diversity contributed to by both cultivated varieties and O. rufipogon (Huang et al. Reference Huang, Young, Reagon, Hyma, Olsen, Jia and Caicedo2017). The contribution of crop–weed gene flow to weedy rice evolution is also demonstrated in other regions in Southeast Asia (Huang et al. Reference Huang, Young, Reagon, Hyma, Olsen, Jia and Caicedo2017). In China, weedy rice collected from Liaoning and Guangdong provinces is more closely related to the cultivated rice varieties that have been planted in the same fields where the weedy rice samples were collected than to other rice varieties planted elsewhere in China and the common Chinese wild rice (Zhang et al. Reference Zhang, Dai, Wu, Song and Qiang2012).
CPS Rice and Its Impact on the IMI-Herbicide Resistance Evolution in Malaysian Weedy Rice
The genetic and morphological similarity between weedy rice and cultivated rice has made chemical, physical, and cultural control difficult, because any herbicide that can inhibit the growth of weedy rice will be detrimental to cultivated rice. Hence, only HR rice herbicides in tandem with IMI-R cultivated rice work best for weedy rice (Barber et al. Reference Barber, Butts, Boyd, Cunningham, Selden, Norsworthy, Roma-Burgos and Bertucci2021). As an option for chemical control of weedy rice, IMI-R rice has been used for weedy rice management in Malaysia since 2010 (Azmi et al. Reference Azmi, Azlan, Yim, George and Chew2012b). CPS rice was first developed in the United States by mutation breeding and was first commercialized in 2002. This technology was introduced quickly to Central and South America and then to Europe (Sudianto et al. Reference Sudianto, Beng-Kah, Ting-Xiang, Saldain, Scott and Burgos2013). In Malaysia, the IMI-R rice varieties were developed by crossing an IMI-R variety (IMI-TR) from the United States (mutant line harboring a mutation at Ser-653 in the AHAS gene) with a Malaysian rice variety MR220. The collaboration between the Malaysian Agricultural Research and Development Institute (MARDI) and BASF (Malaysia) Sdn. Bhd. resulted in the release of two IMI-R rice varieties, ‘MR220CL1’ and ‘MR220CL2’ (Azmi et al. Reference Azmi, Azlan, Yim, George and Chew2012b). MR220CL1 and MR220CL2 have 98.5% and 92.5% similarity with the local parent MR220, respectively (Azmi et al. Reference Azmi, Azlan, Chew, George, Lim, Hadzim and Yim2012a; Sudianto et al. Reference Sudianto, Beng-Kah, Ting-Xiang, Saldain, Scott and Burgos2013), and exhibit resistance to the IMI herbicides imazapic and imazapyr, which are then used with CPS rice to control weedy rice.
CPS rice cultivation practice consists of certified seeds of IMI-R rice varieties, the premix of the IMI herbicides imazapic (52.5%) and imazapyr (17.5%) (OnDuty™ WG,), and surfactant (Tenagam, BASF Malaysia Sdn. Bhd.) to increase herbicide sorption to the soil (Azmi et al. Reference Azmi, Azlan, Yim, George and Chew2012b). The adjuvant is intended to increase the residual activity of herbicides, preventing weedy rice escapes. CPS also comes with the prescribed stewardship guideline. The Clearfield® stewardship guideline is important to ensure proper use of the Clearfield® technology by rice growers. The CPS IMI herbicides are recommended PRE and are most effective when applied within 5 d of sowing. Application later than this is not effective.
The IMIs (imazamethabenz, imazamox, imazapic, imazapyr, imazaquin, and imazethapyr) are acetohydroxyacid synthase (AHAS)-inhibiting herbicides. The IMIs are extensively used in agricultural fields because of their minimal impact on the environment, good crop selectivity, and broad range of target weeds (Ramezani Reference Ramezani2008). Thus, besides controlling weedy rice, the mixture of imazapic and imazapyr (e.g., OnDuty™) also controls other major rice weeds, including barnyardgrass [Echinochloa crus-galli (L.) P. Beauv.], Chinese sprangletop [Leptochloa chinensis (L.) Nees], ricefield flatsedge (Cyperus iria L.), and fimbry [Fimbristylis littoralis Gaudich.].
The implementation of CPS rice technology has given the Malaysian rice industry an upper hand in controlling weedy rice effectively (Azmi et al. Reference Azmi, Azlan, Yim, George and Chew2012b). Nonetheless, there has been a rising concern about gene flow from CPS rice to weedy rice, as has been documented in the first few years of planting Clearfield®® rice in the United States (Shivrain et al. Reference Shivrain, Burgos, Anders, Rajguru, Moore and Sales2007) and Brazil (Roso et al. Reference Roso, Merotto, Delatorre and Menezes2010). The risk of gene flow and subsequent introgression of resistance traits into the weedy population is expected to be higher in tropical regions such as Malaysia, where farmers plant up to three rice crops in 1 yr or five rice crops in 2 yr. The benefit of winterkill, which is helpful in temperate regions, is absent. This situation is exacerbated by the fact that many farmers in developing countries do not have the knowledge, resources, infrastructure, technical support, and institutional support that are needed to follow the CPS stewardship guideline sufficiently (Dilipkumar et al. Reference Dilipkumar, Ahmad-Hamdani, Rahim, Chuah and Burgos2021). The genetic and morphological data by Song et al. (Reference Song, Chuah, Tam and Olsen2014) and Sudianto et al. (Reference Sudianto, Neik, Tam, Chuah, Idris, Olsen and Song2016) demonstrate that introgression of crop traits into weedy populations has occurred in Malaysia (although introgression of the mutant AHAS gene is not yet quantified) due to the previously mentioned mitigating factors. As evidence, SSR marker analysis also shows the occurrence of gene flow between Malaysian CPS rice to weedy rice, although the AHAS gene has not been sequenced (Engku et al. Reference Engku, Norida, Juraimi, Rafii, Abdullah and Alam2016). The samples from Pahang, Malaysia, revealed an outcrossing rate of up to 20.38% based on the survival rate of putative weedy rice outcrosses. The HR gene from CPS rice can be transferred to weedy rice with 0.1% to 3.2% probability (Cao et al. Reference Cao, Lu, Xia, Rong, Sala, Spada and Grassi2006; Clegg et al. Reference Clegg, Giddings, Lewis and Barton1993; Shivrain et al. Reference Shivrain, Burgos, Anders, Rajguru, Moore and Sales2007, Reference Shivrain, Burgos, Gealy, Sales and Smith2009; Singh et al., Reference Singh, Singh, Black, Boyett, Basu, Gealy, Gbur, Pereira, Scott, Caicedo and Burgos2017; Zhang et al. Reference Zhang, Linscombe, Webster, Tan and Oard2006). If that situation occurs, the continued selection of resistant outcrosses with IMI herbicides will facilitate the introgression of resistant alleles into weedy populations, forming stabilized HR weedy rice populations (Burgos et al. Reference Burgos, Singh, Tseng, Black, Young, Huang, Hyma, Gealy and Caicedo2014; Gealy et al. Reference Gealy, Mitten and Rutger2003; Singh et al. Reference Singh, Singh, Black, Boyett, Basu, Gealy, Gbur, Pereira, Scott, Caicedo and Burgos2017; Sudianto et al. Reference Sudianto, Beng-Kah, Ting-Xiang, Saldain, Scott and Burgos2013). Natural hybridization between cultivated rice and its weedy relatives or between wild rice and weedy rice has been documented by many research groups across the globe (Chen et al. Reference Chen, Lee, Song, Suh and LU2004; Majumder et al. Reference Majumder, Ram and Sharma1997; Messeguer et al. Reference Messeguer, Marfa, Catala, Guiderdoni and Melé2004; Oka and Chang Reference Oka and Chang1961; Song et al. Reference Song, Lu, Zhu and Chen2002, Reference Song, Lu, Zhu and Chen2003).
Other than gene flow, resistance evolution in weedy rice populations may also be contributed by selection of resistance-conferring de novo mutation(s). This was evidenced by two populations of weedy rice in Arkansas, USA, containing IMI-R individuals that harbor one de novo resistance-conferring ALS mutation, Gly-654-Glu. These populations were sampled before the adoption of Clearfield® rice (Sales et al. Reference Sales, Shivrain, Burgos and Kuk2008). However, because the occurrence of natural mutations is orders of magnitude rarer than the rate of effective gene flow, what we are witnessing in CPS fields is the evolution of HR populations via crop–weed outcrossing. Farmers in Malaysia, and apparently also in other world regions, are unable to follow all aspects of the CPS stewardship guideline, for various reasons. This is a problem. The inability to rotate rice with other crops, for example, will accelerate the selection of resistant outcrosses by the continued use of the same herbicides. Thus far, selection pressure with IMI herbicides in CPS has not yet been reported to select for de novo ALS mutations other than the one reported in the United States. However, while ALS mutations may not have occurred in weedy rice, other weed species in rice are prone to such selection for resistance to IMI herbicides, as has occurred with Echinochloa spp. (Matzenbacher et al. Reference Matzenbacher, Kalsing, Menezes, Barcelos and Merotto Junior2013; Panozzo et al. Reference Panozzo, Scarabel, Tranel and Sattin2013; Rouse et al. Reference Rouse, Burgos, Norsworthy, Tseng, Starkey and Scott2018), Cyperus spp. (Chiapinotto et al. Reference Chiapinotto, Schaedler, Fernandes, Andres and Lamego2017; Yu et al. Reference Yu, McCullough, McElroy, Jespersen and Shilling2020), Fimbristylis spp. (Ortiz et al. Reference Ortiz, Pérez, Anzalone, Zambrano, Torres, Quintana, López, López and Fischer2017; Schaedler et al. Reference Schaedler, Burgos, Noldin, Alcober, Salas and Agostinetto2015), and many others.
Entering the 10th year of its implementation in Malaysia, CPS rice technology has received mixed feedback from various sectors in agriculture. Growers who have been adopting CPS for at least eight planting seasons started to experience the reduced efficacy of IMI herbicides on weedy rice. In a single-dose CPS IMI-herbicide screening, Ahmad-Hamdani et al. (Reference Ahmad-Hamdani, Juraimi and Mazlan2015) observed a moderate to high level of survival in the three weedy rice populations collected from rice fields in Kedah State. Dilipkumar et al. (Reference Dilipkumar, Burgos, Chuah and Ismail2018) later confirmed that the weedy rice population collected from Pendang, Kedah, has evolved up to 67-fold resistance to IMI herbicides. More recently, resistance to IMI herbicides was also confirmed in weedy rice populations collected from three CPS rice fields in Kedah and Perlis states (Ruzmi et al. Reference Ruzmi, Ahmad-Hamdani and Mazlan2020). Therefore, although the innovation of HR rice offers the ability to selectively control weedy rice, this benefit can be nullified by the evolution of HR weedy rice populations. Rice farmers will then have to revert to traditional, time-consuming practices of managing weedy rice. Herbicides, including the herbicide package in HR crops, are highly effective tools to manage weeds. Nonetheless, to sustain herbicide efficiency, one must integrate herbicides with cultural, mechanical, and biological methods. With the CPS rice technology, farmers are advised to rotate CPS rice with non-CPS rice every two planting seasons to reduce the risk of herbicide persistence and resistance evolution in weedy rice.
Distribution of Resistant Weedy Rice in Malaysia
In Malaysia, rice is grown in granary areas comprising more than 60% of the total rice-planted areas (Department of Agriculture Malaysia 2019). CPS rice has been widely grown in several major rice granaries following its introduction in Malaysia because of its effectiveness in controlling weedy rice. The CPS rice varieties MR220CL1 and MR220CL2 mature early at around 95 to 98 d after sowing and yield higher than other inbred varieties, making them highly popular among rice growers (Harun et al. Reference Harun, Sobri, Sufian and Sulaiman2018; Rosnani et al Reference Rosnani, Tapsir and Azmi2013; Sudianto et al. Reference Sudianto, Beng-Kah, Ting-Xiang, Saldain, Scott and Burgos2013). The adoption of CPS rice rose sharply from 0.9% in 2011 to 56% in 2015 in Peninsular Malaysia (Harun et al. Reference Harun, Sobri, Sufian and Sulaiman2018), following a similar trajectory of adoption in the United States (Hardke Reference Hardke2020). Notwithstanding its popularity among rice growers, the CPS rice technology will not be sustainable if the various constraints to proper adoption are not alleviated and general failure to follow the stewardship guideline continues.
Jaafar et al. (Reference Jaafar, Juraimi, Ahmad-Hamdani, Uddin and Man2014) surveyed weedy rice plants that survived/escaped the IMI herbicides in four selected CPS rice-cropping zones in Peninsular Malaysia. The survey targeted rice fields with a history of CPS rice technology for at least four consecutive planting seasons. Across all surveyed fields (approximately 50 ha, comprising 40 rice fields), 1,240 weedy rice plants survived/escaped the IMI herbicides applied in the dry/off season and 813 weedy rice plants survived/escaped in the main season of 2012. Even though weedy rice resistance to IMI herbicides escaped plants was not verified at that time, our latest nationwide survey indicated that the majority of sampled weedy rice populations are now IMI-R (MS Ahmad-Hamdani, unpublished data). With a continuous CPS rice cycle that allows these survivors to reproduce, these remaining plants will gradually replace the soil seedbank and form the contemporary HR weedy rice populations (Shivrain et al. Reference Shivrain, Burgos, Gealy, Moldenhauer and Baquireza2008; Singh et al. Reference Singh, Singh, Black, Boyett, Basu, Gealy, Gbur, Pereira, Scott, Caicedo and Burgos2017). The high number of consecutive CPS rice plantings and the inadequate removal of weedy rice survivors from CPS rice fields led to the increasing abundance of resistant weedy rice populations in Malaysia, as has been the story in Italy (Busconi et al. Reference Busconi, Rossi, Lorenzoni, Baldi and Fogher2012), the United States (Burgos et al. Reference Burgos, Singh, Tseng, Black, Young, Huang, Hyma, Gealy and Caicedo2014), Greece (Kaloumenos et al. Reference Kaloumenos, Capote, Aguado and Eleftherohorinos2013), and Brazil (Avila et al. Reference Avila, Noldin, Mariot, Massoni, Fipke, Gehrke, Merotto, Tomita, Matos, Facioni and Vieira2021). Of late, several rice fields in Malaysia have been planted with CPS rice for more than eight consecutive seasons; thus, it is expected that IMI-R weedy rice occurs broadly in all the rice granaries in Malaysia. A publication of an extensive study on the in situ geographic distribution of resistant weedy rice populations in CPS rice fields in all rice granaries across Peninsular Malaysia is in progress (MS Ahmad-Hamdani, unpublished data).
Mechanisms Endowing IMI-Herbicide Resistance in Malaysian Weedy Rice
Generally, the mechanisms of resistance to herbicides can be divided into two categories: (1) target-site resistance (TSR) and (2) non–target site resistance (NTSR) (Gaines et al. Reference Gaines, Duke, Morran, Rigon, Tranel, Küpper and Dayan2020). TSR pertains to reduced target-site sensitivity as a consequence of alteration(s) in amino acid sequence(s) of the target enzyme, resulting in reduced herbicide binding. Other than that, resistance involving the target site might be conferred by the overexpression of a target enzyme through modification(s) in the gene promoter or transcription factor (Powles and Yu Reference Powles and Yu2010). Most cases of AHAS-inhibitor resistance in weed species are associated with reduced sensitivity of AHAS to herbicide due to amino acid substitutions in the catalytic site that alter the topology of the binding pocket (Menne and Kocher Reference Menne, Köcher, Kramer, Schirmer, Jeschke and Witschel2007; Powles and Yu Reference Powles and Yu2010; Tranel and Wright Reference Tranel and Wright2002). NTSR generally arises from multiple mechanisms that prevent the herbicide from reaching the target site, limit the amount of herbicide that reaches the target site to a nonlethal dose, or protect the plant from lethal effects of the herbicide. Among these mechanisms are reduced herbicide entry, reduced herbicide translocation, and enhanced herbicide detoxification in the plant (Gaines et al. Reference Gaines, Duke, Morran, Rigon, Tranel, Küpper and Dayan2020).
Among weed species, the first reported case of IMI-herbicide resistance was in rigid ryegrass (Lolium rigidum Gaudin) infesting spring barley (Hordeum vulgare L.) and wheat (Triticum aestivum L.) expressing resistance to imazapyr (Heap Reference Heap2021; Preston et al. Reference Preston, Tardif and Powles1996). Meanwhile, the first IMI- HR weedy rice was detected within two seasons of planting CPS rice in the United States (Heap Reference Heap2021; Rajguru et al. Reference Rajguru, Burgos, Shivrain and Stewart2005). The majority of reported resistance to IMI herbicides is target-site based (Heap Reference Heap2021). Currently, as many as 22 AHAS gene mutations associated with IMI-herbicide resistance have been reported in 94 weed species worldwide (Table 1). These include Ala-122 (8 species), Pro-197 (16 species), Ala-205 (6 species), Asp-376 (8 species), Trp-574 (30 species), Ser-653 (10 species), and Gly-654 (2 species). Of all these, three loci (Ala-122, Ser-653, and Gly-654) are associated with resistance in weedy rice. IMI resistance in weedy rice has been generally attributed to gene flow from HR rice in countries adopting CPS rice technology. Thus far, mutations in weedy rice conferring resistance to IMI herbicides include: Ser-653-Asn in the United States (Rajguru et al. Reference Rajguru, Burgos, Shivrain and Stewart2005; Sales et al. Reference Sales, Shivrain, Burgos and Kuk2008), Italy (Scarabel et al. Reference Scarabel, Cenghialta, Manuello and Sattin2012), and Greece (Kaloumenos et al. Reference Kaloumenos, Capote, Aguado and Eleftherohorinos2013), and Gly-654-Glu, Ser-653-Asn, Ser-653-Asp, and Ala-122-Thr alone or in combination in Brazil (Roso et al. Reference Roso, Merotto, Delatorre and Menezes2010). In Malaysia, IMI-R weedy rice populations collected from CPS rice fields in Kedah and Perlis states harbor a single-nucleotide polymorphism (AGT to AAT) resulting in a Ser-653-Asn substitution in AHAS (Ruzmi et al. Reference Ruzmi, Ahmad-Hamdani and Mazlan2020) as has been reported elsewhere. Regardless, without population genetics data, we cannot rule out the possibility that continuous use of IMI herbicides has selected for this mutation de novo in some cases. Until then, we have to say that IMI-R weedy rice in Malaysia may not all be outcrosses with CPS rice. More recently, based on AHAS gene sequence analysis, an enzyme colorimetric assay, and a genome-wide association study, the coexistence of both TSR and NTSR was speculated in the resistant weedy rice populations collected from CPS rice fields across seven states in Peninsular Malaysia (Yean et al. Reference Yean, Dilipkumar, Rahman and Song2021). Studies to determine NTSR mechanisms are warranted.
Table 1. Summary of acetohydroxyacid synthase amino acid substitution in field-evolved resistant weed species to IMI herbicides worldwide (modified from Heap Reference Heap2021; Tranel et al. Reference Tranel, Wright and Heap2021).
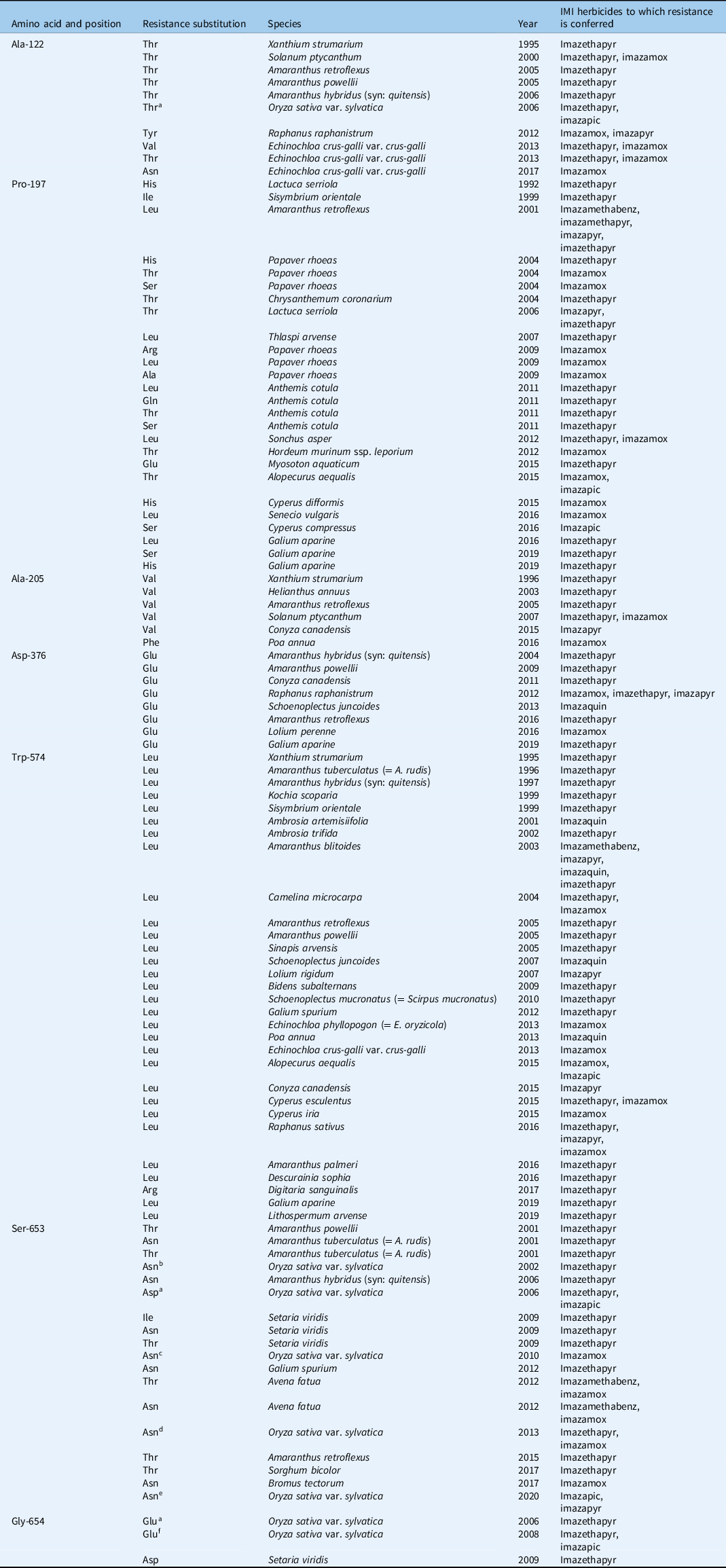
a Additional data from Rajguru et al. (Reference Rajguru, Burgos, Shivrain and Stewart2005); not available in Heap (Reference Heap2021).
b Additional data from Roso et al. (Reference Roso, Merotto, Delatorre and Menezes2010); not available in Heap (Reference Heap2021).
c Additional data from Scarabel et al. (Reference Scarabel, Cenghialta, Manuello and Sattin2012); not available in Heap (Reference Heap2021).
d Additional data from Kaloumenos et al. (Reference Kaloumenos, Capote, Aguado and Eleftherohorinos2013); not available in Heap (Reference Heap2021).
e Additional data from Ruzmi et al. (Reference Ruzmi, Ahmad-Hamdani and Mazlan2020); not available in Heap (Reference Heap2021).
f Additional data from Sales et al. (Reference Sales, Shivrain, Burgos and Kuk2008); not available in Heap (Reference Heap2021).
Management of HR Weedy Rice in Malaysia
After several years of wide-scale, intensive adoption of HR rice technology in Malaysia, the problem of how to manage HR weedy rice populations took center stage. The basic principle of managing HR weeds is to prevent the spread of HR populations and to delay the evolution of resistance by integrating alternative chemical tools and nonchemical practices (Beckie Reference Beckie2006).
The first step is prevention, for which the most effective practice is the use of uncontaminated, certified seeds (Andres et al. Reference Andres, Fogliatto, Ferrero and Vidotto2014; Dilipkumar et al. Reference Dilipkumar, Burgos, Chuah and Ismail2018). Just as this practice prevents the introduction of weedy rice into a field, it also prevents spreading of HR weedy rice to other fields. The sharing of contaminated seeds between farmers has contributed to the spread of weedy rice from infested areas to clean areas (Azmi and Karim Reference Azmi and Karim2008; Chauhan Reference Chauhan2013). Unless rice farmers can be convinced to abandon this custom of seed sharing or access to certified seed can be improved, weedy rice will continue to be spread by farmers among themselves. Similarly, comprehensive sanitation practices surrounding farm equipment and field edges help minimize weed seed immigration into the field (Beckie and Harker Reference Beckie and Harker2017). Implementation of these practices may be may even more challenging than planting certified seed. The time for land preparation and the planting season are short. With severe constraints on time, labor, and machinery, farmers will not be able to practice equipment sanitation.
Cultural practices such as the stale seedbed technique, crop residue burning, the flooding technique, crop rotation, and controlling weedy rice escapes are recommended by most researchers to control weedy rice (Arya and Ameena Reference Arya and Ameena2015; Chauhan Reference Chauhan2013; Chin Reference Chin2001; Rathore et al. Reference Rathore, Singh and Kumar2013). The same practices can likewise prevent the proliferation of resistant weedy rice. Stale seedbed practice is said to be the most effective cultural practice to control weedy rice, because it involves a series of operations, including crop residue burning and preparation of soil to favor weed seed germination for 12 to 15 d, followed by herbicide application to kill the emerged seedlings (Arya and Ameena Reference Arya and Ameena2015). Nonselective herbicides such as glyphosate and glufosinate are highly effective chemical tools in stale seedbed technique, applied 30 to 35 d before rice planting (MSA-H, personal observations). The stale seedbed technique and repeated plowing will reduce the weedy rice seedbank, resistant or not (Chauhan Reference Chauhan2012; Dilipkumar et al. Reference Dilipkumar, Burgos, Chuah and Ismail2018).
Manual removal of weedy rice panicles is also practiced by some farmers in Malaysia. As outlined in MARDI’s cultural practices guidelines for direct-seeded rice, weedy rice must be rouged between 70 and 80 d after planting to prevent new seed deposit and gradually diminish the soil seedbank (Badrulhadza et al. Reference Badrulhadza, Siti Norsuha, Maisarah, Azmi, Allicia, Mohd Fitri and Chong2013). In Malaysia, where the average individual rice farm size is small (1.2 to 1.5 ha), rouging weedy rice panicles before grain filling is doable. Rouging has been included as one of the key checks in the “Farmers Current Agriculture Practices on Paddy Cultivation and Relationship with Work Performance in IADA Batang Lupar, Sarawak, Malaysia” that must be followed by farmers to achieve a yield of 10,000 kg ha−1 (Hassan et al. Reference Hassan, Yussof and Galadima2019). Earlier, Azmi and Abdullah (Reference Azmi and Abdullah1998) proposed a holistic management program to eliminate weedy rice in rice fields that promoted rouging as one of the effective approaches. Today, rouging is still practiced by many farmers in all rice granaries in Malaysia.
Water-seeding and flooding culture has been the primary cultural practice to reduce weedy rice infestation (Chauhan Reference Chauhan2013). Managing water by providing optimum flood depth, duration, and timing will not only reduce the germination of weedy rice but also that of other weed species, especially in a direct-seeded rice system (Arya and Ameena Reference Arya and Ameena2015). The adoption of a 20- to 40-cm water depth in acid sulfate soil areas in Vietnam during the winter–spring season has reduced the weedy rice infestation (Chin Reference Chin2001). In Malaysia, a 5- to 10-cm flooding depth inhibits weedy rice establishment (Azmi and Karim Reference Azmi and Karim2008). Flooding can delay the establishment and proliferation of HR weedy rice populations (Dauer et al. Reference Dauer, Hulting, Carlson, Mankin, Harden and Mallory-Smith2018). At present, water seeding or transplanting is practiced by up to 71.9% of rice farmers (Dilipkumar et al. Reference Dilipkumar, Ahmad-Hamdani, Rahim, Chuah and Burgos2021). The majority practice flooded-rice culture.
Farmers are also encouraged to rotate the crop establishment methods in their rice fields, for example, rotating between wet-seeded rice to transplanted rice to reduce the weedy rice infestation, especially in most Asian countries, where wet-seeding of rice has been practiced indefinitely (Arya and Ameena Reference Arya and Ameena2015). Weedy rice emerging in wet-seeded fields is practically indistinguishable from cultivated rice, because rice seeds are broadcast so the farmers cannot rely on rows to discriminate weedy rice (Chauhan Reference Chauhan2013). In a field that has been severely infested by weedy rice (resistant or not), reverting to the transplanting method can be the only recourse to reduce the infestation (Chauhan Reference Chauhan2013), because it allows farmers to identify and manually remove weedy rice. This method has been recommended by MARDI as reported in the local Sinar Harian newspaper (March 13, 2018, not available online).
Rotation with different crops is highly effective in reducing weedy rice infestation and has been practiced traditionally by rice farmers when they run out of options (Arya and Ameena Reference Arya and Ameena2015; Burgos et al. Reference Burgos, Norsworthy, Scott and Smith2008, Reference Burgos, Singh, Tseng, Black, Young, Huang, Hyma, Gealy and Caicedo2014). Among the best rotational crops with rice are legumes (Burgos et al. Reference Burgos, Norsworthy, Scott and Smith2008; Labrada Reference Labrada2006). There are many practical reasons why farmers practice rice monoculture just as in Malaysian rice granaries. The rice granaries of Malaysia are arable lands that are purposely reserved by the Malaysian government through the National Agricultural Policy as main paddy producing areas. Malaysia has not been self-sufficient in rice production for a long time; thus the establishment of rice granary areas that are monocropped with rice is intended to ensure national food security. To facilitate high production, these rice granaries are centrally irrigated through a systematic and scheduled rice irrigation system by the Department of Irrigation and Drainage, making these areas not suitable for planting other crops. Rice farmers in the granary areas also receive production input (fertilizers and pesticides) subsidies. Thus, the Malaysian CPS stewardship guideline does not recommend rotation of CPS rice variety with other crops, but rather with non-CPS rice varieties. In such situations, farmers have to diversify the herbicide modes of action they use.
As an option for controlling the spread of resistant weedy rice chemically, the non-selective herbicides glyphosate and glufosinate can be applied in the stale seedbed technique (Dilipkumar et al. Reference Dilipkumar, Burgos, Chuah and Ismail2018). Other than that, PRE herbicides like pretilachlor and oxadiazon can also control weedy rice before emergence, provided the herbicides are applied at least 15 to 7 d before sowing (Dilipkumar et al. Reference Dilipkumar, Burgos, Chuah and Ismail2018; Eleftherohorinos and Dhima Reference Eleftherohorinos and Dhima2002; Malaysian Agricultural Research and Development Institute 2020). These herbicides mainly function to reduce the weedy rice seedbank before rice planting. This integrated chemical control method is crucial to avoid overreliance on IMI herbicides in CPS rice.
Intensive monitoring or scouting of CPS rice fields is necessary throughout the growing season. Weedy rice escapes need to be removed manually or chemically (by spot spraying) in every season CPS rice is planted. Outcrosses need to be detected early by observing telltale traits of F1 outcrosses (Burgos et al. Reference Burgos, Norsworthy, Scott and Smith2008). It is important to report resistance cases immediately so that precautions can be taken to prevent seed production from outcrosses and introgression. Using herbicide tactics in conjunction with cultural practices is foremost to thoroughly control resistant weedy rice, either to prevent its spread or to delay resistance evolution. Above all, farmers’ adherence to the stewardship guideline is necessary to ensure the viability and sustainability of HR rice technology. There is a great need to empower and enable the farmers in this regard.
Conclusion
The sole dependence on herbicides for rice weed control, particularly IMI herbicides in the CPS package, has resulted in the evolution of HR weedy rice in Malaysia. Despite the advantages of the CPS package, the evolution and rapid spread of resistant weedy rice has diminished the benefit of CPS and compromised the sustainability of the technology. Molecular and morphological data strongly indicate that HR weedy rice arose from gene flow, but population genetics data are needed to rule out the possibility of selection of de novo mutations. The incidence of HR weedy rice is increasing in Malaysia because of pervasive inability to follow the stewardship guideline. It is necessary to diversify the approach in managing weedy rice by integrating various management strategies, and farmers need to be empowered to do so.
Acknowledgments
The authors would like to express their sincere gratitude to the Ministry of Higher Education Malaysia, under the Fundamental Research Grant Scheme (FRGS/1/2018/WAB01/UPM/02/1: 07-01-18-1961FR; 5540086), for providing financial support for research activities and findings discussed in this review. No conflicts of interest have been declared.




