Carotenoids demonstrate a vast array of biological activities, including vital roles in the eye, both functionally as precursors to retinol in the visual pathway (pro-vitamin A (PVA) carotenoids) and structurally as macular pigments. The major PVA carotenoids in plasma are α-carotene, β-carotene and β-cryptoxanthin. Of these, only β-carotene is found in ocular tissues(Reference Krinsky and Johnson1).
In contrast, lutein/zeaxanthin and lycopene are the major non-PVA carotenoids, i.e. are not retinol precursors, and both are present in ocular tissues at high concentrations. Lutein and zeaxanthin comprise the macular pigments, essential for normal vision and for the protection of photoreceptors from phototoxic blue light, while lycopene is present in high concentrations in the human ciliary body and retinal pigment epithelium/choroid(Reference Khachik, Carvalho, Bernstein, Muir, Zhao and Katz2).
Plasma carotenoid concentrations have been linked to numerous conditions(Reference Voutilainen, Nurmi, Mursu and Rissanen3–Reference Ford, Will, Bowman and Narayan6) including the major blinding conditions – age-related macular degeneration(Reference Cardinault, Abalain, Sairafi, Coudray, Grolier, Rambeau, Carre, Mazur and Rock7, Reference Nilsson, Sundelin, Wihlmark and Brunk8) and cataracts(Reference Gupta, Trivedi, Srivastava, Joshi, Halder and Verma9). To date, the relationship between the major carotenoids and diabetic retinopathy has not been evaluated (Table 1). Consequently, we undertook to investigate the association between plasma carotenoids and diabetic retinopathy.
Table 1 Diabetes-related studies of plasma concentrations of major carotenoids(Reference Coyne, Ibiebele, Baade, Dobson, McClintock, Dunn, Leonard and Shaw5, Reference Ford, Will, Bowman and Narayan6, Reference Neyestani, Shariatzadeh, Gharavi, Kalayi and Khalaji24, Reference Granado, Olmedilla, Gil-Martinez, Blanco, Millan and Rojas-Hidalgo25, Reference Granado-Lorencio, Olmedilla-Alonso, Blanco-Navarro, Botella-Romero and Simal-Antón35, Reference Wang, Liu, Pradhan, Manson, Buring, Gaziano and Sesso37–Reference O'Brien, Watts, Powrie, Shaw and Miller54)

ND, non-diabetic; T1D, type 1 diabetes; T2D, type 2 diabetes; ACAR, α-carotene; BCAR, β-carotene; BCRY, β-cryptoxanthin; LZ, lutein+zeaxanthin; LYC, lycopene; P, prospective; PVA, pro-vitamin A; T, controlled clinical trial; CC, case–control; H, hospital-based; C, cross-sectional; c, community-based; ESRD, end-stage renal disease; ACR, albumin:creatinine ratio (as indicator of glomerular dysfunction).
* Serum not plasma levels.
† Age-adjusted data.
‡ Median values.
Subjects and methods
Diabetes status
Self-reported diabetes status was confirmed biochemically, according to the WHO diagnostic criteria for the classification of diabetes(10).
Subject selection
In order to broaden the range of dietary intakes and lifestyle exposures, we sourced subjects from the Melbourne Collaborative Cohort Study (MCCS), a community-based prospective cohort of 41 528 male and female volunteers, aged 40–69 years at baseline (1989–94), recruited from the electoral roll, and ethnic radio, clubs, and churches(Reference Giles11). We invited 157 men with type 2 diabetes and not taking carotenoid supplements to participate in the present study. Of these, we excluded three (two men with type 1 diabetes and one man with ungradeable photographs). Of the eligible subjects (n 154), 72 % (n 111) participated in the study. Ethics approval was obtained from the MCCS scientific committee and Deakin and Monash Universities (Melbourne, Australia), and written informed consent was obtained from every participant.
Diabetic retinopathy
We used a mydriatic retinal fundus camera (Kowa FX-500S, Japan) to photodocument retinal status. Diabetic retinopathy grading was based on the EURODIAB protocol (validated against the Airlie House classification) in which the overall grading was that of the worse eye and diabetic retinopathy was defined as more than one microaneurysm and/or haemorrhage(Reference Aldington, Kohner, Meuer, Klein and Sjolie12). A medical retina specialist, masked to all other participant information, graded the slides on two separate occasions in order to assess internal validity. Agreement between gradings was excellent (κ value of 0·986).
Clinical measures and retinopathy risk factors
Systolic and diastolic blood pressure was recorded using a Dinamap XL portable automated adult vital signs monitor (model 9300; Critikon, FL, USA). Blood pressure was recorded as the average of the last two of three consecutive readings, obtained from the right arm of seated subjects at 1 min intervals after a 10 min rest period. Weight was measured to within 0·1 kg, using digital electronic scales (UC-300; A.N.D, Tokyo, Japan), before breakfast and following a 12 h fast, with subjects wearing light clothing and no shoes. Height was measured to within 0·1 cm using a wall-mounted stadiometer (Harpenden; Holtain Limited, Crymych, UK). BMI was calculated as weight (kg)/height (m)2. A ‘current smoker’ was defined as a subject who smoked at least seven cigarettes per week at the time of completing the questionnaire.
Plasma biochemistry
A fasting blood sample was drawn between 08.00 and 10.00 hours on the morning of the clinical evaluation. Carotenoids were analysed in plasma separated from blood treated with EDTA as anticoagulant and stored at − 80°C and protected from light until analysed. Extraction was as follows: 200 μl of plasma and 200 μl of 95 % ethanol (containing α-tocopheryl acetate (200 ng/ml) and retinyl acetate (750 ng/ml) as internal standards) were placed in 13 × 100 mm borosilicate tubes (Laboratory Supply, Melbourne, VIC, Australia). Hexane (1 ml) containing 0·01 % butylated hydroxytoluene was added. The different phases were then separated by centrifugation at 2000 g for 10 min. The organic phase was removed by evaporation under N2. The residue was reconstituted in 30 μl chloroform before the addition of 70 μl acetonitrile–methanol (1:1, v/v) and transferred to light-protected vials maintained at 40°C.
A modified HPLC method, developed and reported elsewhere by Su et al. (Reference Su, Rowley and O'Dea13) was used to analyse the prepared samples. Calibration and peak identification for the carotenes were achieved by the use of pure standards obtained from Sigma (St Louis, MO, USA), while calibration and peak identification for lutein/zeaxanthin and β-cryptoxanthin were achieved by the use of pure standards obtained from Hoffman La Roche (Basel, Switzerland). The inter-batch CV was < 10 % for all analytes except lycopene for which the CV was 12 %.
Plasma glucose concentrations were analysed using an automatic analyser (Hitachi model 705, Tokyo, Japan) and a commercial enzymic kit (Boehringer Mannheim GmbH Diagnostica, Mannheim, Germany) by the glucose oxidase method. Plasma cholesterol and TAG concentrations were analysed with an automatic analyser (Hitachi model 705, Tokyo, Japan) using a commercial enzymic kit (Boehringer Mannheim GmbH Diagnostica, Mannheim, Germany).
Urinary biochemistry
Urinary albumin concentration was measured using immunonephelometry (Kallestadt QM300 or Beckman 360 Array nephelometers; inter-assay CV was 3–5 %). Urinary creatinine concentration was measured using an alkaline picrate method (Olympus AU800 autoanalyser; inter-assay CV was 2 %). The urinary albumin:creatinine ratio was then calculated as albumin (mg)/creatinine (mmol).
Statistical analyses
We used SPSS version 13 for Windows (SPSS Inc., Chicago, IL, USA) software to perform the statistical analyses. The data were cross-sectional observations. Descriptive statistics for the exposure and outcome variables were obtained, and variables with distributions that were not normally distributed were log-transformed before analysis. Associations between categorical variables were analysed using χ2 tests.
Initially, variables assessed in univariate analyses, including known risk factors for diabetic retinopathy, were modelled using binomial logistic regression analysis to determine the best clinical predictors of diabetic retinopathy. Plasma carotenoid concentrations were then added to subsequent models that controlled for the major risk factors for diabetic retinopathy. The fit of each model was tested and the Nagelkerke R 2 approximation was compared. P < 0·05 was considered statistically significant.
Results
The mean age of the participants was 64 (range 44–77) years. The clinical and demographic characteristics of the participants, according to retinopathy status, are shown in Table 2. Diabetic retinopathy was significantly associated with established risk factors, i.e. duration of diabetes, HbA1c, use of hypoglycaemic medication, and the albumin excretion rate. A longer duration of diabetes was, however, the only independent predictor of diabetic retinopathy, as demonstrated by multivariate modelling of these factors (data not shown). Mean plasma cholesterol and TAG concentrations were in the normal range and not significantly different between retinopathy and non-retinopathy cases. Consequently, subsequent analyses were not adjusted for cholesterol and TAG concentrations.
Table 2 Characteristics of diabetic subjects according to retinopathy status (Prevalence rates or mean values and 95 % confidence intervals)
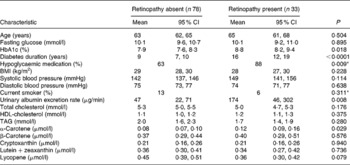
* Pearson χ2P values.
The observed mean concentration of each carotenoid for both retinopathy and non-retinopathy cases was within the range of means reported in other diabetic populations (Table 1). The observed α-carotene concentrations were at the higher end of values reported in other diabetic populations and were associated with diabetic retinopathy. Conversely, lycopene, a non-PVA carotenoid, demonstrated a trend to lower levels in the retinopathy group (Table 2). In addition, a higher plasma non-PVA:PVA carotenoid ratio was inversely associated with diabetic retinopathy (1·6 (95 % CI 1·4, 1·7) v. 1·2 (95 % CI 1·0, 1·4), respectively; P = 0·009).
In multivariate modelling of carotenoids as predictors of diabetic retinopathy (Table 3), the odds of diabetic retinopathy increased with higher plasma concentrations of the PVA carotenoids (model 1). The ratio of non-PVA:PVA carotenoid concentrations was the best carotenoid-related predictor of diabetic retinopathy. A higher combined plasma concentration of lycopene and lutein/zeaxanthin was associated with significantly lower odds of diabetic retinopathy, after adjusting for potential confounding by retinopathy risk factors (model 2).
Table 3 Adjusted regression models of plasma carotenoids (μmol/l) as predictors of diabetic retinopathy*
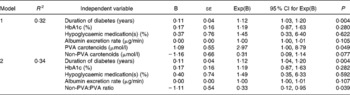
PVA, pro-vitamin A carotenoids (α-+β-carotene+cryptoxanthin); non-PVA, non-pro-vitamin A carotenoids (lutein/zeaxanthin+lycopene).
* Carotenoid and albumin excretion rate data are log-transformed.
Discussion
To date, little is known of the role of carotenoids in diabetes and its complications. Consequently, costly intervention studies of the potential impact of carotenoids on diabetic retinopathy are not yet justifiable, despite evidence of a biologically plausible mechanism, oxidative stress. The present cross-sectional study evaluated the association between diabetic retinopathy and the concentrations of major plasma carotenoids, which in turn are largely, but not exclusively, dependent on dietary intake(Reference Coyne, Ibiebele, McNaughton, Rutishauser, O'Dea, Hodge, McClintock, Findlay and Lee14). Our key finding supports a protective role for a higher combined lutein/zeaxanthin and lycopene concentration against diabetic retinopathy, after adjustment for potential confounders. There are important distinctions between lutein/zeaxanthin and lycopene (non-PVA carotenoids) and the other three major (PVA) carotenoids in humans. Lutein/zeaxanthin and lycopene are absorbed intact, unlike α- and β-carotene and β-cryptoxanthin, which can be cleaved to form retinol and, before absorption, are partly metabolised to vitamin A in the intestinal mucosa(Reference Parker15). Whether competitive inhibition influences PVA carotenoid cleavage at particular intake levels is not well understood. Furthermore, β-carotene is ubiquitous and food sources of various carotenoid combinations differ: lycopene and β-carotene, but not α-carotene, are present in tomatoes, while carrots and pumpkin are good sources of α-carotene and β-carotene, but not lycopene, and cryptoxanthin is primarily found in citrus fruits.
Apart from the key structural role of lutein and zeaxanthin as the macular pigments in the eye, evidence is emerging of a wider role for lutein in chronic disease prevention(Reference Ribaya-Mercado and Blumberg16). In particular, lutein has been shown to attenuate oxidative stress in experimental models of early diabetic retinopathy(Reference Muriach, Bosch-Morell, Alexander, Blomhoff, Barcia, Arnal, Almansa, Romero and Miranda17, Reference Miranda, Muriach, Roma, Bosch-Morell, Genoves, Barcia, Araiz, Diaz-Llospis and Romero18). A role for lycopene in ocular health is also plausible; lycopene is present in high concentrations in human ocular tissues(Reference Khachik, de Moura, Zhao, Aebischer and Bernstein19), is the most potent carotenoid quencher of singlet oxygen(Reference Cantrell, McGarvey, Truscott, Rancan and Bohm20), and has other important functions. In vitro studies have shown that lycopene can inhibit proliferation and induce differentiation of human blood cells, activate genes involved in cell-to-cell communication, and modulate lipoxygenase activity and therefore inflammation and immune function(Reference Heber and Lu21). According to in vitro studies and animal research(Reference Gupta, Trivedi, Srivastava, Joshi, Halder and Verma9), lycopene can attenuate oxidative stress-induced experimental cataract in rat lenses via an antioxidant mechanism involving restoration of levels of endogenous antioxidant enzymes, such as superoxide dismutase and catalase. In one study, lycopene-treated diabetic rabbits demonstrated an increase in antioxidant activity in ocular capillaries and lacrimal fluid(Reference Chesnokova, Grigor'ev, Kuznetsova, Davydova, Olfer'ev, Kost, Nikol'skaia and apitanov22). Low plasma lycopene concentrations have been associated with the very early stages of vascular disease in humans(Reference Rissanen, Voutilainen, Nyyssonen, Salonen, Kaplan and Salonen23), indicating that lycopene contributes to the expression of the disease. Furthermore, lycopene bioavailability is impaired in older individuals(Reference Cardinault, Abalain, Sairafi, Coudray, Grolier, Rambeau, Carre, Mazur and Rock7). In contrast, β-carotene bioavailability is maintained with age, as is the intestinal conversion of PVA carotenoids to vitamin A. Recently, a double-blind placebo-controlled clinical trial found a physiological dose of lycopene for 2 months suppressed oxidative stress and enhanced innate immunity in individuals with type 2 diabetes(Reference Neyestani, Shariatzadeh, Gharavi, Kalayi and Khalaji24).
We also observed an association between retinopathy and plasma PVA carotenoid concentrations. This finding is consistent with one study in which individuals with type 1 diabetes had higher plasma concentrations of PVA (but not non-PVA) carotenoids and lower levels of retinol than their first-degree relatives(Reference Granado, Olmedilla, Gil-Martinez, Blanco, Millan and Rojas-Hidalgo25), suggesting impaired bioconversion of carotenoids to retinol. Diabetes may also promote higher levels of PVA carotenoids via another mechanism, such as down-regulation of the bioconversion of PVA carotenoids to retinol, secondary to a nephropathy-induced accumulation of retinol, levels of which are homeostatically controlled(Reference Gerster26, Reference Thurnham and Northrop-Clewes27) and can be higher in renal disease(Reference Piscator28, Reference Siegenthaler29) and diabetes(Reference Basu and Basualdo30). Importantly, our observation does not demonstrate a negative role of dietary PVA carotenoids. Prospective studies are needed to further evaluate the association between retinopathy and plasma PVA carotenoid concentrations.
Different food sources and intake levels may in part explain the large variation in the ratio of circulating α-carotene: β-carotene reported in previous studies (Table 1). In addition, previous studies point to a complex interplay between carotenoids: non-PVA carotenoids (lutein/zeaxanthin and lycopene) can diminish PVA carotenoid bioavailability. Moreover, synergies have been demonstrated between non-PVA carotenoids(Reference Tyssandier, Choubert, Grolier and Borel31), and competitive inhibition has been demonstrated between carotenoids, for example, for incorporation into chylomicrons(Reference During, Dawson and Harrison32–Reference Van het Hof, West, Weststrate and Hautvast34). It is also noteworthy that while carotenoid levels reflect relatively recent intake, evidence suggests that most individuals maintain relatively consistent eating patterns over time, with seasonal fluctuations affecting only cryptoxanthin and β-carotene levels(Reference Granado-Lorencio, Olmedilla-Alonso, Blanco-Navarro, Botella-Romero and Simal-Antón35).
The main limitation of the present study is its observational nature. The lack of temporal direction prevents us from inferring causality. Consequently, prospective studies of diabetic retinopathy are needed to determine whether over time a higher combined plasma concentration of lutein/zeaxanthin and lycopene and/or a higher plasma non-PVA:PVA carotenoid ratio can reduce the risk of diabetic retinopathy. Residual confounding may have played a role in our observations, although sex, a key determinant of plasma carotenoid levels, was not a confounder in the present study as we selected only men with type 2 diabetes. However, the interaction between different food components may have confounded the present results. Furthermore, plasma carotenoids may be a marker for any of the many non-nutrient food components in vegetables and fruits(Reference Watanabe, Zhuo and Kimira36). Finally, although we used a validated protocol for retinal evaluation, our assessment of retinopathy may have underestimated diabetic maculopathy.
In conclusion, synergies between plasma carotenoids seem to be implicated in diabetic retinopathy, independent of established risk factors. In general, the present study provides additional data concerning the importance of carotenoid-rich foods for health maintenance and gives strength to the recommendation of increasing consumption of lutein- and lycopene-rich foods.
Acknowledgements
The present study would not have been possible without the MCCS and the infrastructure support provided by the Cancer Council Victoria. The National Health and Medical Research Council of Australia part-funded the present study (project no. 124317), which represents the collaboration of and contribution by many.
L. B. contributed to the design, data collection, data analyses, and the writing and revision of the manuscript. K. R. contributed to the design, data collection, and revision of the manuscript. C. I. contributed to the design, data collection, data analyses, and revision of the manuscript. K. O'D. contributed to the design and revision of the manuscript. No conflicts of interest exist.





