Choline is an essential nutrient required for a variety of critical processes including synthesis of membrane phospholipids and acetylcholine, lipoprotein formation and methyl group metabolism( Reference Zeisel and da Costa 1 ). It can be obtained from both dietary and de novo sources, with subclinical symptoms of fatty liver and/or muscle dysfunction resulting from insufficient intake of choline across species( Reference Hove and Copeland 2 , Reference Zeisel 3 ). Choline is widely distributed in food sources, with major sources in the diet reported to include eggs, meat (beef and chicken) and dairy products( Reference Lewis, Subhan and Bell 4 ). In the early postnatal period, the need for exogenous choline increases to meet the demands by the infant for rapidly dividing and developing tissues and to account for the depletion of maternal stores( Reference Gossell-Williams, Fletcher and McFarlane-Anderson 5 ). Both umbilical cord( Reference Molloy, Mills and Cox 6 ) and newborn plasma( Reference Ilcol, Ozbek and Hamurtekin 7 ) choline concentrations are significantly higher than concentrations in the mother’s plasma, suggesting delivery against a concentration gradient to the fetus and infant. Pregnancy and lactation are reported to increase de novo synthesis in rodents( Reference Gwee and Sim 8 , Reference Burdge, Hunt and Postle 9 ); however, it has been demonstrated that lactating female rodents had greater depletion of liver choline metabolites when fed a choline-deficient (ChD) diet as compared with non-lactating females. This suggests that, although there is greater de novo choline synthesis, this period may result in greater sensitivity to changes in dietary choline intake( Reference Zeisel, Mar and Zhou 10 ). Despite increased requirements, our recent cohort study found that the majority (90 %) of lactating women were consuming far below the current daily ‘adequate intake’ recommendations( Reference Lewis, Subhan and Bell 4 ), and it is not clear what effects this may have on their children’s long-term health. The essentiality of choline in the early postnatal period is well established for many organs including the brain and liver (reviewed by Caudill( Reference Caudill 11 )). Given the large amount of cellular expansion occurring within the immune system, it is likely that choline plays a critical role in the development of the immune system; however, the effects of maternal choline deprivation during this critical period have not yet been examined.
The early postnatal period is a critical stage for immune system development, with rapid immune cell expansion and proliferation in rodents( Reference Perez-Cano, Franch and Castellote 12 ) and humans( Reference Field 13 ). It is recognised that nutrients modify immune function( Reference Calder 14 ), and this may be of particular importance during the critical development that occurs during the suckling period( Reference Field 13 ). A study in adult rodents found that feeding a diet without choline (compared with a diet containing 3·5 g choline/kg) for 8 weeks resulted in lower delayed-type hypersensitivity (DTH) response and proliferation of splenocytes after stimulation with Concanavalin A (ConA)( Reference Courreges, Benencia and Uceda 15 ). Similarly, we demonstrated that feeding a diet without exogenous choline during the lactation period significantly impaired the function of the maternal immune system and reduced growth of the suckling offspring( Reference Dellschaft, Ruth and Goruk 16 ). However, the effect of a maternal diet devoid of choline on the pup’s immune system was not measured.
The concept of nutritional programming refers to the effect of environmental factors including diet during a critical period of development on immediate and long-term functions and responses. This concept has not been well established for the development of the immune system, and the potential programming effects of maternal choline intake during the early postnatal period have not been studied. An earlier study found that maternal dietary lipotrope deficiency (choline, methionine and vitamin B12) during suckling resulted in depressed T cell function in the offspring and that it predisposed offspring to bacterial infections (Salmonella typhimurium) in the post-weaning period( Reference Newberne, Wilson and Williams 17 ). Another study examining the effects of age on a marginally methionine–choline-deficient diet (67 % lower in methionine and choline compared with control diet) in weaned rats found that animals at 3 weeks after weaning were most sensitive to methionine–choline deficiency, compared with older animals (12 months after weaning)( Reference Nauss, Connor and Kavanaugh 18 ). These studies suggest that lipotrope deficiency during suckling may result in increased susceptibility to infection. However, there are no reported studies examining the effects of choline insufficiency (independent of other methyl donors in the diet) during suckling on immune function later in life.
Our previous study demonstrated that dams fed a diet devoid of choline during lactation negatively affected maternal immune function and impaired pup growth( Reference Dellschaft, Ruth and Goruk 16 ). Therefore, the first objective of the present study was to determine the effect of maternal choline deficiency on immune system development in suckled offspring. The secondary objective was to determine whether the intake of choline during lactation would have a programming effect on the development and function of the offspring’s immune system later in life. We hypothesised that a choline-deficient maternal diet would be insufficient in supporting the development of the offspring’s immune system, and would have lasting effects on the offspring’s immune system even if provided a choline-sufficient diet after weaning.
Methods
Animals and diets
Expt 1
To examine the effect of maternal choline deficiency on the offspring’s immune system development and function, female Sprague–Dawley rats (nineteen rats) were obtained at 14 days of gestation from Charles River Laboratories. All dams were fed a standard rat chow diet (Lab diet 5001; PMI Nutrition International; containing 1 g choline/kg; Harlan Teklad) throughout pregnancy until parturition. Within 24 h of birth, dams were randomised to one of two isoenergetic, isonitrogenous and nutritionally adequate diets (Table 1). Diets were fed ad libitum throughout the lactation period until 3 weeks postnatally. Animals had free access to food throughout the study period and feed cups were refilled every 2–3 d.
Table 1 Composition of experimental diets
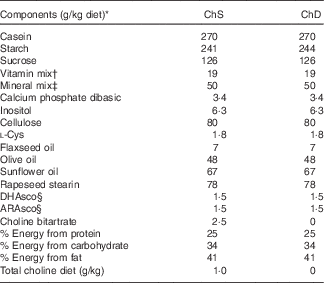
ChS, choline-sufficient diet; ChD, choline-devoid diet.
* All ingredients were purchased from Harlan Teklad, with the exception of the dietary oils that were purchased from Safeway, and rapeseed stearin was donated by Richardson Oilseed Limited.
† American Institute of Nutrition-93-VX vitamin mix( Reference Reeves 19 ).
‡ Bernhart–Tomarelli salt mixture( Reference Bernhart and Tomarelli 51 ).
§ DHAsco is a single-cell oil source of DHA; ARAsco is a single-cell oil source of arachidonic acid.
The two diets differed only in the amount of choline provided (Table 1): ChD (0 g choline/kg diet; n 9) and ChS (1·0 g choline/kg diet; n 10). The choline-sufficient diet contained the recommended amount of choline by The Nutrient Requirements of Laboratory Animals( Reference Reeves 19 ). This amount of choline (1 g/kg diet) is included in American Institute of Nutrition (AIN)-93 standard rodent diet mix( Reference Reeves 19 ). In humans, the daily recommendation (which is reported as an adequate intake) of choline for lactating women is 550 mg/d. The mean intake in this population of humans is approximately 350 mg/d. We have previously reported that approximately the majority of lactating women (90 %) are estimated to be consuming below what is thought to be the adequate level( Reference Lewis, Subhan and Bell 4 ). The diet used in the current study contains what is recommended to meet the needs of pregnant and lactating and growing rodents. At birth, litters were culled to ten offspring (five males and five females when possible) per dam. Dietary intake of dams was recorded throughout the lactation period, and body weight was recorded regularly throughout the study period. This study was concluded at the end of the suckling period (3 weeks), and two offspring from each dam were euthanised, with the study design presented in Fig. 1. Two of the dams and their litters from the ChD group were euthanised before the end of the experiment because of significant weight loss and were not included in data analysis making the final n 7 for the ChD group. Expt 1 was conducted in two complete blocks in two separate years. The experimental unit in the study was the dam. For each n in the study, we used a mean of the measure from two offspring (including one male and one female offspring), and each offspring measure that we report as a unit (n) was the mean of two rodents. The sex of the offspring was not recorded; however, the covariant of variability between the two offspring that we combined for each dam was <10 % for all the parameters. This suggests that there was no significant effect of sex at this age, similar to what we have previously reported( Reference Field, Goruk and Glen 20 ).
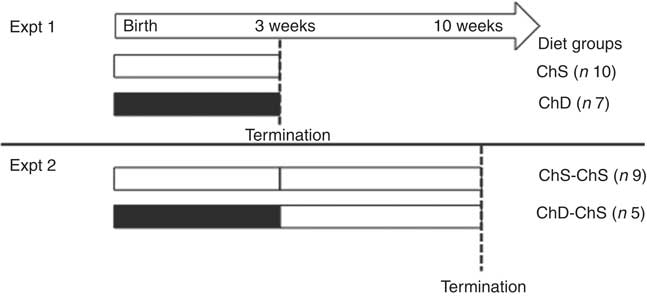
Fig. 1 Experimental study design. Dams were randomly assigned to the choline-sufficient (ChS, ![]() ) or choline-devoid (ChD,
) or choline-devoid (ChD, ![]() ) diets for the duration of the lactation/suckling period. For Expt 1, two offspring (one female and one male) from each dam were terminated at the end of the 3-week suckling period. For Expt 2, two female offspring from each dam were then fed a ChS diet for an additional seven weeks. At 10 weeks, offspring were terminated. The dam represents the experimental unit in this design; therefore, the number of observations within each group is equal to the number of dams, with an average of two offspring per dam for every measurement.
) diets for the duration of the lactation/suckling period. For Expt 1, two offspring (one female and one male) from each dam were terminated at the end of the 3-week suckling period. For Expt 2, two female offspring from each dam were then fed a ChS diet for an additional seven weeks. At 10 weeks, offspring were terminated. The dam represents the experimental unit in this design; therefore, the number of observations within each group is equal to the number of dams, with an average of two offspring per dam for every measurement.
Expt 2
To investigate whether the amount of choline provided during suckling has a programming effect on immune development and function, a new set of dams were randomised (within 24 h of giving birth) to the choline-deficient (n 7, ChD-ChS) or choline-sufficient (n 9, ChS-ChS) diet (Table 1) for 3 weeks, as described in Expt 1. At 3 weeks of age, two female offspring per dam from both the diet groups were weaned to the ChS diet for 7 weeks. All offspring were euthanised at 10 weeks of age (Fig. 1). Rats reach sexual maturity at about 4–5 weeks of age, and therefore female rats in this experiment were sexually mature. Two dams and their litters from the ChD-ChS group were euthanised before the end of the experiment because of significant weight loss and were not included in data analysis making the final n 5 for the ChD-ChS group. The institutional and national guidelines for the care and use of animals were followed, and all experimental procedures involving animals were approved by the Committee of Animal Policy and Welfare of the Faculty of Agriculture, Life and Environmental Sciences at the University of Alberta, Edmonton, AB, Canada.
Tissue collection
At both time points, two offspring from each dam were weighed and euthanised by CO2 asphyxiation in the morning hours. Blood was collected in EDTA-containing tubes via cardiac puncture. Plasma was collected following centrifugation at 3000 g for 10 min. Liver and spleen weight and intestinal length were recorded. Spleens were collected aseptically, weighed and immune cells were isolated for further processing (see below). Stomach contents and livers of the offspring were collected aseptically, weighed, snap-frozen using liquid N2 and then stored at −80°C until analysis.
Choline metabolite analyses of offspring stomach content
The stomach contents of offspring were analysed to reflect the choline content of the dam’s milk and to determine the effect of maternal diet on milk content. Frozen stomach contents were ground with liquid N2 and extracted using a modified Bligh and Dyer method that has been described in detail elsewhere( Reference Zhao, Xiong and Curtis 21 , Reference Xiong, Zhao and Goruk 22 ). Extracts were quantified for all significant choline-containing metabolites and total choline content by HILIC liquid chromatography-tandem MS( Reference Zhao, Xiong and Curtis 21 , Reference Xiong, Zhao and Goruk 22 ) using an Agilent 1200 series HPLC system (Agilent Technologies) coupled with a 3200 QTRAP MS (Ab Sciex).
Immune cell isolation
Isolation of immune cells from the spleen has been previously described( Reference Field, Wu and Metroz-Dayer 23 ). In brief, single-cell suspensions were obtained by disrupting tissue through a nylon mesh screen in sterile Krebs–Ringer HEPES buffer with bovine serum albumin (5 g/l; Sigma-Aldrich Canada Ltd). Ammonium chloride lysis buffer (155 mm-NH4Cl, 0·1 mm-EDTA, 10 mm-KHCO3; Fisher Scientific) was used to lyse erythrocytes. Cells were washed and then re-suspended in complete culture medium (RPMI 1640 media; Life Technologies), supplemented with 5 % (v/v) heat-inactivated fetal calf serum, 25 mm-HEPES, 2·5 mm-2-mercaptoethanol and 1 % antibiotic/antimycotic (pH 7·4; Fischer Scientific). A haemocytometer was used to count live cells using trypan blue dye exclusion (Sigma-Aldrich) and diluted to 1·25×106 cells/ml.
Immune cell phenotype analysis
Immune cell subsets present in freshly isolated splenocytes were identified by direct immunofluorescence assay, as previously described( Reference Field, Wu and Metroz-Dayer 23 , Reference Field, Thomson and Van Aerde 24 ). In brief, 200 000 immune cells were incubated for 30 min at 4°C with pre-labelled monoclonal antibodies applied in combination in order to quantify immune cell phenotypes. The use of four-colour flow cytometry allowed identification of the following combinations of surface molecules in Expt 1 and/or Expt 2: CD28/CD3/CD8/CD4 (Expt 1 and 2), CD3/CD45RA/CD27/OX12 (Expt 1 and 2), OX62/OX6/CD8 (Expt 1 and 2), CD68/CD284/CD11b/c (Expt 1 and 2), CD161/CD3 (Expt 1 and 2), IgG/IgM, IgA, CD3/FOXP3/CD25/CD4 (Expt 1 and 2), CD25/CD152/CD8/CD4 (Expt 1), CD71/CD3/CD8/CD4 (Expt 1), CD80/CD152/CD28 (Expt 1), OX12/OX6/CD27 (Expt 2), CD25CD3/CD4/CD8 (Expt 2) and CD45RA (Expt 2). The CD45RA isoform, also known as leucocyte common antigen, is present only on B cells in rats( Reference Woollett, Barclay and Puklavec 25 ). All antibodies with the exception of IgG, IgM and OX6 (BD Biosciences) were purchased from Cedarlane Laboratories. After incubation, cells were washed and fixed in paraformaldehyde (10 g/l; Thermo Fisher) in PBS. To identify the intracellular protein forkhead box P3 (FOXP3), an indicator of T regulatory cells (Treg), isolated cells were permeabilised before antibody addition, according to the manufacturer’s directions (Cedarlane Laboratories). All the samples were acquired within 72 h of preparation by flow cytometry (FACSCalibur; Becton Dickinson) according to the relative fluorescence intensity determined using Kaluza Software (Beckman Coulter). From the samples acquired, the total lymphocyte population was gated from the forward-scattered light v. side-scattered light plot, and the data presented in the results section represent the percentage of total cells from that mononuclear cell population gate. Representative dot plots illustrating gating for the total mononuclear cell population are shown in the online Supplementary Fig. S1. The online Supplementary Fig. S2 presents the monoclonal antibodies used as isotype controls for flow cytometry analysis, and the online Supplementary Fig. S3 illustrates the unstained splenocytes with the addition of monoclonal antibodies used as isotype controls.
Ex vivo cytokine production by mitogen-stimulated splenocytes
The measurement of the production of cytokines by mitogen-stimulated splenocytes has been previously described( Reference Blewett, Gerdung and Ruth 26 ). In brief, splenocytes (1·25×106 cells/ml) were cultured in 3-ml RMPI-1640 medium for 48 h at 37°C and 5 % CO2 without mitogen (unstimulated) or with mitogen ConA (2·5 µg/ml; MP Biomedicals), lipopolysaccharide (LPS, 100 µg/ml; Sigma-Aldrich) or both CD3 (1 µg/ml) and CD28 (5 µg/ml; both from e-Bioscience Inc.). ConA is a polyclonal T-cell stimulant and monoclonal antibodies CD3/CD28 activate the T cell receptor, with both CD3/CD28 and ConA stimulating the T cell population. LPS activates the antigen-presenting cell population including dendritic cells, macrophages and B cells by binding to their toll-like receptor (CD284). After incubation, cells were centrifuged for 10 min at 1000 rpm, and supernatants were collected and stored at −80°C until analyses. Concentrations of cytokines IL-1β, IL-2, IL-6 and IL-10, TNF-α and IFN-γ were measured using commercial ELISA kits according to the manufacturer’s instructions and as previously described( Reference Blewett, Gerdung and Ruth 26 ). The detection limits for all cytokines were 15·6–4000 pg/ml, except for IFN-γ, for which the detection limit was 9·8–2500 pg/ml (R&D Systems). Cytokine concentrations were quantified using a microplate reader (SpectraMax 190; Molecular Devices), and all measurements were conducted in duplicates, with CV <10 %. The amount of IL-2 in the media after LPS stimulation was below detection levels. IL-1β was only measured in the supernatant of LPS-stimulated cells. To express cytokine response by specific lymphocyte population, the percentage of immune cell phenotype (i.e. CD4+ CD25+) was multiplied by 1·25×106 (number of total cells added to the culture). Next, cytokine production (pg/ml) (i.e. IL-2) was divided by the number of lymphocytes added to the culture (i.e. ×106 of CD4+ CD25+ cells) to express the amount of IL-2 in the media per a specific lymphocyte subset.
Plasma concentrations of cytokines, chemokines and haptoglobin
To determine circulating concentrations of cytokines and chemokines in plasma, an electrochemiluminescent multiplex cytokine kit (Proinflammatory Panel 1 V-PLEX; MesoScale Discovery) was used. IFN-γ, IL-10, IL-13, IL-1β, IL-2, IL-4, IL-5, IL-6, TNF-α and KC/GRO (keratinocyte chemoattractant/human growth-regulated oncogene also known as CXCL1, chemokine ligand 11) were measured according to the manufacturer’s instructions. In brief, standards and samples (50 µl/well) were added in duplicate to a plate pre-coated with capture antibody for each cytokine. Plates were incubated for 2 h at room temperature on a plate shaker. Plates were then washed with wash buffer (3× PBS with 0·05 % Tween 20). Detection antibody was added and incubated for an additional 2 h. Plates were then washed with wash buffer (3×) and a read buffer was added. Plates were read on the MSD Sector Image 6000 (MesoScale Discovery), and all measurements were conducted in duplicates. The lower levels of detection limits were 0·65 pg/ml (IFN-γ), 16·4 pg/ml (IL-10), 1·97 pg/ml (IL-13), 6·92 pg/ml (IL-1β), 0·69 pg/ml (IL-4), 14·1 pg/ml (IL-5), 13·8 pg/ml (IL-6), 0·72 pg/ml (TNF-α) and 1·04 pg/ml (KC/GRO).
Plasma haptoglobin concentrations were determined using a colorimetric assay according to the manufacturer’s instructions (Tridelta Development). The detection limit for haptoglobin was 0·312 mg/ml. All measurements were conducted in duplicates, with CV <10 %. Among the 3-week offspring, discrepancies between total number of offspring (n 17) and the number available for measurements (n 12) of haptoglobin, TNF-α and KC/GRO were due to clotting of the sample, preventing the processing of those samples.
Estimation of lymphocyte proliferation in the absence and presence of mitogens in spleen
In a subset of 3-week-old animals (five dams), proliferation was estimated by the rate of [3H]thymidine (Amersham/Pharmacia Biotech) uptake by spleen lymphocytes (1·25×106/ml) in the presence or absence of mitogens. Lymphocytes were cultured for 48 h in ninety-six-well plates with or without mitogens, as previously described( Reference Field, Van Aerde and Robinson 27 ). The mitogens used were ConA (2·5 µg/ml; MP Biomedicals), LPS (100 µg/ml; Sigma-Aldrich) or both CD3 (1 µg/ml) and CD28 (5 µg/ml; both from e-Bioscience Inc.). Each well was pulsed with 0·037 MBq of [3H]thymidine for 18 h before collecting cells. Proliferation due to LPS was not measured as in our ex vivo conditions as it does not stimulate IL-2 production by splenocytes. All assays were conducted in triplicate for each offspring and two offspring were pooled to obtain a measure of each dam. Stimulation index for each mitogen condition was calculated as follows: amount of [3H]thymidine uptake (dpm) in the presence of each mitogen divided by the amount of [3H]thymidine uptake (dpm) in the absence of each mitogen. Lymphocyte proliferation was not measured in 10-week-old animals as there was no difference in IL-2 production following ConA stimulation.
Statistical analyses
All data are presented as means with their standard errors, unless otherwise noted. As the dam was the experimental unit (n), each n in the data is the mean of two offspring per dam. All measurements were conducted in duplicate or triplicate for each offspring. All data sets were tested for normal distribution using the Kolmogorov–Smirnov test. Parametric data were then analysed for differences using two-tailed t test. Non-parametric data were log-transformed before analysis, and then a two-tailed t test was performed. In some cases, log-transformation of the data did not lead to normal distribution; therefore, groups were analysed using the Mann–Whitney U test. Expt 1 was conducted over 2 years; therefore, we included year as a blocking variable in all analyses. Year was a blocking variable as it was not a variable of interest, but the variability due to the experiments being conducted over 2 years must be taken into account. There was no difference between years (in the block); therefore, data are illustrated as a combination of both years. Statistical analyses were performed using SPSS Statistics (version 21; IBM) and SAS statistical software (version 9.3; SAS Institute Inc.), with a P value of <0·05 considered to be statistically significant for all analyses.
Results
Anthropometric characteristics and total choline stomach content
For Expt 1, two dams and their litters from the ChD group were euthanised before the end of the experiment because of significant weight loss and were not included in data analysis, making the final n 7 for the ChD group. In Expt 1, 3-week-old offspring from ChD dams that survived had mean lower body weight, liver weight and intestine length compared with offspring from the ChS dams (Table 2). Although spleen weight was not significantly different between diet groups, ChD offspring had indications of lymphopenia as demonstrated by a significantly lower number of splenocytes compared with ChS offspring (P<0·05) (Table 2). ChD dams began to consume significantly lower amounts of food beginning on postnatal day 9, which is likely when they started to be deficient in choline. As previously reported, at 21 d postnatally, the ChD dams weighed 17 % less and their food intake was 29 % lower compared with ChS dams( Reference Dellschaft, Ruth and Goruk 16 ).
Table 2 Anthropometric data of 3-week-old offspring from dams fed choline-sufficient (as free choline) (ChS) or choline-devoid (ChD) diets during the suckling periodFootnote † (Mean values with their standard errors)
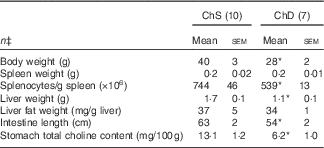
* Mean values within a row are significantly different compared with the ChS group (P<0·05).
† At birth, litters were standardised to ten offspring per dam consisting of five males and five females when possible. Measurements collected at 3 weeks were pooled from one male and one female per dam.
‡ n Refers to the number of dams as they are the experimental unit. This includes two offspring pooled to obtain a measure for each dam, with measurements from each offspring conducted in duplicate.
ChD offspring had a significantly lower total choline concentration in the stomach contents compared with ChS offspring (Table 2). The relative proportion of choline metabolites that contributed to total choline was also different between diets and has been previously reported by Dellschaft et al. ( Reference Dellschaft, Ruth and Goruk 16 ).
For Expt 2, two dams and their litters from the ChD-ChS group were euthanised before the end of the experiment due to significant weight loss and were not included in data analysis, making the final n 5 for the ChD-ChS group. ChD-ChS offspring had lower body weight at weaning (P<0·05) (Table 3). However, after being placed on a ChS diet for the remaining 7 weeks, final body weight was not significantly different compared with ChS-ChS offspring. Food intake, spleen and liver weight, and intestinal length were not significantly different between diet groups at the end of 10 weeks.
Table 3 Anthropometric data from 10-week-old offspring fed a choline-sufficient (as free choline) diet for 10 weeks (ChS-ChS) or offspring from dams fed a choline-devoid diet during the suckling period and then fed a choline-sufficient diet for the remaining 7 weeks (ChD-ChS)Footnote † (Mean values with their standard errors)
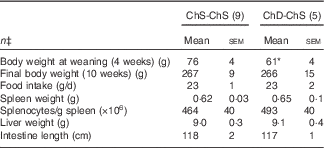
* Mean value within a row is significantly different from the ChS-ChS group (P<0·05).
† At birth, litters were standardised to ten offspring per dam consisting of five males and five females when possible. Measurements collected at 10 weeks were from females only.
‡ n Refers to the number of dams as they are the experimental unit. This includes two offspring pooled to obtain a measure for each dam, with measurements from each offspring conducted in duplicate.
Splenocyte immune cell phenotypes
The phenotypes presented represent a proportion of the total mononuclear cell population that was gated from acquired splenocytes (online Supplementary Fig. S1). The histograms (online Supplementary Fig. S2) and unstained splenocytes with isotype controls (online Supplementary Fig. S3) demonstrate that there was no non-specific binding in our acquired samples. Consistent with the indications of lymphopenia, ChD offspring had 27 % lower total number of T lymphocytes (CD3+) and 47 % lower total number of B lymphocytes (CD45RA+) in the spleen compared with ChS offspring (P<0·05) (Table 4). When examined as a proportion of gated cells, ChD offspring had a higher proportion of CD4+ and CD8+ T cells, which resulted in a significantly higher ratio of T cells (CD3+):B cells (CD45RA+) (Table 4). Although having a significantly lower total amount of lymphocytes, assessment of activation markers on T cells (CD25, CD28, CD152) indicated that ChD offspring had a higher proportion of CD4+ T cells expressing CD28+ and a higher proportion of CD8+ T cells expressing C28 and CD152 cells compared with ChS offspring (P<0·05) (Table 5). There was also a significantly higher proportion of CD4+ and CD8+ cells expressing the proliferation marker CD71 (transferrin receptor) compared with ChS offspring (P<0·05) (Table 5). There was no significant difference in the proportion of FoxP3+ CD25+ cells within the CD4+ T cell population, representing putative Treg cells, between the diet groups (Table 5). There was also a higher proportion of B cells (CD45RA+) with antigen-presenting capacity (OX6+) in ChD offspring compared with ChS offspring (P<0·05) (Table 5).
Table 4 Proportion and the total number of T and B lymphocyte populations in the spleen from 3-week-old offspring from dams fed choline-sufficient (as free choline) (ChS) or choline-devoid (ChD) diets during the suckling period (Mean values with their standard errors)
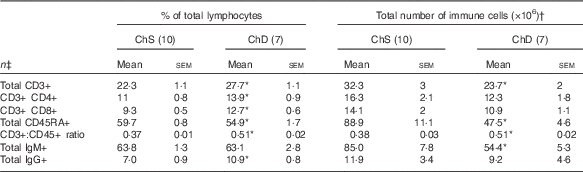
CD, cluster of differentiation.
* Indicates mean within a row that is significantly different from the ChS group (P<0·05).
† The total number of immune cells was calculated by multiplying the percentage of immune cell phenotype in the spleen by the total number of splenocytes isolated (×106).
‡ n Refers to the number of dams as they are the experimental unit. This includes two offspring pooled to obtain a measure for each dam, with measurements from each offspring conducted in duplicate.
Table 5 Splenocyte T and B lymphocyte phenotypes from 3-week-old offspring from dams fed choline-sufficient (as free choline) (ChS) or choline-devoid (ChD) diets during the suckling period (Mean values with their standard errors)
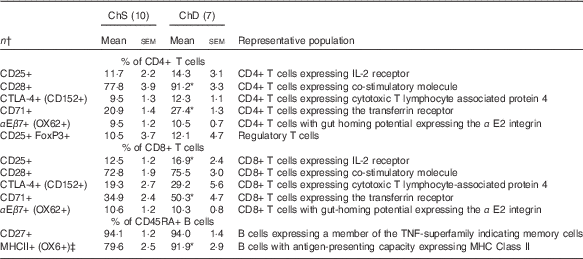
CD, cluster of differentiation.
* Mean values within a row is significantly different from ChS group (P<0·05).
† n Refers to the number of dams as they are the experimental unit. This includes two offspring pooled to obtain a measure for each dam, with measurements from each offspring conducted in duplicate.
‡ % of CD45RA+ cells expressing OX6+ calculated using total OX6+cells–OX6+OX62+ to represent population of CD45RA+cells expressing OX6+.
In Expt 2, feeding a choline-sufficient diet to offspring fed a choline-devoid diet during the suckling period (ChD-ChS offspring) rescued the lymphopenic status, as demonstrated by similar numbers of splenocytes (Table 3) and proportion of total T cells (CD3+) in the spleen between both groups of 10-week-old offspring (Table 6). However, ChD-ChS offspring still had a slight shift in lymphocyte proportions as there was a lower proportion of CD4+ T cells, compared with ChS-ChS offspring (P<0·05) (Table 6). ChD-ChS offspring also had a significantly lower proportion of CD8+ T cells expressing activation marker CD28 (co-stimulatory molecule) (Table 7). ChD-ChS offspring also had a lower proportion of IgA+ cells in the spleen, yet there was no significant difference in the proportion of total B cells (CD45RA+) between groups at 10 weeks (Table 6).
Table 6 Proportion and the total number of T and B lymphocyte populations in spleen from 10-week old offspring fed a choline-sufficient (as free choline) diet for the duration of the 10 weeks (ChS-ChS) or offspring from dams fed a choline-devoid diet during the suckling period then fed a choline-sufficient diet for the remaining 7 weeks (ChD-ChS) (Mean values with their standard errors)
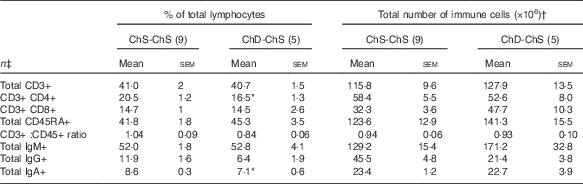
CD, cluster of differentiation.
* Mean values within a row are significantly different from the ChS group (P<0·05).
† The total number of immune cells was calculated by multiplying the percentage of immune cell phenotype in the spleen by the total number of splenocytes isolated (×106).
‡ n Refers to the number of dams as they are the experimental unit. This includes two offspring pooled to obtain a measure for each dam, with measurements from each offspring conducted in duplicate.
Table 7 Splenocyte T and B lymphocyte phenotypes from 10-week-old offspring fed a choline-sufficient (as free choline) diet for 10 weeks (ChS-ChS) or offspring from dams fed a choline-devoid diet during the suckling period and then fed a choline-sufficient diet for the remaining 7 weeks (ChD-ChS) (Mean values with their standard errors)
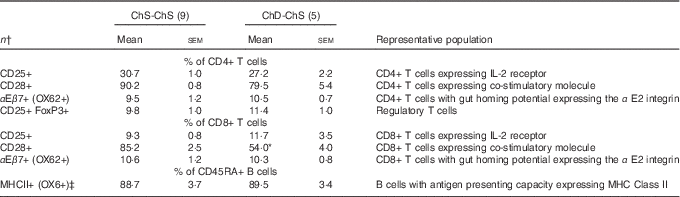
CD, cluster of differentiation.
* Mean values within a row is significantly different from the ChS group (P<0·05).
† n Refers to the number of dams as they are the experimental unit. This includes two offspring pooled to obtain a measure for each dam, with measurements from each offspring conducted in duplicate.
‡ % of CD45RA+ cells expressing OX6+ calculated using total OX6+ cells–OX6+OX62+ to represent population of CD45RA+ cells expressing OX6+.
Ex vivo response to stimulation by splenocytes
At 3 weeks, the absolute rate of [3H]thymidine uptake in the presence of mitogen (ConA or CD3/CD28) or absence of mitogen (unstimulated) was used to calculate the stimulation index. ChD offspring had a significantly lower stimulation index following ConA stimulation compared with ChS offspring (P<0·05) (Fig. 2). When equal numbers of splenocytes were stimulated ex vivo with LPS, ChD offspring produced significantly lower amounts of IL-1β following LPS stimulation (P<0·05) (Fig. 3(a)), with no difference in other cytokines (IL-6, IL-10, IFN-γ and TNF-α) following LPS stimulation (data not shown). Following stimulation with CD3/CD28, a T cell antigen, ChD offspring produced significantly lower amounts of IFN-γ compared with ChS offspring (Fig. 3(b)). There was no significant difference in other cytokines (IL-2, IL-6, IL-10 and TNF-α) following CD3/CD28 stimulation (data not shown). With mitogen ConA, there was no significant difference in cytokine production (IL-2, IL-6, IL-10, IFN-γ and TNF-α) between the ChD and ChS diet groups (data not shown). At 10 weeks, when splenocytes were stimulated ex vivo with ConA or LPS, ChD-ChS offspring produced significantly lower amounts of IL-6 compared with ChS-ChS offspring (P<0·05) (Fig. 3(c) and (d)). There was no significant difference in cytokine production (IL-1β, IL-2, IL-10, IFN-γ and TNF-α) when stimulated with ConA or LPS between the ChD-ChS and the ChS-ChS diet groups (data not shown). There was no significant correlation between IL-2 production and lymphocyte subsets (online Supplementary Table S1). However, when IL-2 was expressed on the basis of the number of lymphocytes thought to be involved in producing this cytokine, there was a significantly lower amount of IL-2 when expressed in relation to the number of CD4+ CD25+ cells and total T cells (CD4+ and CD8+cells) expressing CD25+ in the ChD offspring (online Supplementary Table S2).
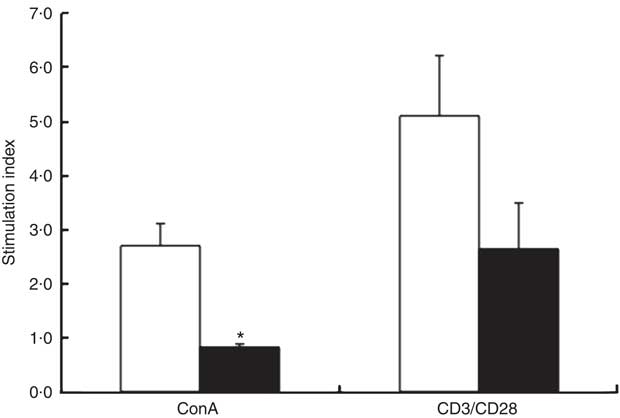
Fig. 2 Response of spleen lymphocytes from the subset of 3-week-old offspring from dams fed choline-sufficient (as free choline) (ChS, ![]() ; n 3) or choline-devoid (ChD,
; n 3) or choline-devoid (ChD, ![]() ; n 2) diets during the suckling period. Stimulation index: amount of [3H]thymidine uptake (dpm) in the presence of each mitogen divided by the amount of [3H]thymidine uptake (dpm) in the absence of each mitogen. This includes two offspring pooled to obtain a measure for each dam, with measurements from each offspring conducted in triplicate. ConA, concanavalin A; n, the number of dams as they are the experimental unit. * Mean value was significantly different from that of the choline-sufficient group (ChS) (P<0·05).
; n 2) diets during the suckling period. Stimulation index: amount of [3H]thymidine uptake (dpm) in the presence of each mitogen divided by the amount of [3H]thymidine uptake (dpm) in the absence of each mitogen. This includes two offspring pooled to obtain a measure for each dam, with measurements from each offspring conducted in triplicate. ConA, concanavalin A; n, the number of dams as they are the experimental unit. * Mean value was significantly different from that of the choline-sufficient group (ChS) (P<0·05).
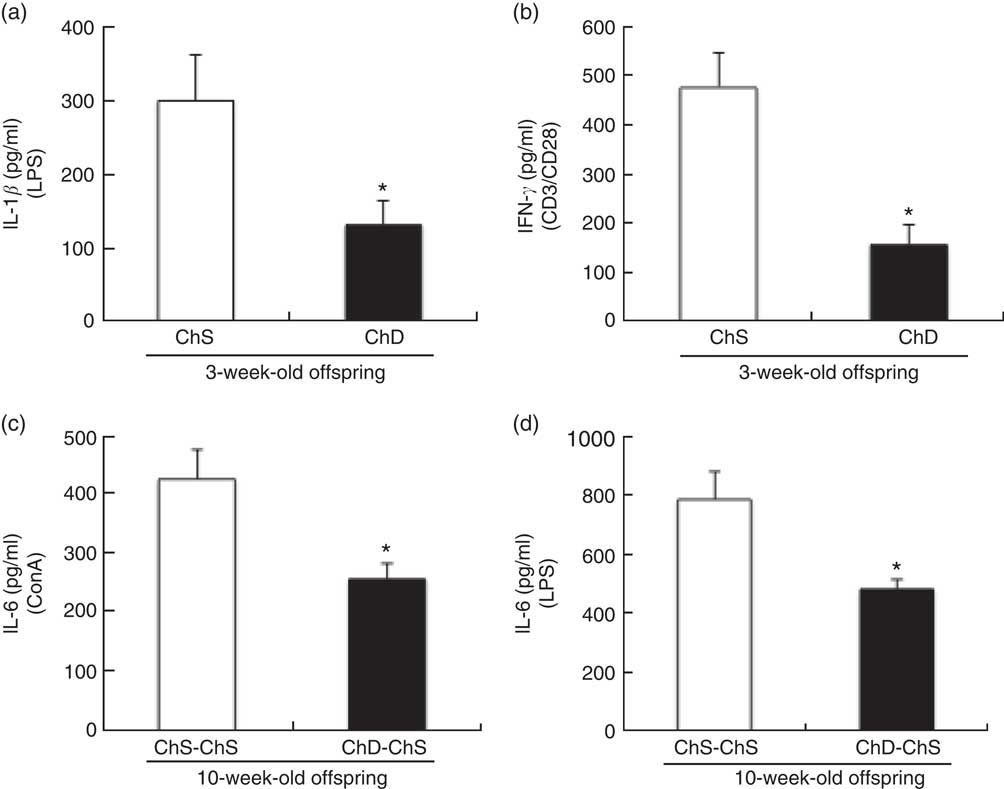
Fig. 3 Ex vivo mitogen-stimulated splenocyte cytokine production from 3-week-old offspring (choline-sufficient diet (ChS) and choline-deficient diet (ChD)) (a, b) and 10-week-old offspring (ChS-ChS and ChD-ChS) (c, d). CD3/CD28 stimulation performed in a subset of animals: ChS (n 5), ChD (n 4). The amount IL-2 in the media after LPS stimulation was below detection levels. IL-1β was only measured in the supernatant of LPS-stimulated cells. Cytokine concentrations in spleen supernatant (pg/ml) after 48-h of culture with mitogen. Each of the measures were conducted in duplicate for each of the two offspring for a dam (CV <10 %). The n represents the mean of two offspring of a dam. * Mean values were significantly different from the choline-sufficient group (ChS or ChS-ChS) (P<0·05). CD, cluster of differentiation; ConA, Concanavalin A; LPS, lipopolysaccharide.
Plasma concentrations of cytokines, chemokines and haptoglobin
Plasma haptoglobin concentrations were significantly lower in ChD offspring compared with ChS offspring (P<0·05) (Fig. 4(a)); however, there was no difference in plasma haptoglobin between ChD-ChS and ChS-ChS offspring at 10 weeks (Fig. 4(b)). Plasma concentrations of TNF-α and KC/GRO were not significantly different between ChS and ChD offspring at 3 weeks of age nor were concentrations different between ChS-ChS and ChD-ChS offspring at 10 weeks of age (data not shown). Circulating plasma concentrations of IFN-γ, IL-10, IL-13, IL-1β, IL-4, IL-5 and IL-6 were all below detection levels for both 3-week-old and 10-week-old offspring.
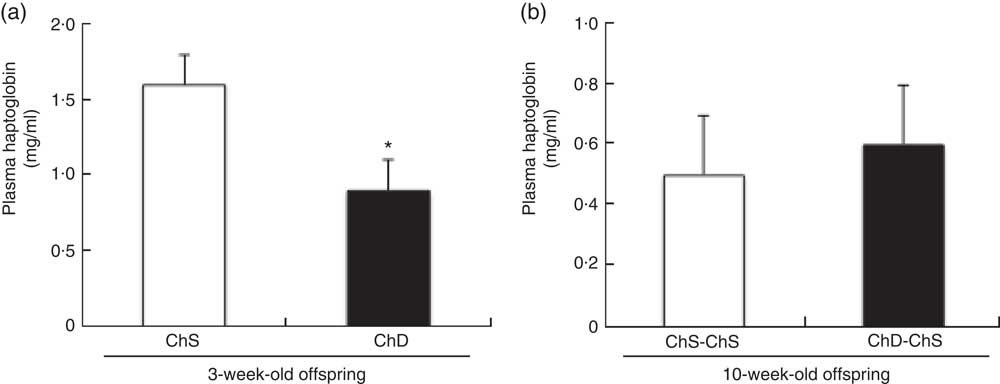
Fig. 4 Plasma concentrations of haptoglobin in 3-week-old offspring (choline-sufficient diet (ChS) and choline-deficient diet (ChD)) (a) and 10-week-old offspring (ChS-ChS and ChD-ChS) (b). Discrepancies between total number of offspring and the number available for measurements of haptoglobin, TNF-α and keratinocyte chemoattractant/human growth-regulated oncogene (KC/GRO) are due to sample errors. * Mean values were significantly different from ChS group (P<0·05).
Discussion
We have demonstrated that endogenous choline synthesis is not sufficient to meet the offspring’s needs for growth and immune system development, and a dietary source is needed in the maternal diet. Consistent with findings from Dellschaft et al.( Reference Dellschaft, Ruth and Goruk 16 ), the absence of choline in the diet during the lactation period resulted in stunted growth likely due to both the reduced supply of choline and the reduced delivery of other essential nutrients owing to a lower intestinal surface area in the pup( Reference da Silva, Kelly and Lewis 28 ). In support of this, plasma haptoglobin concentrations were lower in ChD offspring, suggestive of general malnutrition( Reference Sadrzadeh and Bozorgmehr 29 ). Although conducted in two separate experiments, and therefore invalid for assessment of statistical differences, our studies suggest that providing a ChS diet after weaning reversed some of the negative effects on growth with a ChD in early life. A ChD during suckling significantly reduced offspring growth, but providing choline in the weaning diet enabled catch-up growth to occur, as the mean final body weights of the ChD-ChS offspring were similar to those of the ChS-ChS offspring at 10 weeks of age. This is consistent with a previous study in which maternal dietary lipotrope deficiency (choline, methionine and vitamin B12) resulted in low birth and weaning weights, but a switch to a lipotrope-sufficient diet at weaning corrected body weights at 14 weeks after weaning( Reference Newberne, Ahlstrom and Rogers 30 ). Similarly, a ChS in the weaning period appeared to reverse the symptoms of malnutrition (lymphopenia and lower mean haptoglobin concentration) that occurred with a ChD in the suckling period. A choline-devoid during in the suckling period reduced the proportion of B cells in the spleen of the offspring. However, a ChS diet in the weaning period appeared to reverse these reductions, with similar proportions of B cells in spleen of offspring in both groups at the end of 10 weeks. ChD-ChS also had similar IFN-γ and IL-1β production compared with ChS-ChS offspring. This suggests that the effect of a lower dietary supply of choline during suckling on B cells is reversible by providing what is currently believed to be adequate choline in the weaning diet. Further research is needed to identify the mechanism for the role of choline in B cell function and development during the suckling period.
This study demonstrated that not providing choline in the maternal diet resulted in indicators of lymphopenia in 3-week-old offspring, demonstrated by 34 and 46 % lower number of CD4+ T lymphocytes and B lymphocytes, respectively, similar to what has been reported for other micronutrient deficiencies (Zn( Reference Hosea, Rector and Taylor 31 ) and folate( Reference Williams, Shoot and O’Neal 32 )). As the suckling period is a time of rapid cellular expansion( Reference Perez-Cano, Franch and Castellote 12 ), this may have significant consequences on later immune function. Interestingly, despite a lower total number of lymphocytes, assessment of T cell activation markers (CD25, CD28, CD152) revealed a more activated phenotype in ChD offspring compared with the ChS offspring. ChD offspring had a higher proportion of CD4+ cells expressing CD28. CD28 expression is critical for the interaction of T cells with antigen-presenting cells( Reference Thompson, Lindsten and Ledbetter 33 ) and suggests a more activated and stimulated CD4+ population. There was a higher proportion of CD8+ and CD4+ cells from ChD offspring that expressed the transferrin receptor (CD71), which is involved in cellular Fe uptake and expression, also suggesting cellular activation( Reference Reddy, Eirikis and Davis 34 ). However, despite an activated phenotype, splenocytes from ChD offspring had a significantly lower ex vivo proliferative response (stimulation index) to ConA, a polyclonal T cell mitogen. ChD offspring had a higher proportion of activated T cells and T cells expressing the IL-2 receptor (CD25), yet there was no difference in IL-2 production after either ConA or CD3/CD28 stimulation. Higher proportions of CD8+ CD25+ have been shown to supress IL-2 response( Reference McNally, Hill and Sparwasser 35 ); therefore, this immunosuppression may explain why there was no difference in IL-2 production after stimulation. When the production of IL-2 was expressed on the basis of the number of T lymphocytes expressing the IL-2 receptor (CD25), we observed a significantly lower IL-2 per CD4+ CD25+ lymphocytes in ChD offspring. This suggests that the lymphocytes from ChD offspring may also have reduced ability to synthesise IL-2. Furthermore, ChD offspring had a higher proportion of CD8+ T cells expressing CD152 (cytotoxic T-lymphocyte antigen-4 (CTLA-4), which is a well-characterised co-receptor involved in the regulation of T cell response( Reference Rudd 36 , Reference Rudd, Taylor and Schneider 37 ) that could negatively affect the development of the immune system. CTLA-4 can also be expressed intracellularly, and recently has been demonstrated to play a role in the function of Tregs( Reference Wing, Onishi and Prieto-Martin 38 ); therefore, future studies should examine both surface and intracellular expressions of this protein.
As the proliferative response to CD3/CD28, a mitogen that directly stimulates T cells via the T cell receptor, was not significantly different, our results suggest that the altered ex vivo function may be related to both impaired T cells that do not function as well (on a cell per cell basis) and the lower number of B cells (accessory cells). Similar to what we reported in dams fed the ChD diet( Reference Dellschaft, Ruth and Goruk 16 ), ChD offspring have reduced capacity to produce IFN-γ following CD3/CD28 stimulation. Production of IFN-γ is critical for inhibiting the differentiation and activation of T helper 2 (Th2) cells and promoting a Th1 response. Skewing towards a Th1 response is indicative of immune system maturation in both humans and rodents( Reference Perez-Cano, Franch and Castellote 12 ). Inappropriate Th2 response in early development has been indicated to play a role in the production of allergic inflammation and predisposition towards allergy( Reference Calder 14 ). Insufficient IFN-γ is also associated with increased incidence of infection among newborns( Reference Kotiranta-Ainamo, Rautonen and Rautonen 39 ). This combined with a higher Th2 response would be predicted to impair the ability of the immune system to respond appropriately to challenges. This is supported by previous research that has shown that choline insufficiency results in impaired T cell functions. More specifically, in adult rodents, dietary choline deficiency has been shown to reduce lymphocyte proliferation( Reference Courreges, Benencia and Uceda 15 ), which was accompanied by depression of T cell function. Similarly, 5-month-old offspring from dams fed a choline–methionine-deficient diet during lactation had a decreased stimulation index with ConA or phytohemagglutinin stimulation and greater mortality from a bacterial infection of S. typhimurium ( Reference Williams, Gebhardt and Morton 40 ).
We also have evidence from this study that not providing choline in the maternal diet during suckling may alter the ability of offspring to respond to bacterial antigens. Following ex vivo stimulation with LPS, a bacterial antigen, ChD offspring produced a lower amount of IL-1β. Inadequate production of IL-1β would increase the susceptibility to intracellular pathogens( Reference Hunter, Chizzonite and Remington 41 ) and reduce the Th1 and Th17 response by T cells( Reference Reddy, Eirikis and Davis 34 ). As LPS stimulates antigen-presenting cells including macrophages, B cells and dendritic cells, it is possible that cytokine production in response to LPS stimulation can be attributed to other immune cells, in addition to T cells. However, as the observed changes in immune cell populations (Tables 4 and 5) at 3 weeks of age were primarily in the T lymphocyte population, it is likely that the lymphocyte population had the most profound effect on cytokine production.
Furthermore, this study demonstrates for the first time that insufficient maternal dietary choline during the suckling period results in alterations in the expansion and maturation of the T cell population and the ability to respond to mitogens later in life. Feeding a ChS diet during weaning rescued the lymphopenic status of the ChD offspring at 3 weeks. However, female offspring from dams fed a ChD diet during suckling had a slight shift in lymphocyte proportions at 10 weeks, displaying a lower proportion of CD4+ T cells and lower IL-6 production after ConA or LPS stimulation. Expansion of the CD4+ population is a critical developmental process that occurs during the suckling period( Reference Perez-Cano, Castellote and Marin-Gallen 42 ) and IL-6 has been demonstrated to play a role in this process. IL-6 induces proliferation of T cells in an IL-2-independent manner( Reference Lotz, Jirik and Kabouridis 43 ), promoting differentiation of the CD4+ and CD8+ populations( Reference Okada, Kitahara and Kishimoto 44 ) and the ability of T cells to communicate with accessory cells (B cells)( Reference Eddahri, Denanglaire and Bureau 45 ). Therefore, it appears that it is not only the number of CD4+ cells but also their ability to produce an important cytokine that is affected by maternal choline intake during suckling. In addition, ChD-ChS offspring had a lower proportion of cytotoxic T cells expressing the co-stimulatory molecule CD28, also used as a maturation marker. The reduced proportion of mature T cells may be in part responsible for the impaired ex vivo IL-6 response observed when stimulated. A source of choline has been demonstrated to be essential for macrophage production of IL-6( Reference Tian, Pate and Andreolotti 46 ). Although the proportion of macrophages (CD284+ CD11+) was not altered between the diet groups, reduced IL-6 production may be attributed to impaired macrophage function in addition to T cell function. In the experiment examining the effects of a maternal diet devoid of choline in 3-week-old offspring, we did not examine the possible influence of sex as one male and one female offspring were used for each dam. To our knowledge, there have been no studies examining the difference in choline requirements between sexes in young animals (i.e. during suckling), but previously we did not find an effect of sex on immune cell phenotypes in animals at this age( Reference Field, Goruk and Glen 20 ). The experiment examining the long-term effects of choline-devoid maternal diet during lactation was conducted on female offspring only. It has been shown that female mice, at sexual maturity, have greater synthesis of PC through the PEMT pathway( Reference Noga and Vance 47 ), and mature female rodents are less susceptible to the consequences of low dietary choline compared with male rodents( Reference Tessitore, Sesca and Greco 48 , Reference Saito, Palomba and Rao 49 ). It would be logical to follow-up our findings with male offspring in both studies as we may see a larger programming effect in an animal that has a lower ability to endogenously synthesise choline.
The impaired T cell-mediated functions and maturation observed in both ChD and ChD-ChS offspring are supported by previous studies demonstrating that choline( Reference Dellschaft, Ruth and Goruk 16 ) or lipotrope deficiency (including choline)( Reference Courreges, Benencia and Uceda 15 ) has the most profound effect on the T cell component of the immune system. This impaired T cell function may increase the susceptibility to infection later in life. Newberne et al. demonstrated that when offspring from dams fed a marginally ChD diet during suckling were placed on a ChS diet after weaning, they were still more susceptible to S. typhimurium infection at 100 d after weaning compared with offspring fed a ChS diet. However, Nauss et al.( Reference Nauss, Connor and Kavanaugh 18 ) suggests that age plays a role in the function of the immune system in the context of a methionine–choline-deficient diet. Rats fed a marginally methionine–choline-deficient diet (67 % lower in methionine and choline compared with control diet) during weaning were most susceptible to S. typhimurium infection at a younger age (3 weeks after weaning) compared with older animals (3 and 12 months after weaning). Younger animals also had the lowest amount of lymphocyte proliferation compared with older animals on the same marginally deficient diet. Our results suggest that maternal choline deficiency during the suckling period may impair the development of the T cell population later in life, perhaps with greater effects in the early post-weaning period. Our study focused primarily on surrogate markers of immune function (ex vivo cytokine production, [3H]thymidine uptake and cellular proliferation). As previous studies have found an effect of choline deficiency on T-cell-mediated functions( Reference Courreges, Benencia and Uceda 15 ), future studies should examine not only surrogate markers of immune function but also more specific immune challenges such as susceptibility to infection and DTH response to examine T cell functions and tests that have greater applicability to humans. In addition, we did not perform the proliferation assay on the 10-week-old offspring. In the 10-week-old offspring, there was lower IL-6 production in the offspring from the dams fed the ChD diet, with similar amounts of IL-2 produced compared with the offspring from dams fed the ChS diet during suckling. IL-6 has been demonstrated as a potent regulator and stimulator of lymphocyte proliferation, independent of the proliferation actions associated with IL-2( Reference Lotz, Jirik and Kabouridis 43 , Reference Holsti and Raulet 50 ). In addition, the ChD-ChS offspring had a lower proportion of CD3+ CD4+ and markers indicating activation (CD28+). Therefore, we would predict that the ChD-ChS offspring would have a lower proliferative response following stimulation that is associated with the lower IL-6 production when stimulated with ConA or LPS.
We demonstrated that choline is essential in the maternal diet to support offspring growth and development of the immune system. Maternal choline deficiency during a critical period of development stunted growth and resulted in lymphopenia, which may have contributed to the impaired T and B cell responses in the offspring at the end of suckling. The long-term consequences of a lower supply of choline during this critical period of development resulted in a lower expansion of the CD4+ population and a lower ability to produce IL-6, even after choline was fed in the weaning diet. Together, with findings of a possible suboptimal intake during the lactation period in humans, future research should focus on identifying potential benefits of increasing total choline in the maternal diet to support immune development in infants.
Acknowledgements
The authors acknowledge the technical assistance of Nicole Coursen, Marnie Newell, Yuan Yuan Zhao and Howe-Ming Yu.
Funding for this project came from the Natural Sciences and Engineering Council of Canada (NSERC RGPIN 121610, 386652 and 03932) and Quality Food for Health grant from the ALMA, Alberta Innovates Biosolutions and the Egg Farmers of Alberta (2012Q005R). E. D. L. is a recipient of an Izaak Walton Killam Memorial Scholarship and a Natural Sciences and Engineering Research Council Postgraduate Doctoral Scholarship. C. R. is a recipient of postdoctoral fellow scholarships from Canadian Institutes of Health Research (CIHR), Fonds de Recherche en Santé du Québec and Izaak Walton Killam Memorial Postdoctoral Fellowships. R. L. J. is a CIHR New Investigator. None of the mentioned funders had a role in the design, analysis or writing of this article.
C. J. F., J. M. C. and R. L. J. designed the study; E. D. L., S. G. and N. S. D. conducted the study; E. D. L. and C. R. analysed the data and performed statistical analysis; E. D. L. and C. J. F. wrote the paper; C. J. F. has primary responsibility for final content. All the authors have read and approved the final manuscript.
There are no conflicts of interest.
Supplementary Material
For supplementary material/s referred to in this article, please visit http://dx.doi.org/doi:10.1017/S0007114516002919














