Pb is a well-known neurotoxicant that is pervasive in the environment, found in air, soil and water. Developed countries have tried and succeeded in reducing the level of exposure to Pb since 1980( Reference Muntner, Menke and DeSalvo 1 ). The Chinese government began to ban the use of leaded gasoline nationally since 1 July 2000, which also decreased the Pb levels in air and soil. However, several other sources of Pb exposure remain for Chinese people, one of which is dietary.
Preserved egg (PE), also known as Pidan (Chinese:
![]() $$\raster="rg1"$$
; pinyin: pídàn), is a Chinese preserved food product and delicacy made by preserving duck or chicken eggs in a mixture of clay, ash, salt, quicklime and rice hulls for several weeks to several months. The nutritional value of PE is slightly decreased compared with that of fresh egg, but PE has an extremely long shelf life and a pleasant, fragrant taste that is preferred by most people in Southeast Asian countries(
Reference Wang and Fung
2
). In particular, PE usually contains quite a high amount of Pb because of the use of litharge powder (PbO) in pickle processing. PbO can penetrate through the eggshell and increase the Pb level in eggs. According to an investigation through the national food contamination monitoring system, the average Pb content of PE consumed in China was 1·782 mg/kg, with the maximum of 334·0 mg/kg, which is much higher than any other commonly consumed food(
Reference Jiang, Wang and Yang
3
). Recently, another study showed that among all Chinese food groups the highest Pb exposure was from ‘Eggs and their products’ (42·4–51·6 % of the total exposure), with PE as the main contributor(
Reference Pan, Wang and Peng
4
).
$$\raster="rg1"$$
; pinyin: pídàn), is a Chinese preserved food product and delicacy made by preserving duck or chicken eggs in a mixture of clay, ash, salt, quicklime and rice hulls for several weeks to several months. The nutritional value of PE is slightly decreased compared with that of fresh egg, but PE has an extremely long shelf life and a pleasant, fragrant taste that is preferred by most people in Southeast Asian countries(
Reference Wang and Fung
2
). In particular, PE usually contains quite a high amount of Pb because of the use of litharge powder (PbO) in pickle processing. PbO can penetrate through the eggshell and increase the Pb level in eggs. According to an investigation through the national food contamination monitoring system, the average Pb content of PE consumed in China was 1·782 mg/kg, with the maximum of 334·0 mg/kg, which is much higher than any other commonly consumed food(
Reference Jiang, Wang and Yang
3
). Recently, another study showed that among all Chinese food groups the highest Pb exposure was from ‘Eggs and their products’ (42·4–51·6 % of the total exposure), with PE as the main contributor(
Reference Pan, Wang and Peng
4
).
Various adverse effects of Pb exposure on human health have been established. Disorders of the gastrointestinal, renal, hepatic, haematologic and immunologic systems, as well as cardiovascular systems, have been reported( Reference Patrick 5 , Reference Flora, Gupta and Tiwari 6 ). Neurotoxicity is a well-known clinical feature of Pb exposure. Consistent neuropsychological research has revealed that Pb exposure can result in declines in intelligence, memory, comprehension and reading, visuospatial skills and executive functions( Reference Mason, Harp and Han 7 ). Adverse psychological symptoms also emerged as a result of Pb exposure. Studies on the mental health effects of Pb exposure were mainly in the context of occupational exposures, with levels of exposure much higher than that experienced by the general population. Pb-exposed workers in foundries, battery plants and Pb smelters were reported to experience mood disorders such as anxiety, hostility and depressive states( Reference Schwartz, Lee and Bandeen-Roche 8 , Reference Maizlish, Parra and Feo 9 ). Only one study has examined the relationship between environmental Pb exposure and depression in general populations, which did not demonstrate a consistent association within the investigated blood Pb levels( Reference Golub, Winters and van Wijngaarden 10 ).
For the population at large, which is not occupationally exposed, food and water are the most important sources of exposure to Pb( 11 ). The health impact of long-term Pb exposure through dietary intake, which may cause adverse health effects even at relatively low levels, has attracted researchers’ attention in recent years( Reference Sun, Wang and Wu 12 , Reference Boon, Te Biesebeek and Sioen 13 ). However, few studies have considered the possible link between dietary Pb exposures and mental health outcomes in the general population. Given that dietary Pb exposure is relatively modifiable, this type of potential risk factor for mental health outcomes should be of interest. As a single food with a high content of Pb, PE is quite a preferred candidate for investigating the relationship between dietary Pb exposure and mental health. Therefore, we turned to the comprehensive physical examination data collected in Tianjin to explore the association between consumption of PE and depressive symptoms in a large population of Chinese adults.
Methods
Study participants
This analysis used data from the Tianjin Chronic Low-grade Systemic Inflammation and Health Cohort, which is a large prospective cohort study of inhabitants living in Tianjin, China. The cohort was based on annual health examinations conducted in Tianjin Medical University General Hospital Health Management Center and focused on the relationship between chronic low-grade systemic inflammation and the health status. Participants who had received physical examinations and completed a detailed lifestyle questionnaire were recruited (92·3 % response rate). Recruitment took place from May 2013 to December 2016. Written informed consent was obtained for every participant, and the Institutional Review Board of Tianjin Medical University approved study protocols.
During the study period, a total of 27 946 participants aged 20 years and older were sampled. Participants who did not complete data collection on FFQ or depression scale (n 974), BMI and physical activity (PA) (n 139), or those with a history of CVD (n 1343) or cancer (n 277), were excluded. Thus, a final sample of 25 213 participants (mean age 41·4 (sd 11·8) years; males, 53·9 %) was used in current analyses.
Measures
Assessment of dietary intake
Dietary intake during the previous month was obtained through a validated FFQ that included 100 food items with specified serving sizes. The FFQ included seven frequency categories ranging from ‘never’ to ‘2 or more times/d’ for foods (including PE and egg) and eight frequency categories ranging from ‘never’ to ‘4 or more times/d’ for beverages. By combining the information obtained from the food frequency response with the Chinese food composition tables( Reference Yang, Wang and Pan 14 ), we were able to compute the mean total energy intake. The FFQ have been evaluated with regard to reproducibility and validity( Reference Su, Yu and He 15 )
Assessment of depressive symptoms
Depressive symptoms were assessed by the Chinese version of Self-Rating Depression Scale (SDS), which has been proven to be a valid measure for the Chinese population( Reference Lee, Chiu and Wing 16 ). There are twenty items rated on a four-point scale. Summary scores range from 20 to 80, with higher scores indicating the elevated depressive symptoms. In order to increase sensitivity, two cutoff points (45 and 50) were used to define depressive symptoms in the present study( Reference Xu, Ren and Cheng 17 , Reference Zung 18 ). Scores higher than these cutoff points are considered to reflect moderately or severely depressive symptoms. Continuous scores of SDS were also used in the sensitivity analyses.
Assessment of other variables
Participants completed standardised physical examinations at the Health Management Centers, which included measurements of height, weight, waist circumference, blood pressure, fasting blood sugar and plasma lipids( Reference Su, Yu and He 15 , Reference Yu, He and Zhang 19 ). BMI was calculated as weight (kg) divided by height (m) squared. The metabolic syndrome (MetS) was defined according to the criteria of the American Heart Association Scientific Statement( Reference Alberti, Eckel and Grundy 20 ).
Socio-demographic variables including sex, age, education, occupation, household income and social connections (including marital status, cohabitants and amount of social contact) were also assessed. Smoking and drinking status was obtained from the questionnaire. PA in the most recent week was assessed using the short version of the International Physical Activity Questionnaire( Reference Craig, Marshall and Sjostrom 21 ). For evaluation of total PA, separate metabolic equivalent (MET) hours per week were calculated for walking, moderate and vigorous activities (MET coefficient for each category was 3·3, 4·0 and 8·0, respectively) according to the following formula: MET coefficient of activity×duration (h)×frequency (d)( Reference Craig, Marshall and Sjostrom 21 ). Total PA level was calculated by summing scores for different activities.
Statistical analysis
Descriptive data are presented as the means and 95 % CI or percentages. Four categories of PE consumption were used to classify the participants: <once/week, once/week, 2–3 times/week and ≥4 times/week. Differences in covariates between the PE consumption categories were examined by ANOVA for continuous variables or by logistic regression analysis for categorical variables. Depressive symptoms were analysed as binary variables using the lower (≥45) and higher (≥50) cutoff points. Logistic regression models were fitted to assess the associations between PE consumption categories and depressive symptoms, using the lowest category of PE consumption (<once/week) as the reference group. For all analyses, three different models were fitted: model 1, a crude univariate model; model 2, adjusted for age, sex and BMI; and model 3, adjusted for smoking and drinking, PA, education, employment, household income, cohabitants, social contact, marital, total energy intake, the MetS and egg consumption. The final multivariate logistic analysis was performed with the forced entry of all factors considered to be potential covariates. The interactions between PE consumption and sex for depressive symptoms were tested through the addition of the cross-product term to the final regression model. All P values for linear trends were calculated by using the categories of PE consumption. Significance level was set at 0·05. All the statistical analyses were performed by using SAS (version 9.1; SAS Institute).
Results
A total number of 25 213 participants were included in the final analysis, with the average BMI being 24·5 (sd 3·8) kg/m2. Of all the participants, the mean SDS score was 36·8, with a median of 36·0; 17·0 % were classified as having elevated depressive symptoms when using 45 as a cutoff point, and 6·6 % with the higher cutoff point of 50. Participant characteristics according to categories of PE consumed are presented in Table 1. Most of the participants (88·4 %) in this cohort reported consuming PE less than once/week, whereas only 2·4 % consumed PE more than 4 times/week. Compared with those with least frequent consumption of PE, the higher PE consumers were more likely to be males and younger; were less likely to be married and more likely to be living alone; and had lower household income, a lower PA level, higher BMI and higher prevalence of the MetS. PE consumption was positively associated with smoking and alcohol use, and negatively associated with egg consumption. Mean total energy intake was significantly higher across the PE consumption quartiles.
Table 1 Participant characteristics by frequency of preserved egg consumption (Least square geometric means and 95 % confidence intervals)
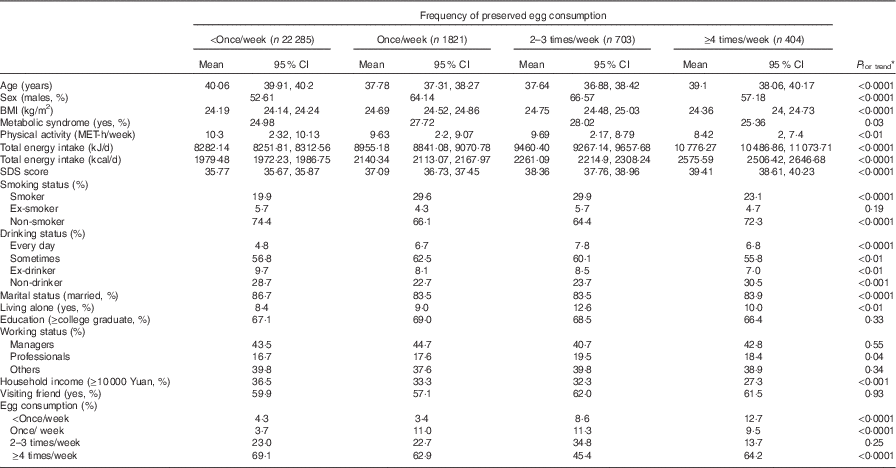
MET, metabolic equivalents; SDS, Self-Rating Depression Scale.
* ANOVA or logistic regression analysis.
The crude and adjusted association between categories of PE consumption and depressive symptoms are shown in Table 2. In all three models, the ORs of the depressive symptoms remarkably increased across PE intake levels. Taking the cutoff point of 50 for example, age-, sex- and BMI-adjusted OR for depressive symptoms across categories of PE consumption were 1·00, 1·65 (95 % CI 1·39, 1·95), 2·51 (95 % CI 1·99, 3·13) and 3·76 (95 % CI 2·89, 4·82), respectively (P for trend<0·0001), indicating a clear dose–response relationship. These results were almost unchanged when adjusted for multiple confounding factors. In the final multivariate logistic models, the adjusted OR for depressive symptoms across categories of PE consumption were 1·00, 1·52 (95 % CI 1·28, 1·81), 2·24 (95 % CI 1·76, 2·81) and 3·31 (95 % CI 2·52, 4·30) (P for trend<0·0001). The prevalence of depressive symptoms was more than threefold for participants who consumed PE≥4 times/week compared with those who consumed <once/week. Similar relations were observed when we used SDS≥45 as a definition of elevated depressive symptoms. We also tested whether similar results would be observed if depressive symptoms were treated as continuous variables. Mean SDS scores for four PE consumption categories were adjusted for all the covariates by using ANCOVA. Adjusted mean SDS scores across categories of PE consumption were 36·2 (95 % CI 34·3, 38·1), 37·3 (95 % CI 35·4, 39·3), 38·5 (95 % CI 36·5, 40·7) and 39·6 (95 % CI 37·4, 41·8), respectively. The mean SDS scores significantly increased with increasing PE consumption (P<0·0001), which are comparable to the categorical analyses.
Table 2 Adjusted associations between frequency of preserved egg consumption and depressive symptoms (Adjusted odds ratios and 95 % confidence intervals)
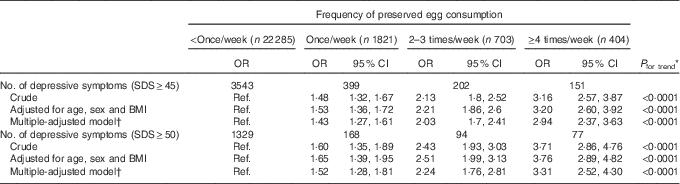
SDS, Self-Rating Depression Scale; Ref., referent values.
* Obtained by using multiple logistic regression analysis.
† Adjusted for age, sex, BMI, smoking, drinking, physical activity, marital status, total energy intake, household incomes, employment, education, visiting friends, living alone, metabolic syndrome and egg consumption.
Additional sensitivity analyses were carried out to exclude the possible bias of the association. No significant interaction between PE consumption and sex was found (SDS≥50, P for interaction=0·81). As depressive status is also related to unhealthy eating habits and appetite( Reference Andreasson, Arborelius and Erlanson-Albertsson 22 , Reference Cassano and Fava 23 ), a sensitivity analysis was added by excluding those who had very low (lower 2·5 %) or high (upper 2·5 %) energy intake. After this exclusion, the fully adjusted OR for depressive symptoms (SDS≥50) across categories of PE consumption were 1·00, 1·48 (95 % CI 1·23, 1·78), 2·21 (95 % CI 1·72, 2·81) and 3·08 (95 % CI 2·22, 4·19) (P for trend<0·0001), which demonstrated a similar pattern of results.
Discussion
In this large-scale study, we have found that increasing consumption frequency of PE was strongly associated with a higher risk of depressive symptoms in adults in China. Compared with participants who consumed PE less than once/week, those who consumed more than 4 times/week had a more than 3-fold-higher risk of increasing depressive symptoms (SDS≥50). The associations were consistent after the adjustment for multiple confounding factors. The trend analyses support the presence of a significant increase in risk across the levels of PE consumption frequency.
To the best of our knowledge, this is the first large population study of the association between consumption of a high-Pb-containing food and depressive symptoms. As PE is quite similar to the nutrient composition of egg( Reference Wang and Fung 2 ), we have adjusted the egg consumption in our regression model. The results did not change after this adjustment, which makes us more confident to infer that it should be the high Pb contents in PE that contribute to the adverse mental health effect.
Pb can enter the body mainly through the respiratory and gastrointestinal tracts. For adults with occupational exposure, the most significant route for absorption is through the respiratory tract( Reference Patrick 24 ). The association between occupational Pb exposure and depression, anxiety and other psychiatric illness has already been well documented( Reference Schwartz, Lee and Bandeen-Roche 8 , Reference Balbus-Kornfeld, Stewart and Bolla 25 , Reference Shih, Hu and Weisskopf 26 ). The link between increased blood and bone Pb levels and psychological symptoms has also been investigated by several studies in the general population. A study of 526 older adults in the Normative Aging Study found that anxiety, depression and phobia were positively correlated with bone Pb level( Reference Rhodes, Spiro and Aro 27 ). Another cross-sectional epidemiologic study found that higher blood Pb levels were associated with increased odds of major depression and panic disorders in US young adults( Reference Bouchard, Bellinger and Weuve 28 ). Recently, a longitudinal study in South Korea with a large sample size found an association between mental disorders and an increased blood Pb level( Reference Yoon and Ahn 29 ). Our study added the evidence that dietary Pb exposure may also increase the risk of mental health problems. This is important as the absorption rates of Pb are varied for different ways of exposure. According to the previous study, the percentage of inhaled Pb reaching the bloodstream is estimated to be 30–40 %, whereas rates of absorption through the gastrointestinal tract for adults are 10–15 % on average( Reference Philip and Gerson 30 ).
We believe the findings to be biologically plausible, as growing literature elucidates the mechanisms of how exposure to Pb may lead to psychological disorders including depression( Reference Dunlop and Nemeroff 31 ). For example, Pb exposure has been found to alter the normal physiology of dopamine and serotonin metabolism in the brain that are frequent targets for medications to treat depression( Reference Kala and Jadhav 32 , Reference Verstraeten, Aimo and Oteiza 33 ). Animal studies have also demonstrated that Pb exposure can disrupt the hypothalamic–pituitary–adrenal axis, leading to altered glucocorticoid and catecholaminergic signalling( Reference Cory-Slechta, Virgolini and Rossi-George 34 , Reference Virgolini, Chen and Weston 35 ), with mood disorder as a proposed consequence( Reference Cory-Slechta, Virgolini and Rossi-George 34 ). Moreover, Pb also has well-known adverse effects on the cardiovascular system( Reference Eum, Nie and Schwartz 36 , Reference Navas-Acien, Guallar and Silbergeld 37 ), and many cardiovascular risk factors have been found to predict depression.
An alternative explanation of the findings is that the relatively small subset of participants who had a relatively high frequency of PE consumption differed from the remainders in ways that might increase the prevalence of depressive symptoms. From the descriptive statistics of the cohort (Table 1), it appears that high PE consumption was associated with many unhealthier characteristics such as higher BMI, lower PA level and higher frequency of smoking and drinking. We addressed this issue by running models adjusting for potential depression risk factors associated with PE consumption (BMI, PA, education level, alcohol consumption, smoking, etc.). The fact that the association between PE consumption and depressive symptoms was not attenuated after these adjustments suggests that effects are unlikely to be attributed to these confounding factors. Moreover, the associations between PE consumption and two different cutoff points for depressive symptoms were tested and similar relationships were consistently observed.
The strength of our study includes the large sample size and the adjustment for a number of potential confounders. One limitation is that the depressive symptoms were assessed with a self-reported questionnaire rather than diagnostic psychiatric interviews. Total scores on the SDS do not correspond with a clinical diagnosis of depression but rather indicate the level of depressive symptoms that may be of clinical relevance. Therefore, a larger population study that uses a standardised comprehensive structured diagnostic interview should be undertaken to confirm the associations with depression. Another limitation is that this is an observational and correlational study, and therefore does not establish a causal relation between PE consumption and outcomes. Although we have adjusted statistically for many potential confounding factors, we may not have fully captured relevant aspects of those factors. Future laboratory studies are needed to confirm the mechanisms involved in the association between dietary Pb exposure and depression.
In summary, the present study provides support for a strong association between the consumption of PE, a high-Pb-containing food and the risk of depressive symptoms in Chinese adults. These findings underscore the need to consider dietary Pb exposure as a risk factor for psychological distress.
Acknowledgements
The authors gratefully acknowledge all the people who have participated in the study.
This study was supported by grants from the National Natural Science Foundation of China (nos 81673166, 81372118, 81372467 and 81302422), the key technologies R&D program of Tianjin (Key Project: nos 11ZCGYSY05700, 12ZCZDSY20400, 13ZCZDSY20200 and 15YFYZSY00020), the National Science and Technology Support Program (no. 2012BAI02B02), 2012 and 2016 Chinese Nutrition Society Nutrition Research Foundation–DSM Research Fund (nos 2014-071, 2016-046 and 2016-023), the Technologies development programme of Beichen District of Tianjin (nos bcws2013-21, bcws2014-05 and 2015-SHGY-02), the technologies project of Tianjin Binhai New Area (nos 2013-02-04 and 2013-02-06), the Science Foundation of Tianjin Medical University (nos 2010KY28 and 2013KYQ24), the Key Laboratory of Public Health Safety (Fudan University), Ministry of Education (no. GW2014-5), the National Training Programs of Innovation and Entrepreneurship for Undergraduates (no. 201510062013) and the National Social Science Foundation (no. 15BSH065), China, Scholarship from China Scholarship Council.
B. Y., Y. W. and K. N. contributed to the conception and design and drafting or revising the article; B. Y., F. Y. and K. N. contributed to the analysis and interpretation of the data; B. Y., J. S., F. Y., G. M., H. W., Y. X., X. B., Y. G., H. S., Y. W. and K. N. contributed to the assembly of the data; B. Y., J. S., F. Y., Q. Z., L. L., G. M., H. W., Y. X., X. B., Y. G., H. S., S. S., X. W., M. Z., Q. J., H. L., Y. W. and K. N. contributed to data collection; B. Y. and K. N. contributed to the approval of the final version of the manuscript.
None of the authors has any conflicts of interest to declare.




