Clinical perspective
What Is New?
Previous evidence from basic research supports folic acid (FA) supplementary therapy as a protective factor for gastric ulcers (GU). Nevertheless, observational studies have not recognised a causal effect of FA supplementary therapy on GU. This Mendelian randomisation (MR) study provides genetic evidence of a causal relationship between FA supplementary therapy and GU.
What Are the Clinical Implications?
These findings confirm the causally decreased risk of GU induced by FA supplementary therapy. According to the evidence, clinicians and researchers should attach great importance to the protective role of FA in the prevention and treatment of GU. Exploration of the underlying mechanism will provide useful guidance for the protection of GU.
Peptic ulcers are a common type of chronic digestive disease, with an estimated 4 million cases occurring worldwide annually. The prevalence of peptic ulcers in the general population is estimated to be between 5 and 10 %(Reference Sverden, Agreus and Dunn1,Reference Lanas and Chan2) , and GU is one of the most common types. GU are characterised by natural relief and recurrence, with a high 5-year recurrence rate of up to 24·3 %(Reference Seo, Hong and Kim3). The high incidence of GU is attributed to a series of induced factors, such as Helicobacter pylori (Hp) infections, abuse of non-steroidal anti-inflammatory drugs, alcoholism and smoking(Reference Allen and Garner4). Despite developing various medicines for the prevention and treatment of GU including proton-pump inhibitors, histamine-2 receptor antagonists as well as prostaglandin analogues(Reference Malfertheiner, Megraud and O’morain5,Reference Scally, Emberson and Spata6) , the incidence of GU remains high.
FA, also known as folate, is a common B-family vitamin, which exists in all kinds of vegetables, fruits, beans and other grains(Reference Chan, Bailey and O’connor7). As an essential nutrient that cannot be made by humans, folate plays a crucial role in DNA and RNA synthesis and is involved in protein metabolism(Reference Crider, Yang and Berry8,Reference Mathers9) . Consequently, it is frequently added to foods as a dietary supplement in the form of FA and sold as a supplement(Reference Choi, Yates and Veysey10). In fact, this form is better than when absorbed from foods. Folate deficiency has been reported to be closely associated with the occurrence of numerous diseases, such as anaemia(Reference Dameshek11), neural tube defects and congenital heart disease(Reference Czeizel, Dudas and Vereczkey12), atherosclerosis(Reference Antoniades, Antonopoulos and Tousoulis13), adverse pregnancy outcomes(Reference Mcnulty, Rollins and Cassidy14) and cancer(Reference Oaks, Dodd and Meinhold15,Reference Pufulete, Al-Ghnaniem and Khushal16) . However, the potential impact of FA on gastric mucosa has received little attention. Several studies have demonstrated that pretreatment with FA can effectively prevent the formation of GU(Reference Ajeigbe, Olaleye and Oladejo17–Reference Ajeigbe, Aibangbee and Saeed21). FA is used in the treatment of GU in the following two aspects: on the one hand, it reduces TNF-α and IL-1β by reducing the infiltration of neutrophils and inflammatory cells but increases IL-4 and IL-10 to play an anti-secretion, anti-oxidation and anti-inflammatory role, reducing the damage to gastric mucosa(Reference Kakhki, Goodarzi and Abbaszade-Cheragheali22); on the other hand, it promotes the healing of gastric mucosa by regulating epidermal growth factor (EGF), epidermal growth factor receptor (EGFR) and nuclear proliferation-associated antigen Ki-67 (Ki-67) to promote epithelial proliferation and increasing vascular endothelial growth factor (VEGF), platelet/endothelial cell adhesion molecule-1 (CD31) and factor VIII expression to promote angiogenesis(Reference Han, Oh and Im23). Nevertheless, most of these studies have been limited to the animal and cellular level and lack of clinical and genetic evidence, which limits their reliability.
MR is a genetic epidemiology method that evaluates the causal association between genetically determined exposure and disease, based on the theory that the random allocation of genes is similar to randomised controlled trials(Reference Liu, Jin and Jiang24–Reference Huang, Lin and He26). MR can avoid the interference of confounding factors, as genetic variation is randomly distributed among individuals and is not influenced by potential social or environmental factors. Compared with observational studies, MR provides more reliable causal inference because observational studies are more susceptible to confounding and selection bias, while MR reduces these biases by utilising genetic variation. Therefore, early access to the results of MR studies before initiating randomised controlled trials can save time, effort and research funding and allow for more informed study design(Reference Ference, Holmes and Smith27,Reference Ference, Kastelein and Ginsberg28) .
Therefore, this study aims to investigate the potential causal effect of FA supplementary therapy on GU from a genetic perspective using MR analysis.
Methods
Study design
We conducted an MR analysis to investigate the potential causal effects of genetically predict FA supplementary therapy on GU. The MR design is a method for testing whether exposure has a causal relationship with the development of diseases in which genetic variations are considered instrumental variables. This method can overcome unmeasurable confounding factors and make stronger causality inferences(Reference Lawlor, Harbord and Sterne29). The design of MR is based on three underlying hypotheses: (1) the genetic variants are closely associated with the exposure; (2) the genetic variants are independent of other confounding factors and (3) the genetic variants are only related to the results of investigated exposure(Reference Emdin, Khera and Kathiresan30). The brief process of this work is displayed in Fig. 1.

Fig. 1. Mendelian randomisation model of FA supplementary therapy and GU. The design is under the assumption that the genetic variants are associated with FA supplementary therapy, independent of other confounders, and the genetic variations affect gastric ulcer only by FA supplement therapy. FA, folic acid; GU, gastric ulcers.
Data source and methods
Summary-level data on the correlations of FA supplementary therapy were obtained from a large-scale genome-wide association study database (GWAS) (https://gwas.mrcieu.ac.uk/datasets/ukb-b-3563/; ICD: ‘ukb-b-3563’). Prior to MR analysis, SNP were rigorously screened to ensure the quality. First, we gathered all SNP using linkage disequilibrium clumping (r2 < 0·01 within windows 1000 kb for variants in the gene locus). Moreover, we retained SNP linked to the opportune exposure at the genome-wide significance threshold (P < 5 × 10–8). Finally, a total of seven independent SNP were genome-wide significant with FA supplementary therapy and were applied to the analysis (Table 1). We conducted a comprehensive search of risk factors for GU from previously published literature. After searching the seven SNP mentioned above on the PhenoScanner V2 web (http://www.phenoscanner.medschl.cam.ac.uk/), the results showed that none of these SNP were related to GU risk factors. Consequently, the ultimate MR analysis included all seven SNP. Summary statistics for the relation between the seven FA supplements-related SNP and GU derived from the GWAS database (https://gwas.mrcieu.ac.uk/datasets/ukb-d-k25/; ICD: ‘ukb-d-k25’). Studies providing data for these GWAS meta-analyses were ethically approved by the relevant institutional review committees. In the present research, we only used the aggregated data of these studies; hence, there is no need for additional ethical approval.
Table 1. The characteristics of seven SNP and their genetic connections with FA supplementary therapy and GU
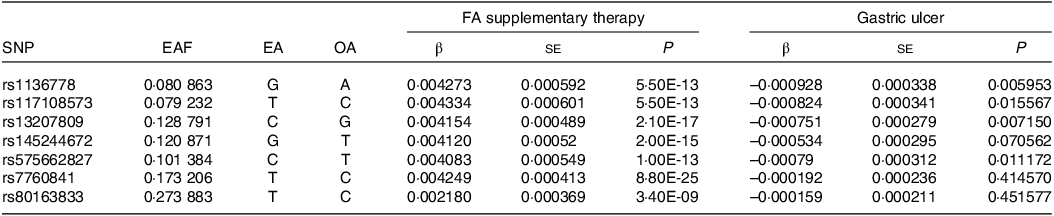
FA, folic acid or folate; GU, gastric ulcers; EAF, frequency of effect allele; EA, effect allele; OA, other allele.
Statistical analysis
Seven MR analysis methods were used in this study. Among them, inverse-variance weighted (IVW) is the primary method of analysis because it provides efficient and accurate causal effect estimates when there are many instrumental variables and the assumptions are met. All instrumental variables were required to meet the MR assumptions in the IVW methods, and the other methods were used for additional sensitivity analyses. A consistent causal assessment could be offered by the weighted median estimator, while more than half of the tool variables were effective. In addition, IVW approaches with MR-Egger intercept and Cochran’s Q statistics were used to evaluate the pleiotropy and heterogeneity of individual SNP. As long as there is no significant difference between the intercept and 0 (P > 0·05), it is considered that the pleiotropic effects do not exist. Cochrane’s Q value was applied to assess the heterogeneity. IVW method with the random-effects model was adopted as the main outcome when the P value of Cochrane’s Q was less than 0·05; otherwise, the fixed-effects model was adopted as the main outcome. MR-Egger regression was also carried out in this study since the pleiotropy could be detected and adjusted by it and then obtaining a causal effect assessment to determine if directional horizontal pleiotropy is accountable to the results. Moreover, leave-one-out analysis was performed to evaluate the robustness of MR analysis results through any outlier SNP. All statistical analyses were carried out using the ‘TwoSampleMR’ package in R version 3.4.2 (R Foundation for Statistical Computing) and a two-tailed P value < 0·05 was regarded as statistical significance.
Results
Results of Mendelian randomisation study
The overall design and summary of the results of this MR study are illustrated in Fig. 2. After matching the GU data, a total of seven SNP as the instruments (FA supplementary therapy) were included in the MR analysis. The final results were analysed using seven MR analysis approaches, including simple mode, weighted mode, IVW multiplicative random effects, IVW fixed-effect, simple median, weighted median and penalised weighted median (Fig. 3). The IVW models of both fixed and random effects showed that FA complementary therapy was related to a decreased risk of GU (OR, 0·870; 95 % CI 0·825, 0·918, P < 0·001; OR, 0·870; 95 % CI 0·826, 0·917, P < 0·001), as indicated in Fig. 4 (Table 2). This causality was also detected in simple median method (OR, 0·835; 95 % CI 0·773, 0·901, P < 0·001), weighted median (OR, 0·854; 95 % CI 0·794, 0·919, P < 0·001), penalised weighted median (OR, 0·849; 95 % CI 0·789, 0·914, P < 0·001), simple mode (OR, 0·826; 95 % CI 0·724, 0·943, P = 0·030) and weighted mode (OR, 0·828; 95 % CI 0·728, 0·941, P = 0·028) as shown in Table 2. Heterogeneity may exist in the IVW analysis (Q = 5·645, P = 0·464) and MR-Egger analysis (Q = 5·172, P = 0·395). MR-Egger regression showed that there was a directed pleiotropy among the genetic variants (intercept, 0·0003; P = 0·529). The leave-one-out sensitivity investigation indicated that the relationship between FA supplementary therapy and GU was not essentially driven by any single SNP (Fig. 5).
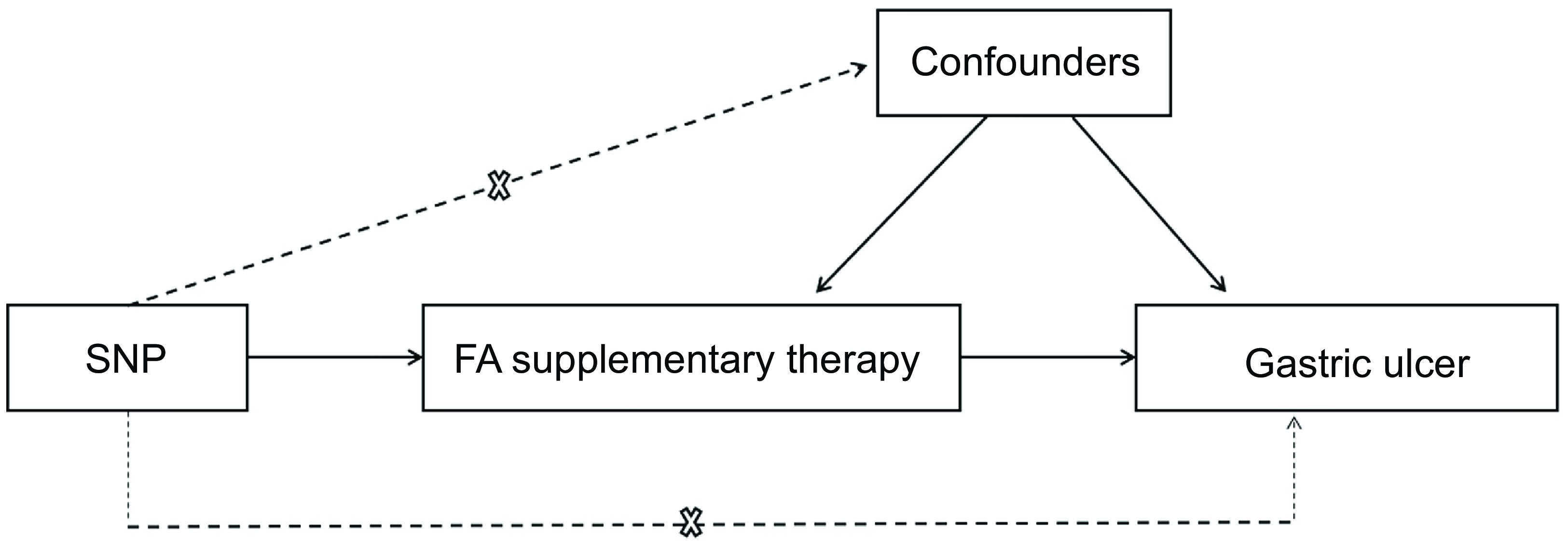
Fig. 2. Mendelian randomisation model of association between FA supplementary therapy and the risk of gastric ulcer. The overall design and summary of the results of this study. FA, folic acid.
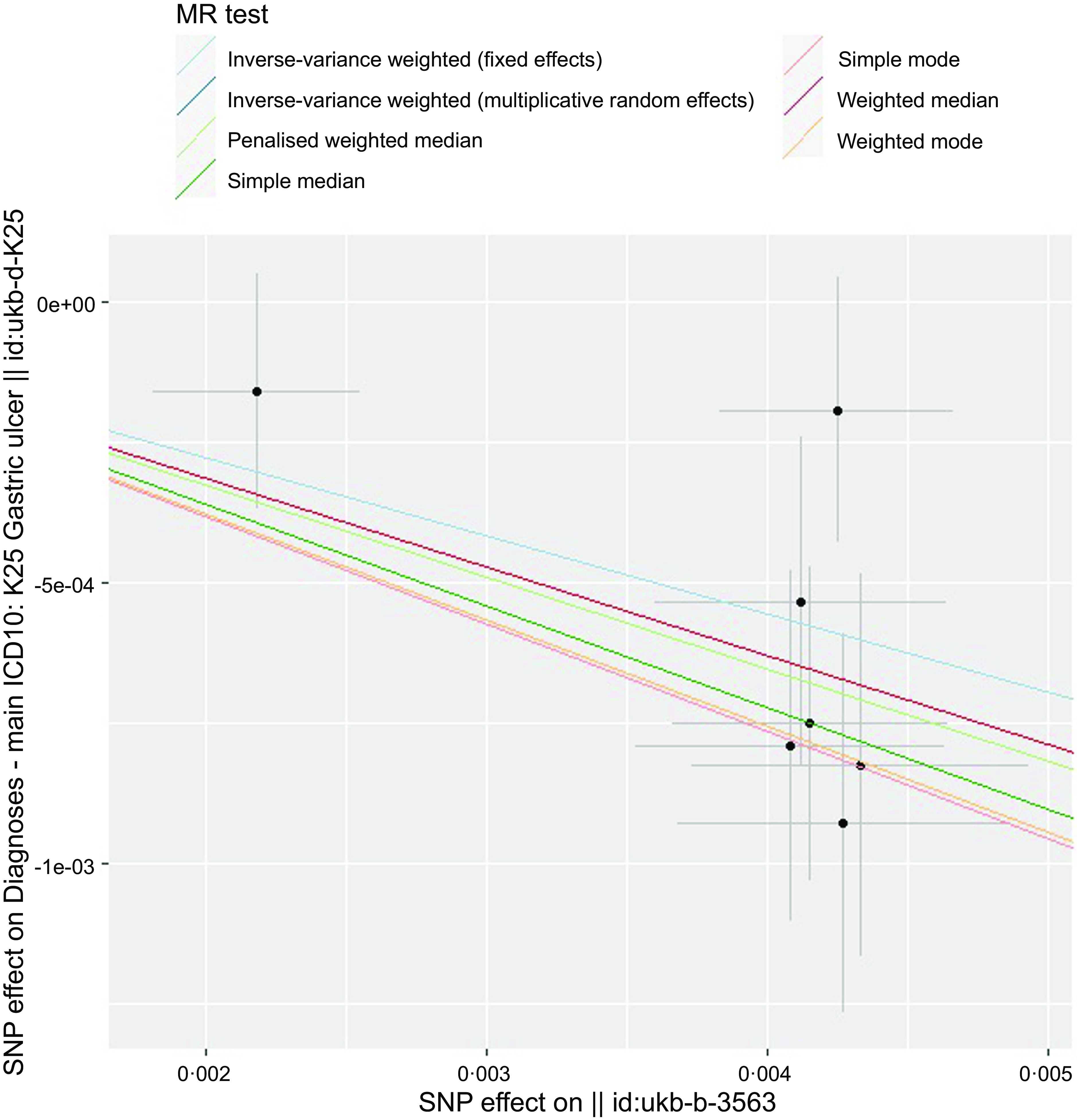
Fig. 3. Scatter plot to visualise the causal effect of FA supplementary therapy on GU genetically. The black dots and bars represented the causal estimation and 95 % CI by means of each SNP and slope of the straight line represents the degree of the causality. FA, folic acid; GU, gastric ulcers.
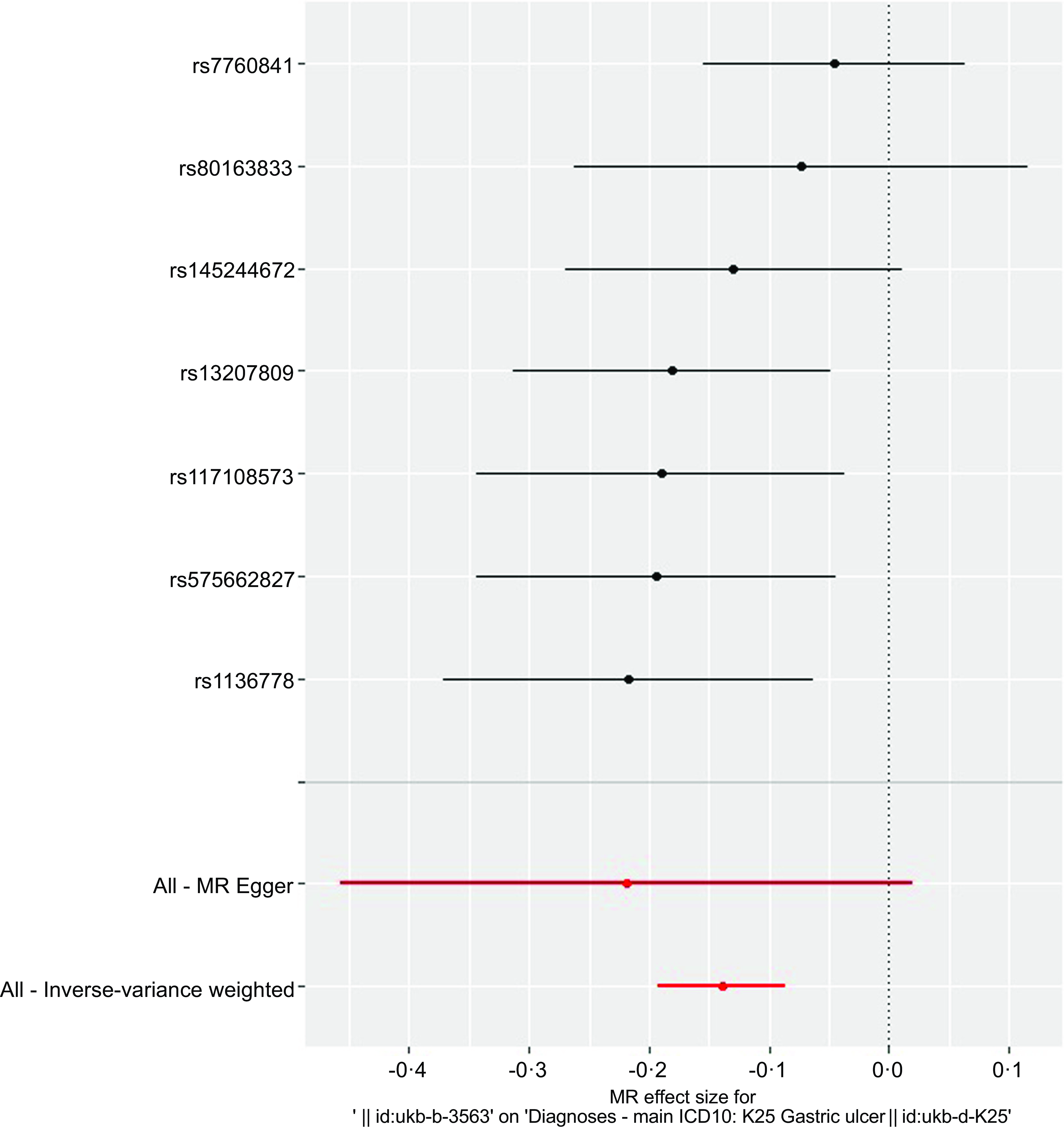
Fig. 4. IVW analysis of fixed effect of causality between FA supplementary therapy with GU. The black dots and bars represented the causal estimation and 95 % CI by means of each SNP. Through MR-Egger and fixed-effect inverse variance weighted method, the red dot and bar represented the overall estimated value and 95 % CI meta-analysed. IVW, inverse-variance weighted; FA, folic acid; GU, gastric ulcers; MR, Mendelian randomisation.
Table 2. The association of FA supplementary therapy with GU using various methods
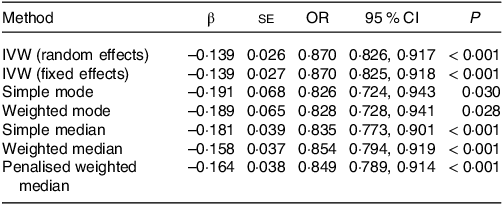
FA, folic acid or folate; GU, gastric ulcers; IVW, inverse variance-weighted.
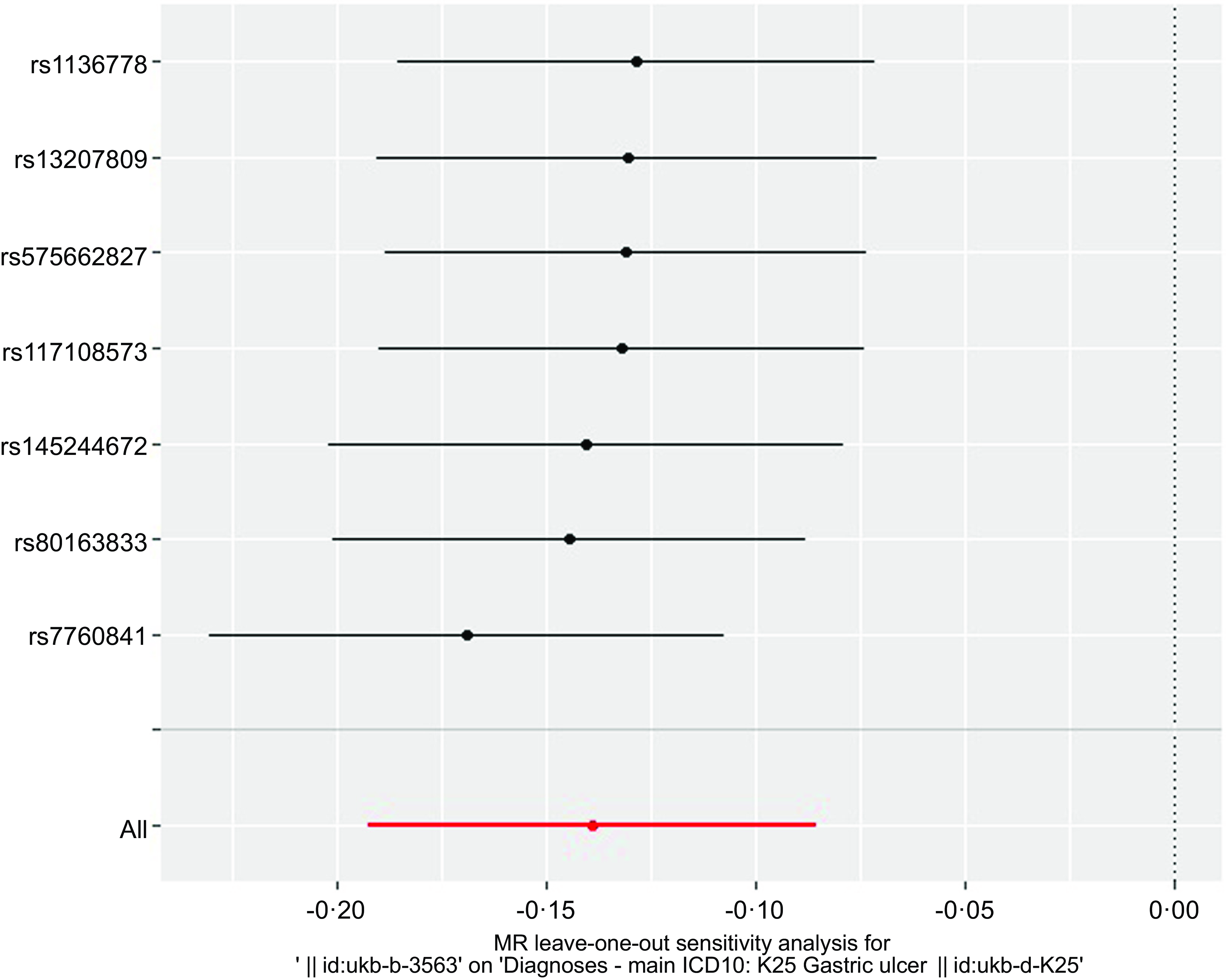
Fig. 5. Sensitivity analysis of MR leave-one-out for GU therapy with FA supplementary therapy. Circles indicate that if each SNP was omitted in turn, MR estimation of FA-assisted GU is performed by inverse-variance weighted fixed-effect method. MR, Mendelian randomisation; GU, gastric ulcers; FA, folic acid.
Discussion
The occurrence despite significant advances in the treatment of GU in recent years, numerous challenges persist in clinical practice. Current therapeutic strategies primarily focus on eradicating Hp, inhibiting gastric acid secretion through proton-pump inhibitors or H2 receptor antagonists, and utilising mucosal protective agents(Reference Gong, Zhao and Zhu31). However, issues such as low Hp eradication rates, increased antibiotic resistance, poor patient adherence, the inability to discontinue non-steroidal anti-inflammatory drugs and high recurrence rates frequently lead to treatment failure. Furthermore, the long-term use of proton-pump inhibitors and histamine-2 receptor antagonist is associated with a range of side effects, including nutrient malabsorption(Reference Teresa Selvin, Thomas and Bikeyeva32), increased fracture risk(Reference Lespessailles and Toumi33), heightened infection susceptibility(Reference Cunningham and Dial34), development of drug tolerance(Reference Sharma35), and potential renal, hepatic, and central nervous system damage(Reference Fossmark, Martinsen and Waldum36). Therefore, exploring more effective and personalised therapeutic strategies is of paramount importance.
This study investigates the role of FA as a potential adjunctive therapy in the prevention and treatment of GU. Previous clinical studies have indicated that patients with GU generally exhibit lower levels of folate(Reference Tamura, Fujioka and Nasu37), and another case report has suggested that FA supplementation may accelerate ulcer healing and alleviate related symptoms(Reference Sharp, Insalaco and Johnson38). Building on this, our study employs MR to assess the impact of FA supplementation on the risk of GU development from a genetic perspective. The results demonstrate that FA supplementation significantly reduces the risk of developing GU, offering a novel perspective on the potential use of FA in the prevention and treatment of GU.
Current research, primarily conducted in animal models and in vitro studies, supports the potential of FA in GU prevention and treatment. First, FA may improve GU through antioxidant mechanisms and inhibition of gastric acid secretion. For example, in a study of indomethacin-induced GU, FA pretreatment significantly increased the levels of superoxide dismutase and mucus, effectively preventing the formation of GU by scavenging free radicals and protecting the mucosa(Reference Ajeigbe, Olaleye and Oladejo17). Second, FA may promote mucosal cell proliferation and angiogenesis, thereby accelerating ulcer healing. One study showed that FA supplementation enhanced the expression of angiogenic factors (e.g. EGF and VEGF) and the cell proliferation marker Ki-67, thereby reducing the severity of GU. Additionally, FA has shown potential in protecting the gastric mucosa from ethanol-induced acute damage through anti-inflammatory and anti-apoptotic mechanisms(Reference Zou, Cui and Xiang39). As an essential substrate for DNA methylation, FA may contribute to mucosal repair by promoting the coordinated methylation of ulcer-healing genes such as TFF2, PPARG and RUNX3 (Reference Hong, Oh and Jung40). However, the potential of FA supplementation to reduce the risk of GU still necessitates further basic research for validation.
The greatest strength of this paper lies in the use of MR, a genetic epidemiological design that is similar to randomised controlled trial(Reference Ference, Holmes and Smith27,Reference Gill, Walker and Martin41,Reference Thanassoulis and O’donnell42) . MR studies are advantageous in that they avoid reverse causality and minimise confounding factors, thereby leading to more reliable causal inferences(Reference Yuan and Larsson43,Reference Bouras, Karhunen and Gill44) . Furthermore, unlike randomised controlled trial that typically assess the effect of short-term treatment, MR studies can reflect the situation of lifetime exposure as the genetic variation is already fixed at the time of conception(Reference Labrecque and Swanson45). Another important advantage of MR studies is their large sample size. In this study, we used seven MR methods to estimate fully the association between FA supplementary therapy and GU in a large sample of more than 360 000 GU cases. The results of MR all seven methods showed that FA was a protective factor for GU, and these findings were statistically significant. Therefore, our work provides more substantial evidence for the current research, indicating that FA supplementary therapy can prevent the occurrence of GU.
Despite the encouraging findings from this study and other related research, it is important to acknowledge the limitations. This study is primarily based on European populations, and its findings may not be generalisable to other racial or ethnic groups, necessitating further validation. Given that our data are derived from European populations, it is important to consider the relationship between diet and FA deficiency within this demographic. Additionally, it is important to note that in MR studies, unmeasured confounders can still impact the stability of the findings. We also cannot entirely rule out the possibility that another condition influenced by FA could act as a confounder. Moreover, the long-term effects and safety of FA at different doses remain uncertain. Some studies have suggested that high doses of FA might cause gastric mucosal damage, leading to gastrointestinal discomfort and other adverse effects(Reference Selhub and Rosenberg46). Therefore, future research should focus on optimising FA dosing, evaluating its long-term effects and safety and assessing its efficacy in diverse populations. Additionally, the potential synergistic effects of FA in combination with existing therapies should be explored to develop more personalised treatment regimens.
In conclusion, FA has shown significant potential in the prevention and treatment of GU, but its precise mechanisms and clinical effects require further validation through high-quality clinical trials and basic research. Future studies should aim to optimise FA treatment protocols to enhance the quality of life and clinical outcomes for patients with GU.
Conclusions
In summary, this study has identified a causal association between FA supplementary therapy and a decreased risk of GU. This significant causal association sheds light on the potential benefits of FA supplementary therapy in managing GU. While there is a dearth of observational evidence, our research contributes to a deeper understanding of the impact of FA supplementary therapy on GU and may serve as a valuable guide for the dietary management of individuals with GU.
Acknowledgements
The authors thank all participants for their great support for UK-Biobank (https://gwas.mrcieu.ac.uk/).
This research was supported by the National Natural Science Foundation of China (81970470), the National Natural Science Foundation of China (82174131) and the National Natural Science Foundation of China (81600426).
Fuhao Li wrote the initial paper; Fuhao Li, Fengming Huang and Yulong Tang analysed and interpreted data; Fuhao Li and Fengming Huang prepared the figures and tables; Bin Lv, Fan Zhang and Hao Jiang revised and reviewed the manuscript. Bin Lv and Jun Chen conceived idea of the study, supervised overall work, reviewed and revised final draft. Bin Lv had primary responsibility for final content. All authors read and approved the final manuscript.
The authors declare no conflicts of interest.
The original contributions presented in the study are included in the article/Supplementary table 1, and further inquiries can be directed to the corresponding authors.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114524002368










