INTRODUCTION
Methicillin-resistant Staphylococcus aureus (MRSA) is a serious public health concern and an economic burden to national healthcare systems [Reference Seybold1, Reference Rubin2]. Various MRSA lineages have emerged in animals over the last 5 years [Reference Weese and van Duijkeren3]. In pigs, two novel MRSA lineages belonging to multi-locus sequence types ST398 and ST9 were first reported in The Netherlands in 2005 [Reference Voss4] and in Hong Kong [Reference Guardabassi5] and China [Reference Wagenaar6] in 2009. Contact with pigs has been shown to be a risk factor for human carriage of ST398 and ST9 [Reference Lewis7–Reference Cui11]. These two MRSA lineages are regarded as emerging zoonotic agents associated with pig farming but display a different geographical distribution. ST398 is the most prevalent porcine MRSA lineage in Europe [12], Canada [Reference Khanna13] and in mid-western USA [Reference Smith14], whereas ST9 is widespread in Asia [Reference Guardabassi5, Reference Wagenaar6, Reference Neela10, Reference Cui11].
Current knowledge of transmission of livestock-associated MRSA (LA-MRSA) is based on field epidemiological studies of ST398. This sequence type is thought to be transmitted through the pig production chain from breeders to finishing farms through purchase of MRSA-positive animals [Reference van Duijkeren15]. The farm environment seems to play an important role in the transmission of MRSA ST398. Dust and air samples taken from pig pens have been shown to harbour MRSA, including ST398 [Reference Van den Broek16, Reference Harper17]. MRSA-negative pigs kept in a MRSA-positive environment have been shown to have an increased risk of acquiring MRSA [Reference Broens18]. Recently, rodents have been identified as possible reservoirs for transmission of MRSA ST398 on pig farms [Reference van de Giessen19]. Little information is available on the transmission dynamics of ST9 within the pig population, since its occurrence in pigs was first described in 2009.
The primary objective of this study was to develop a model to investigate colonization and transmission of LA-MRSA under controlled conditions. Two experimental models were used for this purpose: a nasal-gastrointestinal inoculation model and a sow vaginal inoculation model. As a secondary objective, the colonization properties of four strains belonging to ST398 and ST9 were compared in the two models.
MATERIALS AND METHODS
Bacterial strains and inoculum
Four genetically distinct MRSA strains isolated from the nasal cavity of healthy pigs were used to prepare the inoculum. The four strains differed with respect to multi-locus sequence type (ST398, ST9), spa type (t08, t011, t034, t899) and antimicrobial susceptibility profile (Table 1). For each strain, colonies from an overnight blood agar culture were suspended in saline and the density of the suspension was adjusted to the turbidity of a 0·5 MacFarland standard (about 1·5×108 c.f.u./ml) using a nephelometer (Sensititre, Trek Diagnostic Systems, UK). Equal volumes of the individual strain suspensions were then mixed together to obtain the MRSA inoculation mix.
Table 1. Strains used in the in vivo colonization experiment
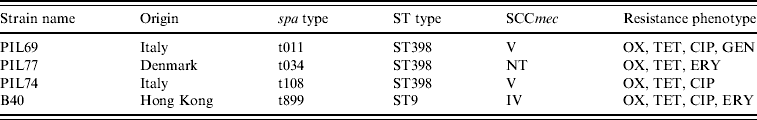
OX, Oxacillin; TET, tetracycline; CIP, ciprofloxacin; ERY, erythromycin; GEN, gentamicin.
Nasal-gastrointestinal inoculation model
Six MRSA-negative, 5-week-old Danish Landrace piglets with starting weights ranging from 8 to 9 kg were purchased from a specific-pathogen-free (SPF) farm in Denmark. The carriage status of each piglet was tested at the farms and confirmed upon arrival at the animal facility. All animals were housed together in a single pen. After 1 week's adaptation, ~800 μl of the MRSA mix was sprayed into each nostril. Thereafter, an endogastric tube, inserted into the stomach via the mouth, was used to inoculate 10 ml of the same MRSA mix. In order to enhance colonization, a standard 7-day tetracycline therapeutic regimen [Terramycin® Vet, Orion Pharma, Denmark, 25 mg/kg body weight (BW)] was given mixed with feed (Fig. 1). Nasal and rectal swabs were collected on days 2, 9, 16, and 23 post-inoculation (Fig. 1). Swabs were enriched in Mueller–Hinton broth (MHB) containing oxacillin (4 μg/ml) and tetracycline (16 μg/ml) for selection of the inoculation strains and azetronam (50 μg/ml) for inhibition of Gram-negative bacteria. Following overnight incubation at 37°C, 10 μl of the enrichment was plated on oxacillin resistance screening agar base (ORSAB, Oxoid, UK) containing tetracycline (16 μg/ml) and a combination of ciprofloxacin (4 μg/ml), erythromycin (8 μg/ml) and/or gentamicin (8 μg/ml) to enable detection of the different strains based on their susceptibility profiles (Table 1). Two denim blue colonies per plate were subcultured on 5% blood agar and strains exhibiting S. aureus colony morphology were confirmed to be MRSA by mecA PCR [Reference Zhang20] and characterized by spa typing [Reference Harmsen21] and pulsed-field gel electrophoresis (PFGE) [Reference Murchan22]. MRSA colonization was defined as four consecutive positive cultures over 4 weeks.
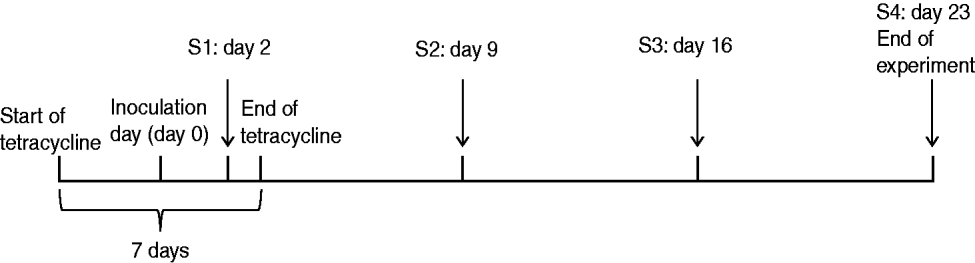
Fig. 1. Nasal inoculation model. Experimental design and sampling times (S1–S4).
Sow vaginal inoculation model
A MRSA-negative 97-day pregnant Yorkshire sow from the same SPF farm supplying animals in the previous experiment was intra-vaginally inoculated with the MRSA mix over three consecutive days (Fig. 2). First, 10 ml of the MRSA cocktail was flushed into the vagina. Thereafter, a tampon was inserted into the vagina and left for up to 6 h to allow the bacteria to be present in the vaginal cavity for a longer period of time. Tetracycline (Terramycin® Vet) mixed with pig feed (25 mg/kg BW) was administered for 18 days until date of farrowing. The partum resulted in seven piglets. Swab samples were taken from the sow's nose, teats, inner vagina and rectum, together with samples taken from the nose, mouth, rectum and a 5 cm×5 cm area of the skin of each of the seven piglets on days 0 (farrowing day), 7, 14, 21 and 28 (end of experiment). A swab was also taken from the inner placenta immediately after farrowing. Samples were cultured and bacterial isolates were characterized as described for the previous experiment. Similar to the nasal-gastrointestinal inoculation model, MRSA colonization was defined as four consecutive positive cultures over 4 weeks.
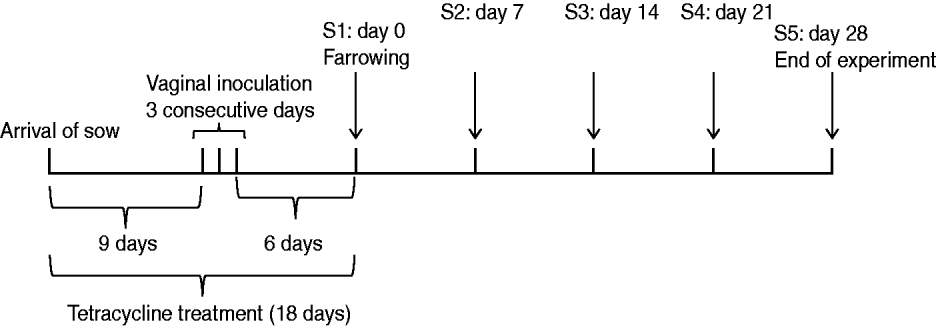
Fig. 2. Sow vaginal inoculation model. Experimental design and sampling times (S1–S5).
In vitro growth competition studies
Two experiments were performed to determine if the four strains used in the MRSA inoculum had antagonistic effects on each other. In the first experiment, equal quantities (103 c.f.u./ml) of each strain were grown in the enrichment broth used for the two in vivo experiments. At 1-h intervals, 100 μl of culture was serially diluted and plated on the different selective agar plates to measure the concentration (c.f.u./ml) of each strain. The resulting viable counts were used to generate growth curves. A second experiment was conducted to determine if any of the strains produced bacteriocins that could inhibit the growth of the other strains. A 50-μl volume of supernatant derived from a centrifuged overnight culture of each strain was spotted onto an agar plate previously inoculated with another strain. All strain combinations were tested. After overnight incubation at 37°C, plates were examined for the presence of inhibition zones indicating bacteriocin production.
Statistical analyses
The frequency of PIL69 and B40 at different body sites was compared using the z test for paired proportions. To assess if there was any difference between the growth curves, MRSA counts for the four strains were compared using Poisson regression taking into account the repeated structure of the data. This analysis was performed using the genmod procedure in SAS v. 9.2 (SAS Inc., USA).
Safety and ethical issues
Animals were housed in a level-2 isolation unit at the Faculty of Life Sciences, University of Copenhagen. Procedures used in the animal experiments were performed in compliance with the Animals Scientific Act, and approved by the Danish National Animal Experiment Inspectorate (License no. 2006/561-1141). The health status of the pigs was monitored twice a day. After completion of the experiments, all animals were euthanized by captive bolt pistol insensibilization and bleeding.
RESULTS
Nasal-gastrointestinal inoculation model
All piglets were sampled in the nose and rectum at the farm prior to arrival at the animal facility, and were MRSA-negative. Two days after inoculation (S1), all nasal swabs and 3/6 rectal swabs were positive for MRSA. On day 9 (S2), only one piglet was positive in the nose. All animals were negative after 16 days (S3). On day 22 (S4), four piglets were positive in the nose only, and included the positive piglet from S2. According to the predefined criteria for colonization, none of the piglets were successfully colonized.
Sow vaginal inoculation model
The sow was sampled from the nose and vagina on the farm prior to arrival at the animal facility and was MRSA-negative. After farrowing, MRSA was isolated from the nose, inner vagina, rectum and teats on all sampling days. A sample taken from the placenta immediately after farrowing was also positive for MRSA. Samples taken after farrowing from the nose, vagina, rectum and teats of the sow were MRSA-positive. All newborn piglets were positive in all sampled body sites except on days 14 and 28. On day 14, only 3/7 piglets were positive on the skin and 6/7 in the rectum. On day 28, only 6/7 piglets were positive in the rectum. All newborn piglets were successfully colonized according to the predefined criteria for colonization.
Strain typing
Based on spa typing, only ST398-t011 (PIL69) was detected in the nasal inoculation model. In the sow vaginal inoculation model, both ST398-t011 and ST9-t899 (B40) were detected. These two strains were also detected in swabs taken from the placenta. PIL77 (t034-ST398) and PIL74 (t108-ST398) were not detected in either models. In 80% of MRSA-positive samples, both PIL69 and B40 were isolated. However, PIL69 was more commonly isolated from rectal samples (23/28) than B40 (5/28), P<0·001 (Table 2). PFGE analysis confirmed that the band patterns of t011 and t899 isolates obtained during the experiment were identical to those of the inoculated strains PIL69 and B40, respectively (data not shown).
Table 2. Distribution of PIL69 (ST398, spa type t011) and B40 (ST9, spa type t899) in the different sampled body sites of the seven piglets obtained by the sow vaginal inoculation model

* For each body site the total number of MRSA-positive samples is indicated in parentheses.
Assessment of antagonistic effects between strains
No statistically significant difference (P=0·89) was observed when comparing the growth curves of the four strains (Fig. 3), indicating no competition between strains during growth. No inhibition zones, indicating bacteriocin production, were observed when supernatant from one strain culture was spotted onto plates previously inoculated with another strain.
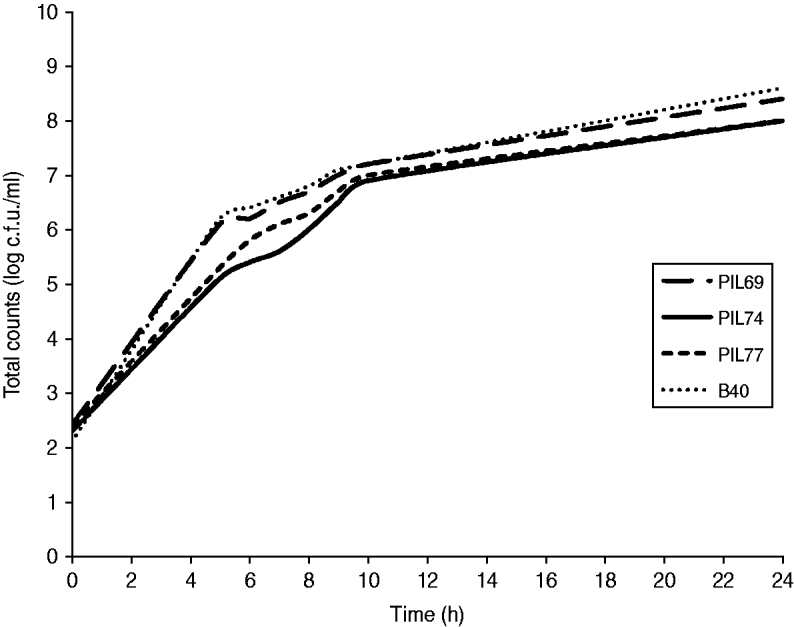
Fig. 3. Competitive growth of the four strains in Mueller–Hinton broth containing oxacillin (4 μg/ml), tetracycline (16 μg/ml), and azetronam (50 μg/ml). PIL69 (ST398-t011), PIL74 (ST398-t108), PIL77 (ST398-t034), and B40 (ST9-t899).
DISCUSSION
Vertical perinatal transmission of MRSA was demonstrated under controlled experimental conditions. All newborn piglets were naturally colonized with MRSA following artificial colonization of the sow's vagina and their carriage status was stable over the 28 days of the experiment, suggesting that MRSA can efficiently be transmitted from sows to their progeny. As this result was based on a single sow/observation, further epidemiological investigation is warranted to assess the importance of this route of MRSA transmission in pig farming. On the contrary, direct nasal and gastrointestinal inoculation of 5-week-old piglets did not result in persistent carriage. This result indicates that experimental colonization is difficult in pigs at this age, possibly due to the already established microbial flora exerting an antagonistic effect on the ‘invading’ MRSA. Light et al. [Reference Light23] showed that in humans, active colonization with a non-pathogenic S. aureus prevented colonization and subsequent infections with virulent strains. Similar results were demonstrated by Allaker et al. [Reference Allaker, Lloyd and Smith24] for S. hyicus in gnotobiotic piglets. Bacterial interference between S. aureus and Streptococcus pneumoniae in children may explain why children are less likely to be persistent carriers of S. aureus in early infancy [Reference Regev-Yochay25, Reference Lebon26]. Failure of the nasal-gastrointestinal inoculation method could also be related to inherent variability in host susceptibility to MRSA colonization, since our experiment was conducted on a small number of genetically related animals. It is also possible that unknown bacterial or environmental factors required to facilitate natural colonization were not provided in the artificial inoculation experiment, illustrating that S. aureus colonization is a complex phenomenon requiring an optimal fit between the host, bacteria and the environment.
In humans, there are two types of carriage status based on rate of nasal elimination and anti-staphylococcal antibody profiles: persistent carriers and others (non-carriers and intermittent carriers) [Reference van Belkum27]. It remains to be fully elucidated why some individuals are resistant to colonization. To date, polymorphisms in at least four human genes that play a role in the immune system have been associated with nasal S. aureus carriage [Reference van den Akker28, Reference Emonts29]. Similar to humans, pig host factors such as breed, sex and other immune factors could influence S. aureus colonization. The effect of pig breed on S. aureus colonization may be difficult to assess due to the widespread use of cross-breeding in intensive pig production. In Denmark, intensively reared pigs are cross-bred between three main breeds (Danish Landrace, Yorkshire, Duroc) to maximize heterosis and increase productivity. Studies are underway to investigate the anti-staphylococcal antibody profiles in sera from MRSA-negative and MRSA-positive pigs.
All pigs were treated with tetracycline to enhance colonization. This was done because the inoculated strains were resistant to this antibiotic. Colonization failed in the nasal-gastrointestinal inoculation model despite treatment with tetracycline. However, the role of tetracycline in MRSA colonization cannot be assessed with the current study design. Weese et al. [Reference Weese30] recently demonstrated under field conditions that colonized piglets could be obtained even from apparently MRSA-negative sows in the absence of antimicrobial use. However, since MRSA colonization in sows was assessed by culture of nasal swabs, it cannot be excluded that they may have been positive in other body sites. The present study shows that MRSA can also be present on other sow body sites including teats, providing a potential route of transmission to piglets during feeding. Shortly after intra-vaginal MRSA inoculation, the sow became positive in various other body sites, highlighting the important role of environmental contamination in the dissemination of MRSA. The environment could also be a potential source for MRSA acquisition by the piglets.
The four strains (PIL69, PIL74, PIL77, B40) used in the colonization models represent the most common spa types among MRSA ST398 (t011, t108, t034) and MRSA ST9 (t899). The results obtained by both models suggest that PIL69 (t011) could be a better colonizer than PIL74 (t108) and PIL77 (t034). This observation is of potential interest since t011 is by far the predominant spa type in both breeding and production holdings in the European Union [31]. However, comparison of larger number of isolates belonging to these three spa types would be needed to assess whether the increased colonization properties of PIL69 are strain-specific or generalized to other strains having this spa type. Limited to the sow vaginal inoculation model, B40 (ST9-t899) appeared be a good nasal and skin colonizer together with PIL69. However, while gastrointestinal carriage of B40 could not be detected 7 days after birth, PIL69 was isolated from rectal swabs throughout the entire duration of the experiment. No antagonistic effects between the strains were observed in the in vitro competition experiments, indicating that failure to detect PIL74 and PIL77 was not a result of suppression by PIL69 or B40. PIL74 and PIL77 appeared to have a slightly slower growth rate than PIL69 and B40, although this could not be determined statistically. Therefore, PIL74 and PIL77 could have been outgrown during enrichment by PIL69 or B40 due to a lower concentration of these strains in the initial sample or slower growth in the enrichment broth.
Animal models are useful to study factors that influence nasal colonization as both bacterial and host colonization factors can be evaluated under controlled conditions. Prior to this study, only two animal models have been described for studying nasal S. aureus colonization. Kiser et al. [Reference Kiser, Cantey-Kiser and Lee32] and Kokai-Kun et al. [Reference Kokai-Kun33] have developed murine S. aureus nasal colonization models. Such models require anaesthetization of the animals prior to inoculation and in one model, long-term nasal carriage was only obtained after antibiotic treatment [Reference Kokai-Kun33]. Furthermore, González-Zorn et al. [Reference González-Zorn34] showed that S. aureus did not grow and multiply in the nasal cavity of mice, illustrating that S. aureus is not a natural colonizer of mice and that this animal is not an ideal model for human S. aureus colonization. The natural occurrence of S. aureus in pigs and the anatomical and physiological similarity between porcine and human skin [Reference Vodicka35] suggest that the pig could be a useful model for studying S. aureus colonization in humans.
By using the vaginal inoculation model described in this study, naturally MRSA-colonized piglets can be easily obtained by implantation of MRSA in the vagina shortly before farrowing. No invasive procedures such as anaesthetization are required. As such the model could represent a valuable tool to investigate MRSA–host interaction during colonization and to test the in vivo efficacy of MRSA decolonization and environmental decontamination strategies.
ACKNOWLEDGEMENTS
The study was supported by the EU FP7 PILGRIM project (Project no. 223050).
DECLARATION OF INTEREST
None.







