Introduction
The first episode of a psychotic disorder is usually preceded by a prodromal period characterized by a progressive decline in functioning and the emergence of attenuated psychotic symptoms. Individuals with these clinical features are said to be at ‘ultra-high risk’ (UHR) for psychosis because 18–36% of them will develop a psychotic disorder within 3 years (Fusar-Poli et al. Reference Fusar-Poli, Bonoldi, Yung, Borgwardt, Kempton, Valmaggia, Barale, Caverzasi and McGuire2012). Early clinical intervention in the UHR population may reduce the risk of later transition to psychosis and improve long-term clinical and functional outcome (McGorry et al. Reference McGorry, Nelson, Amminger, Bechdolf, Francey, Berger, Riecher-Rössler, Klosterkotter, Ruhrmann, Schultze-Lutter, Nordentoft, Hickie, McGuire, Berk, Chen, Keshavan and Yung2009); however, at present it is difficult to predict based on first clinical presentation which UHR individual who will and will not go on to develop psychosis. Recent work has sought to examine if neuroimaging can be used to identify individuals at UHR and predict who will subsequently make transition to psychosis within this population (Koutsouleris et al. Reference Koutsouleris, Meisenzahl, Davatzikos, Bottlender, Frodl, Scheuerecker, Schmitt, Zetzsche, Decker, Reiser, Moller and Gaser2009).
Studies using voxel-based morphometry (VBM) have examined whether UHR subjects are affected by neuroanatomical abnormalities. Cross-sectional studies indicate that, relative to healthy controls, UHR subjects have reduced grey matter (GM) volume in frontal (Meisenzahl et al. Reference Meisenzahl, Koutsouleris, Gaser, Bottlender, Schmitt, McGuire, Decker, Burgermeister, Born, Reiser and Möller2008; Mechelli et al. Reference Mechelli, Riecher-Rössler, Meisenzahl, Tognin, Wood, Borgwardt, Koutsouleris, Yung, Stone, Phillips, McGorry, Valli, Velakoulis, Woolley, Pantelis and McGuire2011), lateral and medial temporal regions (Meisenzahl et al. Reference Meisenzahl, Koutsouleris, Gaser, Bottlender, Schmitt, McGuire, Decker, Burgermeister, Born, Reiser and Möller2008). Studies that used a region of interest (ROI) approach reported GM volume increases (Buehlmann et al. Reference Buehlmann, Berger, Aston, Gschwandtner, Pflueger, Borgwardt, Radue and Riecher-Rössler2010) but also reductions (Phillips et al. Reference Phillips, Velakoulis, Pantelis, Wood, Yuen, Yung, Desmond, Brewer and McGorry2002) in the hippocampus, reductions in the planum polare/temporale, insula and superior temporal gyrus (Takahashi et al. Reference Takahashi, Wood, Yung, Phillips, Soulsby, McGorry, Tanino, Zhou, Suzuki, Velakoulis and Pantelis2009, Reference Takahashi, Wood, Yung, Walterfang, Phillips, Soulsby, Kawasaki, McGorry, Suzuki, Velakoulis and Pantelis2010), increases in the pituitary volume (Büschlen et al. Reference Büschlen, Berger, Borgwardt, Aston, Gschwandtner, Pflueger, Kuster, Radü, Stieglitz and Riecher-Rössler2011), and reductions in the anterior cingulate cortex (ACC) (Röthlisberger et al. Reference Röthlisberger, Riecher-Rössler, Aston, Fusar-Poli, Radü and Borgwardt2012) in the UHR group compared with healthy controls.
In VBM studies, relative to UHR subjects who did not develop psychosis (UHR-NT), those who later became psychotic (UHR-T) had reduced volumes in the inferior frontal cortex, medial and lateral temporal cortex, ACC (Pantelis et al. Reference Pantelis, Velakoulis, McGorry, Wood, Suckling, Phillips, Yung, Bullmore, Brewer, Soulsby, Desmond and McGuire2003), insular, inferior and superior frontal cortex (Borgwardt et al. Reference Borgwardt, McGuire, Aston, Gschwandtner, Pflüger, Stieglitz, Radue and Riecher-Rössler2008). ROI studies suggest that later transition to psychosis is associated with reduced volume in the left parahippocampal gyrus (Mechelli et al. Reference Mechelli, Riecher-Rössler, Meisenzahl, Tognin, Wood, Borgwardt, Koutsouleris, Yung, Stone, Phillips, McGorry, Valli, Velakoulis, Woolley, Pantelis and McGuire2011), the insula bilaterally (Takahashi et al. Reference Takahashi, Wood, Yung, Phillips, Soulsby, McGorry, Tanino, Zhou, Suzuki, Velakoulis and Pantelis2009), the left ACC (Röthlisberger et al. Reference Röthlisberger, Riecher-Rössler, Aston, Fusar-Poli, Radü and Borgwardt2012), and with increased pituitary (Büschlen et al. Reference Büschlen, Berger, Borgwardt, Aston, Gschwandtner, Pflueger, Kuster, Radü, Stieglitz and Riecher-Rössler2011) and hippocampus (Buehlmann et al. Reference Buehlmann, Berger, Aston, Gschwandtner, Pflueger, Borgwardt, Radue and Riecher-Rössler2010) volumes.
Some ROI studies did not find any significant differences between UHR subjects and healthy controls (Wood et al. Reference Wood, Yucel, Velakoulis, Phillips, Yung, Brewer, McGorry and Pantelis2005; Velakoulis et al. Reference Velakoulis, Wood, Wong, McGorry, Yung, Phillips, Smith, Brewer, Proffitt, Desmond and Pantelis2006) or between UHR-T and UHR-NT individuals (Yucel et al. Reference Yucel, Wood, Phillips, Stuart, Smith, Yung, Velakoulis, McGorry and Pantelis2003; Velakoulis et al. Reference Velakoulis, Wood, Wong, McGorry, Yung, Phillips, Smith, Brewer, Proffitt, Desmond and Pantelis2006). Nevertheless, evidence that there may be volumetric differences between the latter subgroups raises the possibility that neuroimaging measures might be able to facilitate the prediction of clinical outcome in UHR subjects.
The vast majority of structural neuroimaging studies in UHR individuals have focused on GM volume. However, neuroanatomical alterations in psychosis may be expressed not only in terms of GM volume but also as subtle changes in cortical thickness. While the analysis of GM volume returns a mixed measure that depends on local cortical thickness as well as cortical folding and gyrification (i.e. cortical surface area), the analysis of cortical thickness is considered to specifically target the presence of cortical atrophy (Hutton et al. Reference Hutton, Draganski, Ashburner and Weiskopf2009). Therefore the two approaches provide complementary information and one can be more sensitive than the other depending on the process underlying neuroanatomical changes. Cortical thickness in the human brain can be examined using the automated data analysis of T1-weighted images (Hutton et al. Reference Hutton, De Vita, Ashburner, Deichmann and Turner2008). Application of this approach in patients with first-episode and established schizophrenia suggests that there is a cortical thinning in the anterior cingulate (Narr et al. Reference Narr, Bilder, Toga, Woods, Rex, Szeszko, Robinson, Sevy, Gunduz-Bruce, Wang, DeLuca and Thompson2005a ; Fornito et al. Reference Fornito, Yucel, Wood, Adamson, Velakoulis, Saling, McGorry and Pantelis2008a ; Schultz et al. Reference Schultz, Koch, Wagner, Roebel, Schachtzabel, Gaser, Nenadic, Reichenbach, Sauer and Schlosser2010b ), prefrontal (Narr et al. Reference Narr, Bilder, Toga, Woods, Rex, Szeszko, Robinson, Sevy, Gunduz-Bruce, Wang, DeLuca and Thompson2005a ; Venkatasubramanian et al. Reference Venkatasubramanian, Jayakumar, Gangadhar and Keshavan2008; Schultz et al. Reference Schultz, Koch, Wagner, Roebel, Schachtzabel, Gaser, Nenadic, Reichenbach, Sauer and Schlosser2010b ), temporal (Narr et al. Reference Narr, Bilder, Toga, Woods, Rex, Szeszko, Robinson, Sevy, Gunduz-Bruce, Wang, DeLuca and Thompson2005a ; Fornito et al. Reference Fornito, Yucel, Wood, Adamson, Velakoulis, Saling, McGorry and Pantelis2008a ) and occipital (Narr et al. Reference Narr, Toga, Szeszko, Thompson, Woods, Robinson, Sevy, Wang, Schrock and Bilder2005b ) cortices. In UHR subjects, reduced cortical thickness has been reported in the prefrontal, ACC, inferior parietal, superior temporal and parahippocampal cortices compared with healthy controls (Jung et al. Reference Jung, Kim, Jang, Choi, Jung, Park, Han, Choi, Kang, Chung and Kwon2011). One study has reported that in UHR individuals who subsequently developed psychosis the ACC was thinner than in UHR-NT (Fornito et al. Reference Fornito, Yung, Wood, Phillips, Nelson, Cotton, Velakoulis, McGorry, Pantelis and Yucel2008b ) and one has reported a progressive thinning in the anterior cingulate, precuneus and temporo-parietal-occipital cortex compared with UHR-NT and healthy controls (Ziermans et al. Reference Ziermans, Schothorst, Schnack, Koolschijn, Kahn, van Engeland and Durston2012). Another study did not find differences in cortical thickness between individuals at UHR, patients with a first psychotic episode and healthy controls, but the groups differed in cortical thickness asymmetry (Haller et al. Reference Haller, Borgwardt, Schindler, Aston, Radue and Riecher-Rössler2009).
The findings from studies of cortical thickness in UHR subjects have thus been inconclusive, which may reflect the relatively small sample sizes examined to date. UHR subjects are difficult to recruit and it is therefore a significant challenge for any single centre to scan a large sample. Multi-centre studies provide a means of addressing this issue with the pooling of data from different sites to produce relatively large samples.
We adopted this approach in the present study. Our aim was to combine magnetic resonance imaging (MRI) data from multiple centres and measure cortical thickness in large samples of UHR subjects and healthy controls. We also sought to compare cortical thickness in UHR subjects who subsequently did or did not developed psychosis. Whole-brain MRIs were acquired from individuals at UHR for psychosis and healthy controls at four psychiatric research centres in London, Basel, Munich and Melbourne. At each site, subjects were scanned at first clinical presentation, and followed up clinically or in the context of research projects for at least 2 years, so that they could be subcategorized according to psychosis outcome. The MRI data from each site were combined to form a large UHR sample, which was subdivided into subjects who later had developed psychosis and subjects who had not. The thickness of the cerebral cortex was assessed using a voxel-based cortical thickness (VBCT) approach that generates maps from MRI data in which each voxel in the GM is assigned a thickness value and regionally specific differences are compared on a voxel-by-voxel basis (Hutton et al. Reference Hutton, De Vita, Ashburner, Deichmann and Turner2008, Reference Hutton, Draganski, Ashburner and Weiskopf2009). This method differs from others reported in the literature, as it does not require the construction of a three-dimensional model for extracting cortical thickness values. For instance, surface-based techniques involve the generation of surface models that are driven by image information and surface geometry to fit the GM and white matter (WM) surfaces of the image (Fischl & Dale, Reference Fischl and Dale2000; Hutton et al. Reference Hutton, De Vita, Ashburner, Deichmann and Turner2008). Cortical thickness is subsequently defined at surface points and is computed based on the measure of the distance between them. Another approach involves extracting only the surface between the GM and the WM and then mapping towards the surface the thickness values that are derived by calculating the distance between voxels in the cortex and the surface (Lerch & Evans, Reference Lerch and Evans2005; Hutton et al. Reference Hutton, De Vita, Ashburner, Deichmann and Turner2008). In contrast, using the VBCT technique, GM and WM boundaries are defined on the basis of whole voxel information (Jones et al. Reference Jones, Buchbinder and Aharon2000; Hutton et al. Reference Hutton, De Vita, Ashburner, Deichmann and Turner2008) and cortical thickness is calculated at every volumetric point within the cortex and based on the length of the trajectory from one boundary to another (Hutton et al. Reference Hutton, De Vita, Ashburner, Deichmann and Turner2008).
Our first prediction, based on data from previous studies (Narr et al. Reference Narr, Bilder, Toga, Woods, Rex, Szeszko, Robinson, Sevy, Gunduz-Bruce, Wang, DeLuca and Thompson2005a ; Fornito et al. Reference Fornito, Yucel, Wood, Adamson, Velakoulis, Saling, McGorry and Pantelis2008a ,Reference Fornito, Yung, Wood, Phillips, Nelson, Cotton, Velakoulis, McGorry, Pantelis and Yucel b ; Schultz et al. Reference Schultz, Koch, Wagner, Roebel, Schachtzabel, Gaser, Nenadic, Reichenbach, Sauer and Schlosser2010b ; Jung et al. Reference Jung, Kim, Jang, Choi, Jung, Park, Han, Choi, Kang, Chung and Kwon2011; Ziermans et al. Reference Ziermans, Schothorst, Schnack, Koolschijn, Kahn, van Engeland and Durston2012), was that the UHR group as a whole would show differences in cortical thickness relative to controls in areas that have previously been identified in studies of cortical thickness in UHR subjects and patients with first-episode psychosis: the frontal, anterior cingulate, parahippocampal, temporal, parietal cortices and the precuneus. Our final prediction was that within the UHR sample, subjects who developed psychosis subsequent to scanning would show more pronounced cortical thickness abnormalities in these regions (Narr et al. Reference Narr, Bilder, Toga, Woods, Rex, Szeszko, Robinson, Sevy, Gunduz-Bruce, Wang, DeLuca and Thompson2005a ; Fornito et al. Reference Fornito, Yucel, Wood, Adamson, Velakoulis, Saling, McGorry and Pantelis2008a ,Reference Fornito, Yung, Wood, Phillips, Nelson, Cotton, Velakoulis, McGorry, Pantelis and Yucel b ; Schultz et al. Reference Schultz, Koch, Wagner, Roebel, Schachtzabel, Gaser, Nenadic, Reichenbach, Sauer and Schlosser2010b ; Jung et al. Reference Jung, Kim, Jang, Choi, Jung, Park, Han, Choi, Kang, Chung and Kwon2011; Ziermans et al. Reference Ziermans, Schothorst, Schnack, Koolschijn, Kahn, van Engeland and Durston2012) than those who did not.
Method
Sample
All the UHR subjects were recruited from specialized clinical services for this group in London [Outreach and Support in South London (OASIS)], Basel [Clinic for Early Detection of Psychosis (FEPSY) at the Psychiatric Outpatient Department, University Hospital], Melbourne [Personal Assessment and Crisis Evaluation (PACE) Clinic] and Munich [Early Detection and Intervention Centre for Mental Crisis (FETZ), Department of Psychiatry and Psychotherapy, Ludwig-Maximilians-University]. Criteria used to identify participants at UHR for psychosis were comparable across the different sites as reported in Supplementary Table S2. Specifically, the London and Melbourne sites used as a screening instrument the Comprehensive Assessment for at Risk Mental State (Yung et al. Reference Yung, Yuen, McGorry, Phillips, Kelly, Dell'Olio, Francey, Cosgrave, Killackey, Stanford, Godfrey and Buckby2005); the Basel site used as a screening assessment the Basel Screening Instrument for Psychosis (Riecher-Rössler et al. Reference Riecher-Rössler, Aston, Ventura, Merlo, Borgwardt, Gschwandtner and Stieglitz2008); and the Munich site used the Bonn Scale for the Assessment of Basic Symptoms (Gross et al. Reference Gross, Huber and Klosterkotter1987). In addition they also assessed attenuated psychotic symptoms and brief limited intermittent psychotic symptoms as defined by the PACE criteria (Yung et al. Reference Yung, Yuen, McGorry, Phillips, Kelly, Dell'Olio, Francey, Cosgrave, Killackey, Stanford, Godfrey and Buckby2005). Data were combined from these four sites. This sample overlaps with a multi-centre sample that was previously used to investigate GM volume in UHR subjects (Mechelli et al. Reference Mechelli, Riecher-Rössler, Meisenzahl, Tognin, Wood, Borgwardt, Koutsouleris, Yung, Stone, Phillips, McGorry, Valli, Velakoulis, Woolley, Pantelis and McGuire2011), and includes subjects that participated in previous single-centre studies of GM volume in this population. In total there were MRI data from 167 UHR subjects. MRI data acquired from healthy volunteers from the same geographical area as the UHR subjects at each site were combined to form a control dataset. Healthy controls were excluded if there was a past or present personal or familiar history of neurological and/or psychiatric conditions. The total control sample comprised 150 subjects, and was comparable with the total UHR sample with respect to gender, age and ethnicity (Table 1).
Table 1. Sociodemographic data of the study samples
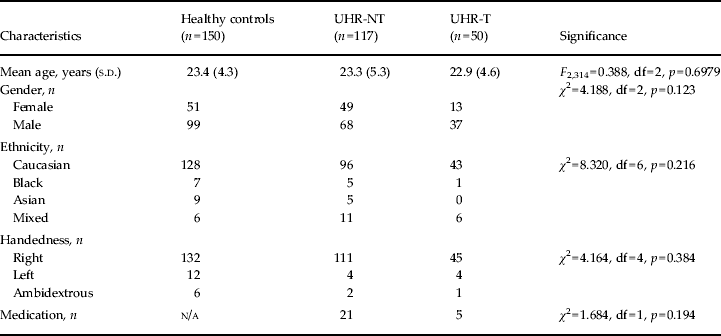
UHR-NT, Ultra-high risk without disease transition; UHR-T, UHR with disease transition; s.d., standard deviation; df, degrees of freedom; n/a, not applicable.
Subsequent to MRI scanning, the UHR subjects were followed up and assessed regularly for at least 2 years at all four sites. UHR subjects who developed a first episode of psychosis during this period were identified using standardized transition criteria (McGorry et al. Reference McGorry, Yung and Phillips2003; Yung et al. Reference Yung, Phillips, Yuen and McGorry2004). Each site used this information to subdivide their UHR sample into a group that had made a transition to psychosis (UHR-T), and a group that had not (UHR-NT). In the following 30.6 (s.d. = 10.4) months, 50 (30%) of the UHR individuals developed psychosis (UHR-T) and 117 did not (UHR-NT). Transition to psychosis during the follow-up period was established according to Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) criteria based on clinical consensus between at least two experienced psychiatrists. Most of the UHR group (147/167, 84%) had never taken antipsychotics at time of scanning; 20 (12%) had been exposed to antipsychotics; the mean antipsychotic medication exposure time was 7.5 (s.d. = 11.1) weeks. UHR participants that were receiving medication were scanned at the London or Basel sites. Inclusion and exclusion criteria for the two experimental groups, clinical characteristics and sociodemographic of the UHR subjects are reported in the Supplementary material (Supplementary Tables S1–3, S5–8).
Image acquisition
At all four sites, volumetric MR images were acquired using scanner field strengths of 1.5 T and a T1-weighted protocol. Two sites used General Electric scanners (i.e. London and Melbourne) and two used Siemens scanners (i.e. Basel and Munich). The details of the image acquisition sequence are reported in Supplementary Table S4.
Data analysis
Sociodemographics
Sociodemographic differences between groups were examined using one-way analysis of variance for parametric data, and by χ2 test for non-parametric data, as implemented in SPSS 19.0 for Windows (IBM, USA) (Table 1, Supplementary Tables S5–8).
Pre-processing
Pre-processing for the analysis of cortical thickness was carried out using the procedure described by Hutton et al. (Reference Hutton, De Vita, Ashburner, Deichmann and Turner2008, Reference Hutton, Draganski, Ashburner and Weiskopf2009). In brief, all the images were visually checked and re-sampled to a voxel size of 1 mm3 using trilinear interpolation. Using the unified segmentation procedure implemented in SPM8 (http://www.fil.ion.ucl.ac.uk/spm), the images were segmented into GM, WM and cerebrospinal fluid (CSF) (Ashburner, Reference Ashburner2007). For each subject, this resulted in a set of three images in the same space as the original T1-weighted image, in which each voxel was assigned a probability of being GM, WM and CSF, respectively. A VBCT map was created for each subject using the GM, WM and CSF segments created in the previous step (Hutton et al. Reference Hutton, De Vita, Ashburner, Deichmann and Turner2008). This method is implemented as a toolbox for SPM (Hutton et al. Reference Hutton, De Vita, Ashburner, Deichmann and Turner2008, Reference Hutton, Draganski, Ashburner and Weiskopf2009). In brief, it uses the input tissue probability maps and a transformed labelled brain atlas (http://www.fil.ion.ucl.ac.uk/spm/ext/~IBASPM). Starting from the initial estimate of the GM/WM boundary, layers of one voxel in thickness are successively added to surround the WM allowing voxels to be identified where the GM from different sides of a sulcus was in contact. Once all GM voxels have been processed in this way, Laplace's equation is solved for all voxels between the final GM/WM and GM/CSF boundaries resulting in a scalar field that makes a smooth transition from one boundary to the other. The gradient of this field at each point forms a unique trajectory connecting the two boundaries, and the thickness at each point is calculated by integrating along these trajectories. The resulting VBCT maps are created in the space of the original input images which contain cortical thickness values within voxels identified as cortical GM and zeros outside the cortex. dartel (Ashburner, Reference Ashburner2007), an algorithm for diffeomorphic image registration which is implemented as a toolbox for SPM8, was used to warp the VBCT maps into a new group-specific reference space representing an average of all the subjects. This procedure uses the GM and WM segments estimated from the original T1-weighted images to calculate a group-specific template and the deformation fields required to warp data from each subject to the new template. Each VBCT map was warped to the new template using the corresponding subject-specific deformation field and was re-sampled to an isotropic voxel size of 1.5 mm3 using trilinear interpolation. The warped VBCT maps were scaled by the Jacobian determinant of the deformations to account for stretching and compression and subsequently smoothed with a 6 mm Gaussian kernel then divided by a binary mask of each original VBCT map which had been identically warped, scaled and smoothed (see Supplementary Fig. S1). A Gaussian kernel of 6 mm was chosen because, when investigating cortical thickness, it is important to keep smoothing to a minimum so that any abrupt changes in thickness that may occur at the boundaries between cortical areas are not obscured (Hutton et al. Reference Hutton, De Vita, Ashburner, Deichmann and Turner2008). This procedure results in smoothed warped VBCT maps for which the Gaussian smoothing kernel applied in the warped space has been projected into the native space of the subject, and the cortical thickness values are preserved over a region the size of the smoothing kernel.
Statistical analysis
Statistical analysis was performed using SPM8 software (Wellcome Trust Centre for Neuroimaging, UK) on the smoothed warped VBCT maps. An analysis of variance was used to compare cortical thickness in UHR-T, UHR-NT and healthy control subjects. Scanner site was modelled as an additional factor, resulting in a total of 12 experimental groups. Including scanner site as a factor in the statistical analysis allowed us to model scanner-related variance in the data, which had the effect of reducing error variance and increasing statistical sensitivity. We also modelled age, gender, ethnicity and handedness as covariates of no interest to minimize any confounding effect of these variables on the findings. An ROI approach was used to examine between-group differences in areas where abnormalities in cortical thickness have been identified in MRI studies of individuals at UHR or with a first episode of psychosis, excluding studies that included data from subjects who participated in the present investigation. These comprised the parahippocampal gyrus, inferior frontal gyrus, anterior cingulate, superior temporal gyrus, inferior parietal gyrus and the precuneus (Narr et al. Reference Narr, Bilder, Toga, Woods, Rex, Szeszko, Robinson, Sevy, Gunduz-Bruce, Wang, DeLuca and Thompson2005a ; Schultz et al. Reference Schultz, Koch, Wagner, Roebel, Schachtzabel, Gaser, Nenadic, Reichenbach, Sauer and Schlosser2010b ; Jung et al. Reference Jung, Kim, Jang, Choi, Jung, Park, Han, Choi, Kang, Chung and Kwon2011; Ziermans et al. Reference Ziermans, Schothorst, Schnack, Koolschijn, Kahn, van Engeland and Durston2012). Using WFU PickAtlas (http://www.nitrc.org/projects/wfu_pickatlas/) we created a mask that included the six chosen ROIs and comprised a total of 11 700 voxels. Within this mask, statistical inferences were made using a statistical threshold of p < 0.05 after familywise error (FWE) correction for multiple comparisons as calculated in SPM8. Trends that did not survive correction for multiple comparisons (p < 0.001 uncorrected) are reported but not discussed. For completeness we also performed a whole-brain analysis using a statistical threshold of p < 0.05 after FWE correction for multiple comparisons.
Results
Sociodemographics
No statistically significant differences were noted among the UHR-T, UHR-NT and control groups in age, gender, ethnicity and handedness (Table 1).
Differences in cortical thickness between UHR subjects and healthy controls
Within the ROIs, the cortex was thinner in the UHR group than in controls in the right parahippocampal gyrus [p < 0.05 FWE corrected; z score = 4.06; Montreal Neurological Institute (MNI) coordinates x = 25, y = –4, z = –13; see Fig. 1]. There was also a trend (p < 0.001 uncorrected) for a thinner cortex in the UHR group compared with controls in the inferior part of the left parahippocampal gyrus (z score = 3.42; MNI coordinates x = –20, y = –7, z = –30). Plotting of the cortical thickness values revealed that the reduction in right parahippocampal cortical thickness was evident in the data from each of the four sites (Fig. 1). In contrast, there were no areas in which UHR individuals had a thicker cortex than healthy controls. The whole-brain analysis did not identify significant differences in cortical thickness between the UHR group and healthy controls at p < 0.05 (FWE corrected).
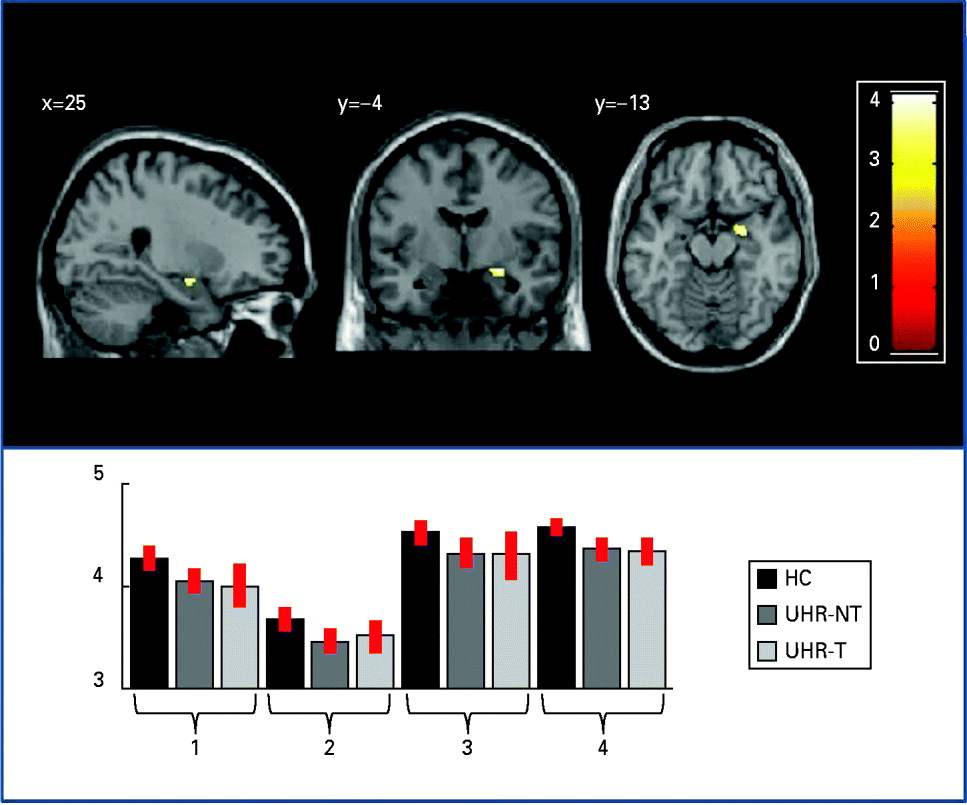
Fig. 1. Cortical thickness differences between the ultra-high risk (UHR) and healthy control (HC) groups: right parahippocampal region where the total UHR sample showed cortical thinning relative to HCs (p < 0.05 after familywise error correction). For visualization purposes, effects are displayed at p < 0.001 uncorrected. The plot shows cortical thickness values for the HC group and the two UHR subgroups (UHR-T, transition to psychosis; UHR-NT, no transition to psychosis) at each site (x axis: 1 = London; 2 = Basel; 3 = Melbourne; 4 = Munich); values on the y axis refer to millimetres. Error bars represent standard deviations.
Differences in cortical thickness between the UHR-T and UHR-NT groups
No differences were observed for the comparison between the UHR-T and UHR-NT groups at p < 0.05 after FWE correction. At a less conservative statistical threshold (p < 0.001 uncorrected), there was a trend for cortical thinning in the UHR-T group in the orbital part of the left inferior frontal gyrus (z score = 3.32; MNI coordinates x = –33, y = 32, z = –15). The whole-brain analysis revealed no significant differences between the UHR-T and UHR-NT groups at p < 0.05 (FWE corrected).
Discussion
Previous neuroimaging studies have reported cortical thinning in schizophrenia, as well as in first-episode psychosis and in subjects at UHR for psychosis (Narr et al. Reference Narr, Bilder, Toga, Woods, Rex, Szeszko, Robinson, Sevy, Gunduz-Bruce, Wang, DeLuca and Thompson2005a ; Fornito et al. Reference Fornito, Yucel, Wood, Adamson, Velakoulis, Saling, McGorry and Pantelis2008a ,Reference Fornito, Yung, Wood, Phillips, Nelson, Cotton, Velakoulis, McGorry, Pantelis and Yucel b ; Schultz et al. Reference Schultz, Koch, Wagner, Roebel, Schachtzabel, Gaser, Nenadic, Reichenbach, Sauer and Schlosser2010b ; Jung et al. Reference Jung, Kim, Jang, Choi, Jung, Park, Han, Choi, Kang, Chung and Kwon2011; Ziermans et al. Reference Ziermans, Schothorst, Schnack, Koolschijn, Kahn, van Engeland and Durston2012). However, the results of these studies have not always been consistent; this may reflect the recruitment of relatively small samples, the use of different study designs, and the investigation of samples that were heterogeneous with respect to age, duration of illness and exposure to treatment. In the present study, we sought to reduce the impact of these potential methodological pitfalls by assessing cortical thickness in a relatively large sample of individuals at UHR for psychosis, all of whom were scanned at a similar stage of illness, when they first presented with clinical high-risk symptoms. Most of the sample had not been treated before. Our first hypothesis was that the UHR group as a whole would show differences in regional cortical thickness relative to controls, and that these would be most evident in areas that have previously been identified as sites of cortical thickness or volume abnormalities in studies of UHR and first-episode subjects (i.e. the temporal, frontal, parietal, anterior cingulate, parahippocampal cortices and the precuneus). This hypothesis was in part confirmed, in that we found that the right parahippocampal cortex was thinner in the UHR group than in controls. This finding is consistent with those of a recent investigation which also found reductions in thickness in other cortical areas (Jung et al. Reference Jung, Kim, Jang, Choi, Jung, Park, Han, Choi, Kang, Chung and Kwon2011). Our result is also in line with evidence that the density of the parahippocampal gyrus is altered in UHR and familial high-risk subjects (Job et al. Reference Job, Whalley, McConnell, Glabus, Johnstone and Lawrie2003). Furthermore, in first-episode schizophrenia, altered right parahippocampal–lingual cortical folding and reduced cortical thickness have been found (Schultz et al. Reference Schultz, Koch, Wagner, Roebel, Nenadic, Gaser, Schachtzabel, Reichenbach, Sauer and Schlosser2010a ). Finally, this region has also been identified as a site of functional (Allen et al. Reference Allen, Seal, Valli, Fusar-Poli, Perlini, Day, Wood, Williams and McGuire2011) alterations in UHR subjects and is one of the most robust site of volume reduction (Seidman et al. Reference Seidman, Pantelis, Keshavan, Faraone, Goldstein, Horton, Makris, Falkai, Caviness and Tsuang2003) and neuropathological abnormalities (Shenton et al. Reference Shenton, Dickey, Frumin and McCarley2001) in schizophrenia.
In our previous volumetric study in an UHR sample that overlapped with that in the present study, we found differences between the UHR sample and controls in the ventral prefrontal cortex and ACC, but not in the parahippocampal cortex (Mechelli et al. Reference Mechelli, Riecher-Rössler, Meisenzahl, Tognin, Wood, Borgwardt, Koutsouleris, Yung, Stone, Phillips, McGorry, Valli, Velakoulis, Woolley, Pantelis and McGuire2011). However, in that study the subgroup of UHR subjects that subsequently developed psychosis had smaller left parahippocampal volumes than the UHR subjects who did not make a transition to psychosis. This volumetric finding was in a similar part of the parahippocampal gyrus to the site of the difference in cortical thickness between all UHR subjects and controls in the present study, although in the opposite hemisphere. Differences in the location of alterations in cortical thickness and cortical volume may reflect the fact that abnormalities in these two measures are not necessarily manifestations of the same underlying neuroanatomical changes. This would be consistent with recent evidence that cortical thickness and surface area are genetically and phenotypically independent and that the local cortical volume is more closely related to surface area than to cortical thickness (Winkler et al. Reference Winkler, Kochunov, Blangero, Almasy, Zilles, Fox, Duggirala and Glahn2010). Therefore differences between the results of our present study and our previous VBM study may be partially explained by the fact that the cortical thickness method does not take surface information into account. The volumetric reduction of the left parahippocampal gyrus reported in our VBM study (Mechelli et al. Reference Mechelli, Riecher-Rössler, Meisenzahl, Tognin, Wood, Borgwardt, Koutsouleris, Yung, Stone, Phillips, McGorry, Valli, Velakoulis, Woolley, Pantelis and McGuire2011) may denote a marker of progression consistent with the results of earlier investigations (Pantelis et al. Reference Pantelis, Velakoulis, McGorry, Wood, Suckling, Phillips, Yung, Bullmore, Brewer, Soulsby, Desmond and McGuire2003; Job et al. Reference Job, Whalley, Johnstone and Lawrie2005), whereas the thinning of the right parahippocampal gyrus may represent either a vulnerability marker (evident not only in the UHR stage but also in the earlier asymptomatic stage) or a specific marker of the UHR stage (not evident in the earlier asymptomatic stage). These two alternative options could be investigated by acquiring longitudinal neuroimaging data from individuals at high genetic risk.
Our second hypothesis was that UHR subjects who went on to develop psychosis would already at baseline show more pronounced cortical thickness abnormalities than UHR subjects who did not. We did not find any differences that survived correction for multiple comparisons. Two previous studies have investigated cortical thickness in UHR subjects in relation to clinical outcome. One study, which restricted its analysis to the ACC, reported cortical thinning in this region in the UHR-T group compared with the UHR-NT group (Fornito et al. Reference Fornito, Yung, Wood, Phillips, Nelson, Cotton, Velakoulis, McGorry, Pantelis and Yucel2008b ). We included the ACC as one of our ROIs but did not replicate this finding, although this may be in part explained by methodological differences in thickness measurements and in ROIs investigated. The second study used MRI to scan UHR subjects at two time points and compared longitudinal changes in cortical thickness in UHR individuals who did and did not make a transition to psychosis. The UHR-T group showed longitudinal reductions in several regions including anterior cingulate, precuneus and temporo-parietal-occipital areas compared with controls, whereas the UHR-NT group did not (Ziermans et al. Reference Ziermans, Schothorst, Schnack, Koolschijn, Kahn, van Engeland and Durston2012). In contrast, no differences in cortical thickness were reported between UHR-T and UHR-NT subjects at baseline. The inconsistency between previous studies and our investigation could be in part explained by the use of different techniques to measure cortical thickness. In particular, the studies by Fornito and colleagues and Ziermans and colleagues used two different surface-based methods (Fischl & Dale, Reference Fischl and Dale2000; Kim et al. Reference Kim, Singh, Lee, Lerch, Ad-Dab'bagh, MacDonald, Lee, Kim and Evans2005; Fornito et al. Reference Fornito, Yung, Wood, Phillips, Nelson, Cotton, Velakoulis, McGorry, Pantelis and Yucel2008b ; Ziermans et al. Reference Ziermans, Schothorst, Schnack, Koolschijn, Kahn, van Engeland and Durston2012) while in the present investigation, a voxel-based method was used to derived cortical thickness values (Hutton et al. Reference Hutton, De Vita, Ashburner, Deichmann and Turner2008). Another study reported no baseline differences in cortical thickness between UHR and first-episode psychosis and healthy controls, although the analysis of cortical asymmetry revealed significant group differences (Haller et al. Reference Haller, Borgwardt, Schindler, Aston, Radue and Riecher-Rössler2009). The lack of significant differences between UHR-T and UHR-NT subjects may seem surprising, given that the present sample was relatively large, and abnormalities in functional and in other structural measures between these subgroups have previously been identified (Pantelis et al. Reference Pantelis, Velakoulis, McGorry, Wood, Suckling, Phillips, Yung, Bullmore, Brewer, Soulsby, Desmond and McGuire2003; Borgwardt et al. Reference Borgwardt, McGuire, Aston, Gschwandtner, Pflüger, Stieglitz, Radue and Riecher-Rössler2008, Mechelli et al. Reference Mechelli, Riecher-Rössler, Meisenzahl, Tognin, Wood, Borgwardt, Koutsouleris, Yung, Stone, Phillips, McGorry, Valli, Velakoulis, Woolley, Pantelis and McGuire2011; Allen et al. Reference Allen, Luigjes, Howes, Egerton, Hirao, Valli, Kambeitz, Fusar-Poli, Broome and McGuire2012). One possible explanation for the absence of significant differences in the present study is that there was some heterogeneity within our UHR sample. Individuals can meet the UHR inclusion criteria through different patterns of clinical and cognitive symptoms, and it is unknown if these reflect distinct pathophysiological processes. Unfortunately, a stratification of the statistical analysis by type of UHR inclusion criteria was not possible in the present investigation, as incorporating this additional factor would require much larger single-centre sample sizes. The sample may also have been clinically heterogeneous with respect to the co-morbid anxiety, depression and substance abuse that are often present in UHR subjects (Svirskis et al. Reference Svirskis, Korkeila, Heinimaa, Huttunen, Ilonen, Ristkari, McGlashan and Salokangas2005).
Another possibility is that some subjects who met transition criteria subsequently have returned to lower levels of psychotic symptoms and higher levels of functioning, while some who did not meet criteria for transition actually have deteriorated in functioning over time. This latter group may be more likely to develop schizophrenia than the former group (Yung et al. Reference Yung, Nelson, Thompson and Wood2010). Thus, the non-transitioned but low functioning group may show brain imaging changes consistent with the ones observed in schizophrenia, but the high functioning transitioned cases may not.
The age range of our UHR individuals varied considerably across the four sites (age range 15–37 years). Previous work indicates that the pattern of neuroanatomical findings in schizophrenia with a relatively early onset (e.g. before the age of 18 years) differs from that in schizophrenia with onset in adulthood and that the longitudinal trajectory of brain abnormalities varies with the age of onset (Gogtay et al. Reference Gogtay, Vyas, Testa, Wood and Pantelis2011). Although we modelled age as a covariate of no interest in the statistical analysis, the fact that the UHR sample comprised individuals at different stages of brain development might have reduced the likelihood of detecting reliable differences.
A further potential contributory factor is that the MRI data we studied were collected on different scanners, using different acquisition sequences. However, we are confident that our results were not significantly affected by the use of different scanners for several reasons. First, only MRI data collected with T1-weighted sequences were used and scanner site was modelled as an independent factor in the statistical analysis. Second, a comparable proportion of control, UHR-NT and UHR-T subjects were scanned at each scanning site. Third, plotting of GM values suggested that the differences between UHR and healthy control groups were evident also within each site and therefore cannot be explained by inter-scanner differences (Fig. 1). Finally, the present approach to the integration of multi-scanner data has been employed successfully in previous studies that also combined different scanners and acquisition sequences (Stonnington et al. Reference Stonnington, Tan, Kloppel, Chu, Draganski, Jack, Chen, Ashburner and Frackowiak2008; Segall et al. Reference Segall, Turner, van Erp, White, Bockholt, Gollub, Ho, Magnotta, Jung, McCarley, Schulz, Lauriello, Clark, Voyvodic, Diaz and Calhoun2009; Suckling et al. Reference Suckling, Barnes, Job, Brenan, Lymer, Dazzan, Marques, MacKay, McKie, Williams, Williams, Lawrie and Deakin2010). Nevertheless, we cannot completely exclude an effect of scanner on the findings, and ideally multi-centre studies should employ the same acquisition sequence, and calibrate data across scanners using phantoms and common subjects to minimize the risk of scanner-related effects (Jack et al. Reference Jack, Bernstein, Fox, Thompson, Alexander, Harvey, Borowski, Britson, Whitwell, Ward, Dale, Felmlee, Gunter, Hill, Killiany, Schuff, Fox-Bosetti, Lin, Studholme, DeCarli, Krueger, Ward, Metzger, Scott, Mallozzi, Blezek, Levy, Debbins, Fleisher, Albert, Green, Bartzokis, Glover, Mugler and Weiner2008).
The whole-brain analysis did not identify significant differences in cortical thickness between the UHR group and healthy controls or between UHR-T and UHR-NT subjects at p < 0.05 (FWE corrected). This could be in part explained by the heterogeneity of the UHR sample (e.g. symptoms, level of functioning, age), or by the use of different scanners and sequences. Nevertheless, when restricting the analysis to ROIs, the method employed was able to detect a thickness reduction in the right parahippocampal gyrus in the UHR group compared with the control group at a statistical threshold of p < 0.05 corrected (FWE). The reduction was evident across all the different scanning sites. Using the whole-brain approach did not enable us to detect differences at a corrected level; this might indicate that group differences in the UHR population are distributed and diluted across the brain cortex.
In conclusion, we observed right parahippocampal thinning in subjects at UHR for psychosis, suggesting that thickness reduction in this region is related to the UHR symptomatology rather than the onset of psychosis. This cortical alteration could represent a marker of vulnerability to psychosis or, alternatively, a specific and distinctive marker of the UHR stage. No reliable differences in cortical thickness were found between subjects who did and did not go on to develop psychosis. Future multi-centre work in this area would benefit from the subcharacterization of UHR individuals with different clinical and cognitive profiles, the recruitment of participants at the same stage of neurodevelopment and the use of a standardized image acquisition sequence.
Supplementary material
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0033291713000998.
Acknowledgements
This work was supported by the National Alliance for Research on Schizophrenia and Depression Independent Investigator Award to A.M.; the National Health and Medical Research Council of Australia (grant no. 970391, 981112, 145737, 145627, 350241, 566529); the National Health and Medical Research Council Clinical Career Developmental Award (grant no. 359223 to S.W.); the National Alliance for Research on Schizophrenia and Depression Young Investigator Award to S.W.; the National Health and Medical Research Council Senior Principal Research Fellowship (grant no. 628386 to C.P.); the National Alliance for Research on Schizophrenia and Depression Distinguished Investigator Award to C.P.; the National Health and Medical Research Council Senior Research Fellowship (grant no. 566593 to A.Y.) and the Wellcome Trust (grant no. 091593/Z/10/Z to C.H.).
Declaration of Interest
None.




