Introduction
This review focuses on methods that can be exploited to disrupt the diapause response. Many insects, including major pest species and disease vectors, rely on diapause to coordinate their seasonal development with favorable times of the year. Disrupting this seasonal rhythm has the potential to manipulate insect populations through untimely diapause entry or termination. As discussed in this review, diapause manipulators that prevent diapause also offer utility for mass rearing programs used to generate the huge numbers of individuals needed for sterile insect release, as well as commercial silk or food production. At the other extreme, manipulators that might be capable of maintaining the diapause state could be useful for enhancing the shelf life of biological control agents or for long-term, low-cost maintenance of genetic stocks.
In some cases, variability inherent in the diapause response within an insect population can be exploited to obtain selected lines with traits that are attractive for experimentation or laboratory rearing. This is sometimes the easiest way to develop lines that lack diapause or have other diapause traits that may be attractive, thus I briefly discuss this approach as well as the use of various chemical agents and physical manipulations capable of altering the diapause response.
The literature contains numerous references to agents capable of disrupting diapause. Many such agents were discovered inadvertently, and many of these tricks work selectively on only a few closely related species. This can be both an advantage as well as a disadvantage. Selectivity is a highly desirable trait for agents that might potentially be used for insect pest management, but selectivity also means that diapause disruptors that are highly effective in one species may not work in that capacity for others. My goal in this review is to summarize some of the known diapause disruptors, with the hope that this will prompt investigators to test these and other agents in species where altering the diapause response may be an attractive option. A wide range of physical and chemical tricks are known. Most reports are for manipulators that break diapause, but of equal interest are manipulations that can prevent diapause entry, manipulations that can block termination of diapause, or treatments capable of inducing a diapause-like state.
Selecting for nondiapause or desirable diapause traits
For experimental or commercial purposes it is often attractive to have an insect colony that can be propagated continuously without the interference of diapause. Such a colony can frequently be generated by selecting for nondiapause. In many insect colonies maintained in the laboratory, the diapause response is diminished or lost entirely after a few years, thus yielding a nondiapause line.
For example, newly field-collected flesh flies such as Sarcophaga crassipalpis or S. bullata show a robust diapause that can be induced by short daylengths at 25 °C (Denlinger, Reference Denlinger1972), while it may become difficult to induce diapause in colonies that have been in the laboratory for a few years, and frequently the diapause response is completely lost at 25 °C and temperatures may have to be lowered to 18 °C to induce a reasonably high incidence of diapause. Selection for nondiapause in S. bullata can result in a nearly total loss of diapause within five generations (Henrich and Denlinger, Reference Henrich and Denlinger1983) or within ten generations for the tropical flesh fly Poecilometopa spilogaster (Denlinger, Reference Denlinger1979), and years of laboratory rearing can yield a completely nondiapausing line of S. bullata (Goto et al., Reference Goto, Han and Denlinger2006). Selection for diapause in S. bullata can be a bit more challenging but equally successful (Henrich and Denlinger, Reference Henrich and Denlinger1983).
Even with an obligate diapause, it is not unusual to have a few individuals failing to enter diapause. This anomaly can be exploited to select a nondiapausing line, as nicely exemplified for the gypsy moth Lymantria dispar (Hoy, Reference Hoy1977). Most pharate first instar larvae of the gypsy moth overwinter in an obligate diapause, but a few individuals develop without entering diapause, thus providing the genetic basis for the development of a nondiapausing strain. But, as demonstrated in the gypsy moth example, not all selected strains, especially strains selected for nondiapause, thrive under long-term laboratory rearing, suggesting loss in fitness may be a cost of selection. Maintaining expression of diapause may be necessary for some species.
Numerous attempts, many of which were successful, have been made to select for nondiapausing or diapausing strains, as nicely reviewed by Danks (Reference Danks1987). Not only can selection be made for diapause or nondiapause, but other diapause-related traits, such as critical photoperiod and diapause duration, are also responsive to selection under controlled laboratory conditions. Thus, the natural variation of diapause traits evident in most populations can be exploited and used to select for desirable diapause attributes. Similarly, one can imagine the use of maladapted phenotypes that have the wrong interpretation of local environmental cues as agents that could be reared and released for control purposes.
Breaking diapause
The ability to break diapause on demand is particularly useful for shortening the duration of diapause, thereby making it possible to maintain an insect colony in a nearly continuous state of development. Of course, for many insects averting diapause can be achieved by manipulating the photoperiod and rearing temperature, but for insects that have an obligate diapause (one that is genetically programmed and not responsive to environmental cues) the ability to break diapause can be especially useful for maintaining continuous development without waiting for the diapausing insect to reach its normal time for termination. But, the utility of having a highly effective diapause terminator can also be extremely useful for insects with a facultative diapause (one that is environmentally programmed). This offers the possibility to store such insects in diapause and break the diapause as needed. Such tools are especially useful for probing fine molecular and physiological details involved in the transition from diapause to diapause termination. Potent diapause terminators allow investigators to develop valuable timelines detailing in a fine-scale the molecular events associated with this transition. One caveat is that a chemical agent used to terminate diapause could trigger some responses that may not fully represent the process of diapause termination elicited by a more natural signal, generated by, for example, elevated temperature or a switch in daylength, as outlined by Koštál (Reference Koštál2006). Errors of this type would most likely occur shortly after the application of a chemical agent. Once the elicitor sets in motion the endocrine cascade leading to a resumption of development, the events monitored would likely be valid regardless of the method used to terminate diapause. Thus far, few experiments have compared immediate or long-term consequences of terminating diapause naturally or with chemical tools.
Endocrine signals that break diapause
The most obvious and best understood factors that break diapause are the hormones that naturally regulate diapause. Although there are exceptions, most types of diapause are the consequence of a hormone deficiency (Denlinger et al., Reference Denlinger, Yocum, Rinehart and Gilbert2012). Most larval and pupal diapauses are initiated and persist as long as environmental cues prevent synthesis and/or release of ecdysteroids from the prothoracic gland, while most adult diapauses are the consequence of a failure of the corpora allata to produce a juvenile hormone (JH). Thus, the developmental hiatus can usually be broken when ecdysteroids are injected into diapausing larvae or pupae, and adult diapauses are routinely terminated by the application of JH. Many examples demonstrate the efficacy of such hormone treatments for diapause termination, but such methods are not without challenges. The major challenge is finding a dose that is neither too small nor too large, and successful breaking of diapause may require being able to sustain an elevated hormone titer for several days. Dosages of ecdysteroids that are too high can lead to hyperecdysonism, a condition in which development is initiated but not appropriately synchronized, leading to generation of abnormalities that prevent successful completion of development through to adulthood (Williams, Reference Williams1968, Ždárek and Denlinger, Reference Ždárek and Denlinger1975). A more moderate dose can be chosen to prompt immediate diapause termination with no adverse side effects. Doses that are too small will not break diapause immediately but may shorten the duration of diapause, as noted in the flesh fly S. crassipalpis (Ždárek and Denlinger, Reference Ždárek and Denlinger1975). In some examples using JH to terminate adult diapause, the hormone prompts the start of reproductive events such as egg laying, but the response is not always sustained and the adult reverts to a diapause-like state, as noted in the Colorado potato beetle Leptinotarsa decemlineata (Schooneveld et al., Reference Schooneveld, Sanchez and de Wilde1977). But, by tailoring and fine-tuning the dosage and duration of application to the test insect, diapause can usually be successfully broken using commercially available hormones or hormone analogs.
JH does not immediately terminate pupal diapause in the flesh fly S. crassipalpis, as do the ecdysteroids, but it can halve the duration of diapause if applied to third instar larvae just prior to diapause entry (Denlinger, Reference Denlinger, Bhaskaran, Friedman and Rodriquez1981). This effect is likely due to the elevated metabolic rate noted in such pupae (Denlinger et al., Reference Denlinger, Shukla and Faustini1984), an effect causing a more rapid depletion of energy reserves and consequently diapause termination (Hahn and Denlinger, Reference Hahn and Denlinger2011).
One of the more unusual endocrine diapause terminators is the neuropeptide diapause hormone (DH), a hormone first described for its action as an inducer of embryonic diapause in the commercial silkmoth Bombyx mori (Yamashita, Reference Yamashita1966). Curiously, this same neuropeptide terminates pupal diapause in species within the moth genera Heliothis and Helicoverpa, a large complex of agricultural pests (Xu and Denlinger, Reference Xu and Denlinger2003). A number of DH analogs have been generated, some which are as much as 50× more potent than DH in breaking diapause (Zhang et al., Reference Zhang, Nachman, Kaczmarek, Zabrocki and Denlinger2011). Although advances have been made in developing DH analogs capable of penetrating the insect cuticle (Zhang et al., Reference Zhang, Nachman, Kaczmarek, Kierus, Zabrocki and Denlinger2015), this still remains a formidable challenge. Attempts to incorporate DH and its analogs into food have thus far been unsuccessful in preventing or breaking diapause, thus injection still remains the best way to administer the neuropeptide, a feature that has obvious drawbacks for large scale use. Recent success in fusing DH with the protein transduction domain (PTD) of the human immunodeficiency virus-1 transactivator of transcription (TAT) provides a tool that prevents rapid degradation of DH in the insect gut, thus allowing new possibilities for oral administration of DH (Zhou et al., Reference Zhou, Li, Yuan, Zhang and Qu2015). Although oral administration of TAT-PTD-DH retards larval development, it has not so far been successful in altering the diapause response.
Like other insects with a pupal diapause, diapause in Heliothis/Helicoverpa can also be terminated with ecdysteroids, but the actions of ecdysteroids and DH are not identical. Ecdysteroids can break diapause at any temperature, but DH is effective only at temperatures of 21 °C or higher (Xu and Denlinger, Reference Xu and Denlinger2003). Diapause termination by ecdysteroids and DH share some common RNA and microRNA responses but also some distinctions (Reynolds et al., Reference Reynolds, Nachman and Denlinger2019), suggesting that different pathways may evoke mechanisms leading to the same developmental response.
Mediators of the endocrine signaling cascade can also be expected to trigger diapause termination. For example, cyclic nucleotides, especially cyclic AMP (cAMP) and cyclic GMP (cGMP), function as second messengers used by neuropeptides to deliver the message from membrane receptors to the nucleus within the cell. Such second messengers can thus be expected to mediate the action of brain-based neuropeptides such as prothoracicotropic hormone, allatostatin or allatoinhibins in activating or inhibiting endocrine glands such as the prothoracic gland or corpora allata. cGMP, for example, can terminate pupal diapause in the flesh fly S. crassipalpis, and co-injection of cGMP with 20-hydroxyecdysone is more potent than either agent alone (Denlinger and Wingard, Reference Denlinger and Wingard1978). cGMP co-injected with 20-hydroxyecdysone elicits a similar diapause-terminating effect on pupal diapause in the Bertha armyworm Mamestra configurata (Bodnaryk, Reference Bodnaryk1978). In both of these examples, cAMP is ineffective as a diapause terminator, but curiously cAMP is highly effective in breaking pupal diapause in the silkmoths Hyalophora cecropia and Antheraea peryni (Berry, Reference Berry1981). Such mediators, along with their analogs and antagonists, are potent manipulators of diapause, but the fact that there are species difference in the response highlights the diversity that may be expected. Many of the chemical triggers that can terminate diapause, as discussed below, also likely target one of the links in the endocrine cascade.
Synthetic analogs of juvenile hormone, especially methoprene and fenoxycarb, have been used successfully in a small-scale field trial to disrupt adult diapause of the apple blossom weevil Anthonomus pomorum (Ždárek et al., Reference Ždárek, Ctvrtecka, Hovorka and Koštál2000). Diapausing females exposed to methoprene show elevated locomotory activity, accompanied by a decrease in dry weight, elevated water content, depletion of carbohydrate stores, a decrease in cold hardiness and ultimately 100% mortality within four weeks.
Chemical triggers that break diapause
An array of chemical agents have been used to break diapause, as summarized in table 1. The earliest reports of such agents, summarized by Lees (Reference Lees1955), come from French and Japanese experiments with the commercial silkmoth Bombyx mori (Henneguy, Reference Henneguy1904; Kogure, Reference Kogure1933). Brushing or immersion of diapausing silkmoth eggs in warm dilute hydrochloric or sulfuric acids is quite effective in breaking diapause in this species, a technique enabling continuous propagation of this important commercial species. Diapausing eggs of the grasshopper Melanoplus differentialis treated with xylol and then supplied with water promptly initiate development (Slifer, Reference Slifer1946). In this species, the natural event responsible for terminating diapause appears to be access to water, and the addition of xylol destroys the waterproof coating on the chorion, allowing the diapausing embryo to access the water trigger.
Table 1. Non-hormonal chemical agents capable of breaking or preventing diapause
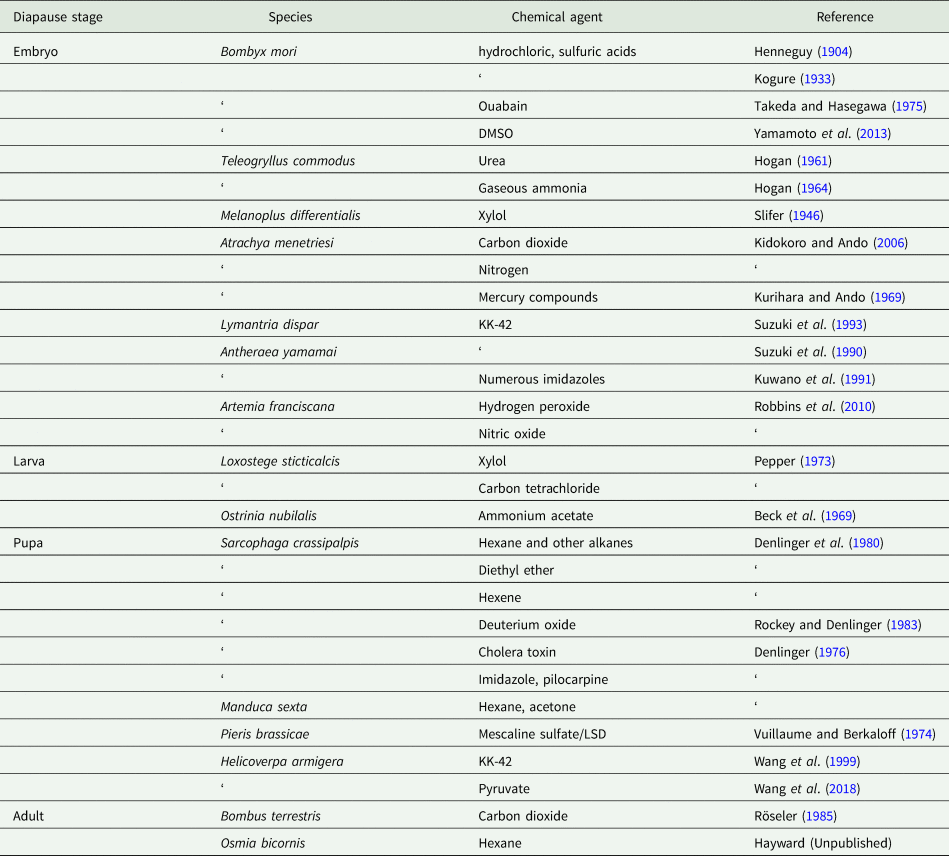
Vapors of xylol or carbon tetrachloride are capable of breaking larval diapause in the sugarbeet webworm Loxostege sticticalis (Pepper, Reference Pepper1973), and vapors or topical applications of a range of organic solvents are potent terminators of pupal diapause in the flesh fly S. crassipalpis (Denlinger et al., Reference Denlinger, Campbell and Bradfield1980). This effect on fly pupae was discovered by accident when acetone was used as a carrier for the application of juvenile hormone (Ždárek and Denlinger, Reference Ždárek and Denlinger1975). A more systematic survey of related solvents revealed that alkanes such as hexane, pentane, heptane, isooctane and cyclohexane are all extremely active, as is the alkene hexene and diethyl ether (Denlinger et al., Reference Denlinger, Campbell and Bradfield1980). A number of other chemicals also successfully break diapause in the flies (e.g. linoleic acid, dichloromethane, benzene), but mortality occurs during pharate adult development or around the time of adult emergence from the puparium, making them less useful as diapause terminators. Hexane is among the most effective agents that both breaks diapause and allows successful adult eclosion and subsequent reproduction. Hexane can be applied either topically, by immersion or by vapor exposure. As little as a 2 μl topical application to the surface of the pupa, a 15 min immersion of the intact puparium, or a 4 h exposure of the intact puparium to hexane vapors is adequate to elicit the effect. Within 15 min of a topical application of hexane the rate of oxygen consumption spikes dramatically (100-fold increase) in the fly pupae and then continues at a high rate consistent with the onset of development. Hexane has proven to be a valuable tool for generating detailed timelines of molecular events surrounding diapause termination and the onset of development in flesh flies. Head-ligated pupae or pupae that have been debrained do not respond to hexane, implying that the brain and/or prothoracic gland are essential for coordinating the response. Topical application of solvents is not effective in terminating pupal diapause in the tobacco hornworm Manduca sexta, but injection of either hexane or acetone is modestly effective (Denlinger et al., Reference Denlinger, Campbell and Bradfield1980). Hexane vapors are also modestly effective in breaking the obligate adult diapause of the red mason bee Osmia bicornis, but it is effective only on males and only after they have been chilled for several months (Scott Hayward, personal communication).
Urea is a potent diapause terminator in eggs of the cricket Teleogryllus commodus (Hogan, Reference Hogan1961), as is an application of gaseous ammonia (Hogan, Reference Hogan1964). High concentrations of urea are effective in breaking diapause in an embryo that has already been in diapause for some time, but much lower concentrations of urea can avert diapause if administered shortly after the diapause-programmed embryo has been deposited. Injection of ammonium acetate breaks larval diapause in the European corn borer Ostrinia nubilalis (Beck et al., Reference Beck, Shane and Garland1969). How urea and the ammonia compounds work is unclear, but ammonium acetate boosts excretion, suggesting a possible diapause link to the excretory system.
Pyruvate terminates pupal diapause in the cotton bollworm H. armigera (Wang et al., Reference Wang, Geng, Guan and Xu2018). Low pyruvate is linked to diapause initiation, while high pyruvate is associated with nondiapause development or the resumption of development at the termination of diapause. Simulating diapause termination by injecting pyruvate into diapausing pupae is effective in breaking diapause, a result suggesting an intriguing role for pyruvate in diapause regulation.
Overwintering diapause in queens of the bumblebee Bombus terrestris can be readily broken with CO2 narcosis, a treatment that prompts egg laying within one week after treatment (Röseler, Reference Röseler1985). As discussed later, CO2 narcosis not only breaks diapause but can be used to avert diapause entry as well (Amsalem and Grozinger, Reference Amsalem and Grozinger2017). It is not clear whether narcosis acts directly on the corpora allata (Larrere et al., Reference Larrere, Lavenseau, Tsei and Couillaud1993), but at some point JH synthesis is needed to initiate egg laying. Regardless of the mode of action, the utility of this technique allows year-round laboratory rearing, a feature that has greatly facilitated use of this species for experimentation (e.g. Amsalem et al., Reference Amsalem, Galbraith, Cnaani, Teal and Grozinger2015).
Anoxia, brought on by either CO2 or N2, is also effective as a diapause terminator in embryos of the false melon beetle Atrachya menetriesi, but in this case, the anoxia treatment works only on embryos that have already been chilled for 50 days at 7.5 °C (Kidokoro and Ando, Reference Kidokoro and Ando2006), suggesting perhaps that the treatment accelerates post-diapause development rather than actually terminating diapause. Another mechanism for breaking embryonic diapause in A. menetriesi is to place the diapausing eggs on filter paper saturated with mercuric chloride or other mercury compounds, at a concentration of approximately 5 ppm (Kurihara and Ando, Reference Kurihara and Ando1969). Dipping eggs in a mercuric chloride solution is equally effective (Kidokoro et al., Reference Kidokoro, Iwata, Takeda and Fujiwara2006).
In an insect relative, the brine shrimp Artemia franciscana, embryonic diapause normally requires freezing and/or desiccation for diapause termination, but the natural termination process can be short-circuited with a 4-h exposure to either hydrogen peroxide (H2O2) or nitric oxide (NO) (Robbins et al., Reference Robbins, Van Stappen, Sorgeloos, Sung, MacRae and Bossier2010). NO is effective at a lower concentration, but H2O2 results in more of the embryos breaking diapause.
The imidazole derivative KK-42 (1-benzyl-5-[(E)-2,6-dimethyl-1,5-heptadienyl] imidazole) is an effective diapause terminator in several insect species. KK-42 (Suzuki et al., Reference Suzuki, Minagawa, Kumagai, Naya, Endo, Osana and Kuwano1990), as well as numerous other imidazole compounds (Kuwano et al., Reference Kuwano, Fujisawa, Suzuki and Eto1991), are highly effective in breaking the pharate first-instar diapause of the wild silkmoth, Antheraea yamamai, presumably by suppressing the ecdysteroid titer. The topical application of imidazoles offers a simple tool that can be used to propagate this commercially important species without the delay of diapause. Diapause in the pharate first-instar larvae of the gypsy moth L. dispar can also be terminated promptly with KK-42 (Suzuki et al., Reference Suzuki, Nakamura, Yanbe, Kurihara and Kuwano1993, Bell, Reference Bell1996, Lee and Denlinger, Reference Lee and Denlinger1996). The gypsy moth diapause is maintained by a high ecdysteroid titer and a drop in the titer is required for diapause termination (Lee et al., Reference Lee, Valaitis and Denlinger1997). KK-42 likely exerts its diapause terminating effect by lowering the effective concentration of ecdysteroids in the embryo, thus providing an endocrine trigger for diapause termination and subsequent hatching of the larva. Having a tool such as KK-42 that works on the gypsy moth is especially attractive because this species has an obligate diapause that is otherwise difficult to terminate without a long time delay. Pupal diapause of H. armigera can also be broken with KK-42 (Wang et al., Reference Wang, Gong and Qin1999), but this response is not as easily explained. If KK-42 acts solely to suppress the ecdysteroid titer, one would not expect this to result in the termination of a pupal diapause. Attempts to break pupal diapause of the flesh fly S. crassipalpis with KK-42 have not been successful (Liu et al., Reference Liu, Zhang and Denlinger2015), thus this agent, like many diapause terminators, exerts its effect only within a narrow taxonomic range of species.
Physical triggers that break diapause
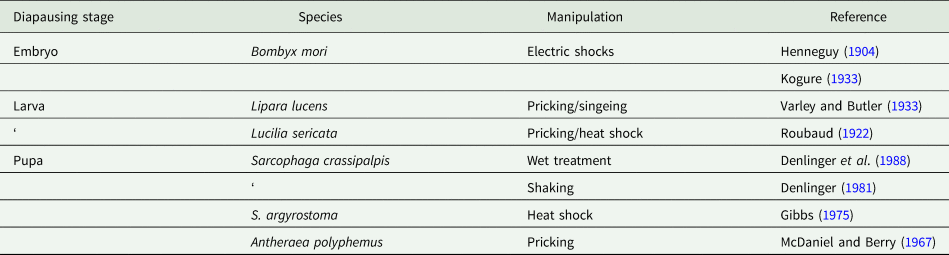
As with chemical triggers reported to break diapause, a number of physical manipulations are also documented as diapause terminators (table 2). Electric shocks have been known for quite some time to trigger diapause termination in embryos of the commercial silkmoth (Kogure, Reference Kogure1933). Although we still cannot say for certain how electric shocks work, they possibly disrupt electrical properties in membranes encapsulating neurosecretory products, thus prompting premature discharge of neuropeptides that stimulate development. Early reports on larval diapauses of the chloropid fly Lipara lucens (Varley and Butler, Reference Varley and Butler1933) and blow fly Lucilia sericata (Roubaud, Reference Roubaud1922) also show the efficacy of pricking and singeing as a trigger for diapause termination. The physical injury usually boosts the metabolic rate, and in diapausing pupae of the saturniid A. polyphemus injury from pricking is capable of breaking diapause (McDaniel and Berry, Reference McDaniel and Berry1967), an effect not noted in diapausing pupae of another saturniid, H. cecropia, or the flesh fly S. argyrostoma (Denlinger et al., Reference Denlinger, Willis and Fraenkel1972).
Table 2. Physical manipulations capable of breaking or preventing diapause
Preventing diapause entry
Preventing entry into diapause can be even more attractive than waiting until the insect has entered diapause and then attempting to break the arrested state. This, of course, completely short-circuits diapause, enabling continuous progression of development, akin to the developmental fate of a nondiapausing insect. For a facultative diapause, preventing diapause can easily be achieved by providing environmental cues that are not diapause inducing, commonly long daylengths and moderate temperatures for many of the insects from temperate latitudes. But, the challenge is greater for an insect with an obligate diapause or for an insect with a facultative diapause that has already been environmentally programmed. As we have seen above, the insect's own endocrine system can be engaged to subvert diapause, and a number of tools capable of breaking diapause (discussed above) are also effective in preventing diapause entry.
Insects appear to be particularly vulnerable to disruption of diapause at the very onset of diapause (Denlinger et al., Reference Denlinger, Giebultowicz, Adedokun, Sehnal, Zabza and Denlinger1988). For example, while an ED50 of 1.7 μg 20-hydoxyecdysone is needed to break pupal diapause in the flesh fly S. crassipalpis 8 days after diapause entry, an ED50 of 0.01 μg can prevent entry into diapause if injected at the onset of diapause. Similarly, one-day heat exposure to 35 °C around the time of diapause entry is highly effective in preventing diapause, but 10 days later a 16 day exposure at the same high temperature is required to break diapause. A similar vulnerability is seen in early embryos of the band-legged ground cricket Dianemobius nigrofasciatus (Fukumoto et al., Reference Fukumoto, Numata and Shiga2006). The embryos are highly sensitive to a cold (O °C) or heat (35 °C) pulse around the time of egg deposition but not later. A cold pulse administered to long-day crickets on the day of deposition boosts the diapause incidence in long-day crickets but is not effective one day later. Conversely, a heat pulse (35 °C) delivered to a short-day cricket on either the day of deposition or one day later decreases diapause incidence. Thus, capitalizing on the insect's vulnerability at the point of diapause entry can be especially attractive for altering the diapause response.
Light at night
Photoperiodic cues dominate in determining the timing of diapause entry (Danks, Reference Danks1987, Denlinger, Reference Denlinger2002). Most temperate latitude species rely on short daylength to program diapause entry. It is thus possible to use long daylengths in the laboratory to prevent diapause in insects with a facultative diapause, and several field trials have been at least partially successful in preventing diapause entry in Lepidoptera by using artificial light to either extend daylength in the field (Ankersmit, Reference Ankersmit1968, Hayes et al., Reference Hayes, Sullivan, Oliver and Schechter1970, Schechter et al., Reference Schechter, Hayes and Sullivan1971, Berlinger and Ankersmit, Reference Berlinger and Ankersmit1976) or by using pulses of light at strategic times during the night (Barker et al., Reference Barker, Cohen and Mayer1964, Hayes et al., Reference Hayes, Cawley, Sullivan, Adler and Schechter1974). Diapause can be prevented in the Kanzawa spider mite Tetranychus kanzawai using a 1-h light pulse with a light intensity of 2.5 kJm−2 at wavelengths of 350–1050 nm in mid-scotophase (Shah et al., Reference Shah, Suzuki, Ghazy, Amano and Ohyama2011), a treatment that would likely prove too costly for field application. The growing problem of light pollution at night (Gaston, Reference Gaston2018) is suspected to be a potential problem affecting diapause and other life-history traits as demonstrated in the noctuid moth M. brassicae (van Geffen et al., Reference van Geffen, vanGrunsven, van Ruijven and Berendse2014). Issues of light intensity and photoperiod duration can also alter the efficacy of parasitoids used in greenhouses to combat whiteflies (Zilahi-Balogh et al., Reference Zilahi-Balogh, Shipp, Cloutier and Brodeur2006).
Hormonal, chemical and physical factors that can prevent diapause
When administered at the onset of diapause, the insect hormones that can break diapause, as discussed above, are also capable of preventing entry into diapause. Thus, ecdysteroids delivered at low levels can prevent most larval or pupal diapauses, DH and its analogs administered at this time can prevent entry into pupal diapause in the heliothine moths, and JH can prevent diapause entry in most adults.
Although pupal diapauses are primarily regulated by the absence of ecdysteroids, application of either an ecdysteroid or a JH analog can prevent pupal diapause in the flesh fly S. crassipalpis (Denlinger, Reference Denlinger1976). A rather high dose (10 μg) of the JH analog methoprene applied topically to newly ecdysed third instar (final) larvae prevents entry into pupal diapause. An ecdysteroid injection of 1 μg into post-fed third instar larvae averts pupal diapause and an even lower dose (0.01 μg) is effective if injected into young pupae immediately before the onset of diapause. Diapause can also be prevented in S. crassipalpis by injecting cholera toxin into larvae 24 h before pupariation (Denlinger, Reference Denlinger1976). Cholera toxin is an activator of adenylate cyclase, thus the elevated levels of cAMP generated likely trigger this effect, mimicking the action of a second messenger system that mediates the action of prothoracicotropic hormone or other neuropeptides involved in promoting continuous development. Other agents that are effective in averting pupal diapause when injected into post-feeding third instar larvae of S. crassipalpis include imidazole and pilocarpine, although neither are as effective as cholera toxin (Denlinger, Reference Denlinger1976).
Carbon dioxide narcosis, a treatment that breaks adult diapause in the bumble bee B. impatiens can also prevent diapause entry, allowing the queen bumble bee to completely bypass diapause and initiate egg laying (Amsalem and Grozinger, Reference Amsalem and Grozinger2017). This treatment for preventing diapause appears to be devoid of negative side effects that might impair flight, stress responses, or longevity. The effect appears to be a response to the carbon dioxide rather than the absence of oxygen, but it is still unclear how the effect is mediated.
Another manipulation effective in averting pupal diapause in the flesh fly S. crassipalpis is to rear larvae on a diet supplemented with deuterium oxide (Rockey and Denlinger, Reference Rockey and Denlinger1983). Deuterium oxide, heavy water, alters circadian cycles and thus likely averts diapause by preventing accurate discrimination between long and short daylengths. An injection of mescaline sulfate or lysergic acid diethylamide (LSD) into larvae of Pieris brassicae during their photosensitive period prevents entry into pupal diapause (Vuillaume and Berkaloff, Reference Vuillaume and Berkaloff1974). The effectiveness of these psychodysleptics is restricted to the photosensitive stage, suggesting the effect may be achieved by disrupting measurement of daylength or transduction of the daylength cue. Injection of ouabain, an inhibitor of active transport of certain inorganic cations, into adult females of B. mori prevents the female from laying diapausing eggs (Takeda and Hasegawa, Reference Takeda and Hasegawa1975), an effect possibly blocking the release of DH from the subesophageal ganglion. Dimethyl sulfoxide (DMSO) is also a potent preventer of diapause in B. mori (Yamamoto et al., Reference Yamamoto, Mase and Sawada2013), but the active window is rather brief. For DMSO to prevent diapause it must be administered within 24 h of oviposition. DMSO is as effective in diapause prevention and termination in B. mori eggs as is the long-time standard used for this purpose, HCl.
Once the programming of diapause has been completed, there are relatively few physical manipulations that can reverse the outcome. In the flesh fly S. crassipalpis, 5 days of wet treatment, desiccation for 3 days on silica gel, exsanguination (removing 15 μl of blood), inflation with air, starvation, cold shock and a 1-day heat shock at 33 °C are all ineffective in preventing the onset of pupal diapause (Denlinger, Reference Denlinger1976). Heating post-fed, third instar larvae at 33 °C for 2 days, however will avert pupal diapause (Denlinger, Reference Denlinger1976), as will heat treatment immediately after pupariation (Gibbs, Reference Gibbs1975). Also, if the wet treatment continues beyond 5 days, until the larvae are actually forced to pupariate in a wet substrate, pupal diapause incidence drops approximately 50% (Denlinger et al., Reference Denlinger, Giebultowicz, Adedokun, Sehnal, Zabza and Denlinger1988). Both high temperatures and a wet environment are conditions that a diapausing pupa would not be able to survive for very long, thus these unfavorable conditions apparently trigger a fail-safe reversal of the diapause program. One additional manipulation that is effective is to physically shake the larvae: fly larvae mounted on an oscillating platform shaker (160 oscillations min−1) enter diapause at a significantly lower rate than undisturbed controls (Denlinger, Reference Denlinger, Bhaskaran, Friedman and Rodriquez1981).
Prolonging the duration of diapause
Tools that might prolong the natural duration of diapause would, of course, be attractive as agents for disrupting the insect's natural life cycle. Delaying overwinter emergence could be effective in desynchronizing a pest species with an agricultural crop, and increasing the duration of diapause may be useful for increasing the shelf life of a commercial biological control agent or pollinator. But, few agents with such actions have been reported. One agent does elicit such an effect on pupal diapause of the corn earworm H. zea (Zhang et al., Reference Zhang, Nachman, Kaczmarek, Zabrocki and Denlinger2011). Diapause in this species can be broken with an injection of DH and a number of agonists have been developed that are quite potent in terminating diapause, but in addition, an antagonist has been developed that blocks the termination of diapause by DH. The antagonist was developed by incorporating a dihydroimidazole trans-Proline mimetic motif into one of the DH agonists, thereby converting the agonist into an antagonist.
In pupae of the Chinese silkmoth A. pernyi, injection of luzindone, a melatonin receptor antagonist, inhibits the release of prothoracicotropic hormone, causing a delay in the timing of diapause termination, a result suggesting a role for melatonin in the photoperiodic termination of diapause in this species (Wang et al., Reference Wang, Mohamed and Takeda2013). The imidazole derivative KK-42 exerts a similar effect on A. pernyi and H. zea (Liu et al., Reference Liu, Zhang and Denlinger2015): when injected into diapausing pupae, KK-42 delays diapause termination by 1–2 months. Most likely, other agents can be found or developed with similar activities.
Inducing a diapause-like state
Is it possible to induce diapause or a diapause-like state in an insect that lacks diapause or has not been programed environmentally to enter diapause? Again, the endocrine system can indeed be targeted to achieve this goal, although we still lack examples where this has been done on a large scale that may prove useful for control or other practical purposes.
In the silkmoth B. mori DH exerts its arresting effect through an accumulation of sorbitol within the yolk of the egg, as demonstrated by the fact that a diapause-like state can be induced in nondiapause-programed silkworm embryos by bathing the embryos in culture media supplemented with sorbitol (Horie et al., Reference Horie, Kanda and Mochida2000). Conversely, development can be stimulated in diapausing embryos by removing sorbitol from the culture medium. The cause of arrest appears to be the presence of sorbitol in the medium rather than some other effect of DH, thus diapause can be mimicked by the simple addition of sorbitol.
Yamamarin, a palmitoyl conjugate of an insect pentapeptide (DILRGa) isolated from diapausing embryos of the giant silkmoth A. yamamai, is capable of inducing embryonic diapause in the progeny of the silkmoth B. mori injected as pupae (Yang et al., Reference Yang, Abe, Sato, Yamashita, Matsuda and Hamayasu2007). This interesting compound has been tested extensively on insect and human cell cultures, and is highly effective in arresting cell proliferation and respiration (Sato et al., Reference Sato, Yang, An, Matsukawa, Ito, Imanishi, Matsuda, Uchiyama, Imai, Ito, Ishida and Suzuki2010), common attributes of diapause. A compound with these features has obvious potential as a pharmacological agent. Whether Yamamarin is also capable of inducing diapause in insects other than B. mori remains to be tested. Several additional chemical agents are capable of inducing diapause in B. mori. Bathing eggs not programmed for diapause with formalin, hydrogen peroxide, and cinnamaldehyde elicits a diapause response in the embryos, a response likely evoked by activation of a thermosensitive transient receptor potential (TRP) channel, an early step in the temperature-sensitive pathway leading to DH release (Sato et al., Reference Sato, Sokabe, Kashio, Yasukochi, Tominaga and Shiomi2014).
Pupal diapause in the cabbage armyworm M. brassicae can be induced by feeding Dopa, a dopamine precursor, to larvae during their last larval instar (Uryu et al., Reference Uryu, Ninomiya, Yokoi, Tsuzuki and Hayakawa2003). Dopa feeding results in higher concentrations of dopamine in the hemolymph and central nervous system, an effect that possibly inhibits synthesis or release of PTTH, the neuropeptide that is an essential trigger for adult development. A screen of genes upregulated in both Dopa-fed pupae and pupae induced to enter diapause in response to short daylength, reveals one highly upregulated gene common to both groups: RACK, a receptor for activated protein kinase C, a prominent gene in the protein kinase C (PKC) family, a family of genes linked to a wide range of biological responses.
To simulate an adult diapause the corpora allata must be shut down so that JH is no longer providing the needed stimulant for reproduction. Precocene, the dimethylchromene extracted from seeds of the common garden flower Ageratum houstonianum, chemically destroys the corpora allata, thus removing the source of JH (Bowers, Reference Bowers and Gilbert1976). When applied to nondiapausing adults of the Colorado potato beetle L. decemlineata, adult beetles burrow into the soil mimicking the state of diapause. Suppression of other components of the JH signaling pathway can elicit similar effects. For example, suppression of insulin-like peptide-1, an upstream regulator of JH synthesis, results in a diapause-like state in adult females of the northern house mosquito Culex pipiens (Sim and Denlinger, Reference Sim and Denlinger2009), and suppression of the genes encoding Methoprene-tolerant (Met) and Taiman (Tai), components of the JH signaling pathway, mimic the effect of adult diapause in the linden bug Pyrrhocoris apterus (Smykal et al., Reference Smykal, Bajgar, Provaznik, Fexova, Buricova, Takai, Hodková, Jindra and Doležel2014).
Similarly, agents that could suppress synthesis or secretion of prothoracicotropic hormone or ecdysteroids could cause a diapause-like state in larvae or pupae. Artificial reduction of an insect's ecdysteroid titer is not easy to achieve, but one interesting agent capable of doing so is ecdysteroid-22-oxidase (E22O) (Kamimura et al., Reference Kamimura, Saito, Niwa, Niimi, Toyoda, Ueno, Kanamori, Shimura and Kiuchi2012). This compound, extracted from the fungus Nomuraea rileyi, is capable of suppressing the ecdysteroid titer in a number of species. In larvae of the crambid moth Haritalodes basipunctalis injection of E22O induces a diapause-like state, which, as with a normal diapause, is broken by chilling.
The imidazole derivative KK-42 does not force a long-day (nondiapause-programmed) insect to enter diapause, but it can significantly boost the incidence of pupal diapause under short-day (diapause-programmed) conditions, as demonstrated for both the moth H. zea and the flesh fly S. crassipalpis (Liu et al., Reference Liu, Zhang and Denlinger2015). In H. zea, the effect is elicited by topical application to the final larval instar, and in S. crassipalpis the effect can be achieved by incorporating KK-42 into the larval diet. The fact that KK-42 is active topically or by feeding is an attractive feature that can easily be exploited. The polyamine putrescine elicits a similar effect in H. armigera: diet supplementation elevates the diapause incidence at short daylengths (Wu et al., Reference Wu, Wang and Zhang2010). Other natural compounds capable of inducing a diapause-like state or boosting the diapause incidence likely await discovery.
Conclusions
From this brief review, it is evident that genetic selection can be used to develop strains with desirable diapause attributes, and numerous types of chemicals and physical treatments are capable of breaking diapause, preventing entry into diapause, extending the duration of diapause, or generating a diapause-like state in an insect that would otherwise develop without diapause. Chemical agents that act as diapause disruptors represent broad classes of chemical compounds, including both natural products as well as synthesized compounds. What is also clear is that there likely are no compounds that consistently exert the same effect on diverse species or even in closely related species that share the same diapausing stage. This diversity in responses likely reflects the rich evolutionary origins of insect diapause and the accompanying complex and diverse regulatory networks that have evolved to shape and coordinate the diapause response. What this means is that appropriate tools for manipulating diapause will have to be discovered for each species of interest, but hopefully the literature provided here will offer ideas that might be tested for achieving the desired manipulation. The fact that so many different tools are already known offers hope that appropriate tools can be found for other species as well.
Acknowledgements
I appreciate the thoughtful comments provided by four anonymous reviewers.





