Introduction
The world population is currently in excess of 7 billion people and is expected to reach 9 billion by 2050. Global demand for agricultural products is expected to double in the coming decades (Godfray et al., Reference Godfray, Beddington, Crute, Haddad, Lawrence, Muir, Pretty, Robinson, Thomas and Toulmin2010); hence there is a need to produce more food per unit area to satisfy demand. Much of this increase will depend on rain-fed systems in developing countries in sub-Saharan Africa. Smallholder farmers living in southern Africa are resource-constrained and traditionally practice conventional tillage-based agriculture systems using mouldboard ploughs or hand hoes, despite increasingly dwindling maize grain yields (Thierfelder and Wall, Reference Thierfelder and Wall2009; FAO, 2018). Smallholder farmers in Africa have small plots of land, with an average of 2 ha, where they grow crops using mostly family labour (Muyanga and Jayne, Reference Muyanga and Jayne2006). Recent figures show that the yield of maize, the major subsistence cereal in southern Africa, can be as low as 0.8 t/ha (Baudron et al., Reference Baudron, Tittonell, Corbeels, Letourmy and Giller2012) against yield potential of 10–15 t/ha (Thierfelder et al., Reference Thierfelder, Baudron, Setimela, Nyagumbo, Mupangwa, Mhlanga, Lee and Gérard2018). Climate variability has exacerbated the situation and increased the pressure on smallholder farmers, making most of them food insecure (Seo et al., Reference Seo, Mendelsohn, Dinar, Hassan and Kurukulasuriya2009; Thierfelder et al., Reference Thierfelder, Chivenge, Mupangwa, Rosenstock, Lamanna and Eyre2017).
Since the late 1990s, more sustainable cropping systems including conservation agriculture (CA) have been promoted with the aim of improving smallholder farmers' livelihoods in Africa (Wall et al., Reference Wall, Thierfelder, Ngwira, Govaerts, Nyagumbo, Baudron, Jat, Sahrawat, Kassam and da Silva2013). CA is defined as a cropping system based on three key principles: (a) minimum soil disturbance; (b) maintenance of a permanent soil cover and (c) diverse crop rotation and/or crop interaction (Kassam et al., Reference Kassam, Friedrich, Shaxson and Pretty2009). In addition, CA systems need to be supported by good management to function (Thierfelder et al., Reference Thierfelder, Baudron, Setimela, Nyagumbo, Mupangwa, Mhlanga, Lee and Gérard2018). CA has many benefits such as improved and more stable yields, improved rain water use efficiency, increased microbial activity and, in the longer term, improved soil fertility (Twomlow et al., Reference Twomlow, Urolov, Jenrich and Oldrieve2008; Kassam et al., Reference Kassam, Friedrich, Shaxson and Pretty2009; Mashavakure et al., Reference Mashavakure, Mashingaidze, Musundire, Nhamo, Gandiwa, Thierfelder and Muposhi2019a, Reference Mashavakure, Mashingaidze, Musundire, Nhamo, Gandiwa, Thierfelder and Muposhi2019b). Improvement in soil biology is associated with increases in soil organic carbon, which may sequester under CA in the longer term (Guo et al., Reference Guo, Lin, Liu, Cao and Li2016).
The fertility status of soil is a product of various processes in the soil (e.g. biological, physical and chemical) (Green et al., Reference Green, Stott, Cruz and Curi2007). Biological activity involves living organisms, which facilitate decomposition and breakdown of organic matter, thus making essential nutrients available for plant growth (Hesammi et al., Reference Hesammi, Farshidi, Sadatebrahimi and Talebi2014). Improvement in chemical soil fertility is vital in increasing crop yields within an agricultural system (Vanlauwe et al., Reference Vanlauwe, Six, Sanginga and Adesina2015). Organic matter can hold large concentrations of nutrients which are released through decomposition, making them available for plant growth (Berg, Reference Berg2000). However, the release of nutrients from organic matter requires soil biological activity to help in its build-up and decay (Wardle et al., Reference Wardle, Bardgett, Klironomos, Setälä, van der Putten and Wall2004). Organic matter also contributes to greater soil stability through increased aggregation (Oades, Reference Oades1984) with associated benefits on increased water infiltration, reduced run-off and soil erosion.
Improvement in soil biological activity can be achieved in different ways: (a) through application of manure, compost, crop residues and crop rotations that provide food for the organisms; (b) maintenance of a chemically balanced soil that provides nutrients for soil organisms and (c) practising reduced tillage systems that improve water infiltration (Stout, Reference Stout1973; Thierfelder and Wall, Reference Thierfelder and Wall2009) and are mechanically less disruptive. Often the availability and concentration of organic resources for soil amendment is critical and usually in short supply. Retention of crop residues and reduced tillage may also have detrimental effects in the soil, leading to increases in pests and diseases (Ishijima et al., Reference Ishijima, Motobayashi, Nakai and Kunimi2004).
Increased soil fauna diversity and evenness is expected under CA due to the maintenance of permanent soil cover and diverse crop rotations. TerAvest et al. (Reference TerAvest, Carpenter-Boggs, Thierfelder and Reganold2015) observed increases in earthworms and termites under high residue cover and diverse crop rotations in Malawi, which are fundamental principles of the system (Thierfelder and Wall, Reference Thierfelder and Wall2010; Mutema et al., Reference Mutema, Mafongoya, Nyagumbo and Chikukura2013). Both aspects increase the availability of food for macro- and micro-organisms (Kladivko, Reference Kladivko2001). Furthermore, minimum soil disturbance reduces the destruction of faunal communities, which benefits the life-cycle and reproduction of soil faunal communities (Tsiafouli et al., Reference Tsiafouli, Thébault, Sgardelis, de Ruiter, van der Putten, Birkhofer, Hemerik, de Vries, Bardgett, Brady, Bjornlund, Jørgensen, Christensen, Hertefeldt, Hotes, Gera Hol, Frouz, Liiri, Mortimer, Setälä, Tzanopoulos, Uteseny, Pižl, Stary, Wolters and Hedlund2015). However, soil fauna can also become pests if they are not in balance, for example high termite activity has been shown to damage maize crop in Mozambique (Nyagumbo et al., Reference Nyagumbo, Munamati, Mutsamba, Thierfelder, Cumbane and Dias2015).
The association of different macro- and micro-organisms found on or beneath the soil surface increases the decomposition rate of crop residues and soil organic matter (Van Veen and Kuikman, Reference Van Veen and Kuikman1990). Macrofaunal organisms such as termites are the first to start decomposing crop residues (primary decomposers). They break down the crop residues and increase the surface area for micro-organisms to continue the decomposition (Schuurman, Reference Schuurman2005). Earthworms feed on crop residues and their casts help to retain nutrients in the soil, finally leading to improvements in soil structure and fertility (Curry and Schmidt, Reference Curry and Schmidt2007). Dung beetles and centipedes feed on soft-bodied insects, which help reduce detrimental pests in agriculture. All these faunal groups have potential to improve crop production; however, there is little knowledge about how they are affected by different CA systems in Africa.
The objective of the current study was to monitor the effect of different CA systems and conventionally ploughed (CP) control practices on below-ground biodiversity and soil fauna over a 4-year period. The study tested the following research hypothesis: CA and no-tillage (NT) with crop residue retention systems lead to greater faunal activity in the soil surface compared with tillage-based cropping systems.
Materials and methods
Site description
A field experiment was conducted in an ongoing long-term trial located at the Monze Farmer Training Centre (MFTC), Monze District, southern province of Zambia (16°24′S, 27°26′E, 1103 m a.s.l.) established in 2005 by CIMMYT (Thierfelder and Wall, Reference Thierfelder and Wall2010). This study was carried out in four consecutive cropping seasons (2009/10 to 2012/13). The site is characterized by soils classified as Lixisols according to the FAO classification (Hartemink et al., Reference Hartemink, Krasilnikov and Bockheim2013). Soil characteristics of the site have been previously described in Thierfelder and Wall (Reference Thierfelder and Wall2009). The site receives an annual average rainfall of 748 mm (Fig. 1) and the rainy season in this region normally starts at the beginning of November and ends in April. Farmers in the surrounding areas practice an integrated crop-livestock farming system, rearing livestock including cattle (beef and dairy), goats and sheep through free-roaming grazing and fed crop residues. Most farmers depend on natural rainfall to grow food and cash crops including maize (Zea mays L.), sorghum (Sorghum bicolor L.) soybean (Glycine max L. Merr), cotton (Gossypium hirsutum L.) and cowpea (Vigna unguiculata L. Walp), as well as green manures such as sunn hemp (Crotalaria juncea L.) and velvet bean (Mucuna pruriens L.) among other crops (Thierfelder and Wall, Reference Thierfelder and Wall2010).
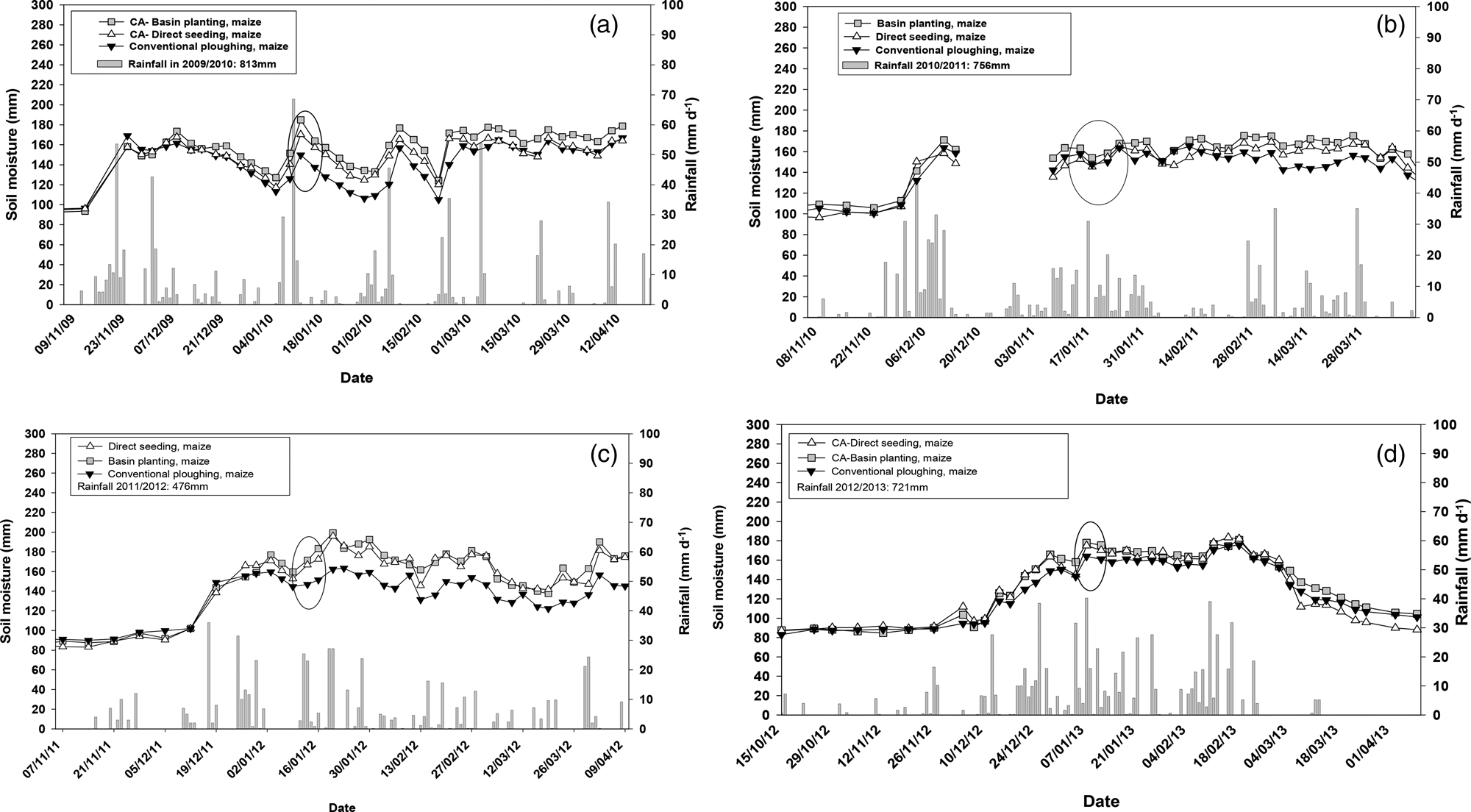
Fig. 1. Rainfall distribution and soil moisture content (top 60 cm) for 2009/10, 2010/11, 2011/12 and 2012/13 seasons at research sites in Monze, Zambia. The circles in the moisture graphs indicate the sampling period. CA, conservation agriculture.
Description of experiment
The experiment at MFTC had different treatments under both tillage and NT (Thierfelder and Wall, Reference Thierfelder and Wall2010). The treatments involved three levels of rotation with up to three phases, determined by the number of crops involved within each crop rotation, and tillage system. The rotation level and tillage system were used to create a new factor, rotation-tillage (RotTill) (Table 1).
Table 1. Description of the treatments in the 2011–13 cropping seasons in Zambia
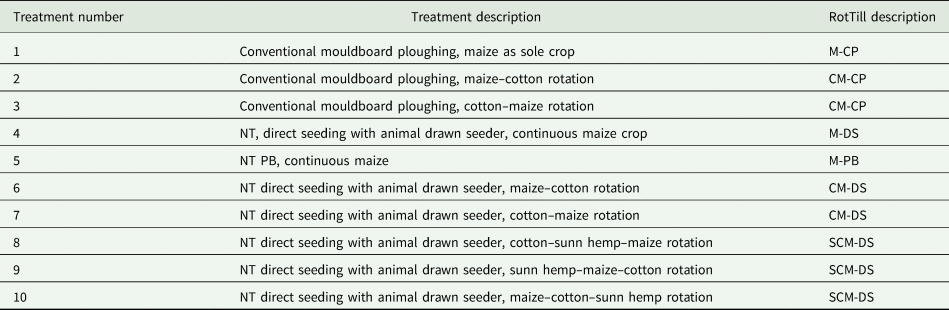
First letters in the abbreviations for treatments indicate the crop: M, maize; C, cotton; S, sunn hemp; the second letter indicates tillage/NT methods: CP, conventional ploughing; DS, direct seeding; PB, plant basins.
Treatments 2 and 3 were added to the trial in the 2010/11 season. All treatments were established in a randomized complete block design and replicated four times. Each plot measured 10 × 30 m (300 m2) and all plots were separated by 1 m pathways. In each year, all phases of the rotation were grown in each block (e.g. MC-DS started with maize then cotton whereas CM-DS started with cotton then maize, and both treatments were rotated thereafter).
Plot management
The harvested crop residues were removed from the plots of CP treatments soon after harvesting the crop, i.e. MCP, MC-CP and CM-CP. For CP treatments, the land was tilled (at 10–15 cm depth) using an animal-drawn single row mouldboard plough. Maize was spaced at 0.90 × 0.50 m in CP treatments with three seeds later thinned to two plants per station (44 444 plants/ha target population). The NT treatments consisted of planting basins (PB) (15 × 15 × 15 cm) dug using a hand hoe during the dry season (May to August), for distribution of labour, and spaced at 0.9 × 0.70 m (to achieve a plant population of 15 873 plants/ha). The direct seeded (DS) treatments used an animal drawn Fitarelli® direct seeder (Fitarelli Máquinas Agrícolas, Brazil) pulled by two cattle, calibrated to drop seed and fertilizer in one line at a seed spacing of 0.25 m. With a 0.9 m row spacing this aimed to achieve a population of 44 444 plants/ha for maize. The commercial maize hybrid MRI 624, which takes 135 days to reach maturity, was sown across all treatments in all years.
In the rotation treatments, cotton (F135 variety) was planted at 0.90 × 0.50 m with five seeds per station and thinned to two plants per station (44 444 plants/ha target population). Local sunn hemp seed (C. juncea L. from 2009 to 2012 and Crotalaria ochroleuca G. Don in 2012–13) was dribbled into rip lines spaced at 0.45 m, at a rate of 40 kg seeds/ha.
All crops except for sunn hemp received a basal dressing fertilizer in the form of compound D [10 kg nitrogen (N) : 20 kg phosphorus pentoxide (P2O5) : 10 kg potassium oxide (K2O)] at 16.3 kg N/ha, 14.2 kg phosphorus (P)/ha, 13.5 kg potassium (K)/ha that is 163 kg/ha compound D. Top dressing in the form of urea (460 g/kg N) was applied to maize only at the rate of 92 kg N/ha, applied in equal splits at 4 and 7 weeks after sowing. Initial weed control was done using glyphosate at 2.5 litres/ha after planting followed by subsequent manual hoe weeding during the growing season. Depending on the cropping season, two to three weeding sessions were necessary to keep the crop stand weed-free. Crop residues were applied in all NT and CA systems at approximately 3–5 t/ha depending on the quality of the previous cropping season.
Sampling and data collection
Sampling for soil fauna was done on three sampling points in each plot in all seasons in January when there was adequate soil moisture (Fig. 1), using a metal frame of 0.25 × 0.25 m placed randomly in each plot. Soil layers of 10 cm each were excavated separately using a straight-edged spade. The soil sampling depths were 0–10, 10–20 and 20–30 cm. All crop residues in the area were removed and examined carefully for any macro-organisms present. The soils from different soil depths of the reported treatments were hand-sorted for all macro-organisms and placed in small, labelled containers with alcohol for further determination at a later stage.
Soil moisture was measured with a capacitance probe (PR-2) (Delta-T Devices Ltd., UK) using the access tubes installed in respective treatments, which measured moisture content to a depth of 1 m. Soil moisture measurements were taken twice a week during the cropping season. The soil moisture measurements were recorded in volume percentage which was converted to millimetres for the top 60 cm soil depth. Maize grain was harvested at physiological maturity in each plot. The grain was dried, weighed and converted to kg/ha, corrected to 125 g/kg moisture content.
Data analysis and statistical methods
Since two treatments were missing in the 2009/10 cropping season and treatments should be compared under equal conditions, all data from this season was removed before the statistical analysis.
The relationship between the six RotTill treatments, as defined in Table 1, and mean density of earthworms (Lumbricus terrestris), termites (Coptotermes formosanus), dung beetles (Scarabaeus viettei) and centipedes (Lithobius forficatus) was examined through principal component analysis (PCA). For this analysis, the 6 × 4 matrix of means was used, with RotTill treatments in rows and variables in columns. Each column was initially standardized to zero mean and unit variance, i.e. the PCA was based on the correlation matrix. The significance of the principal components was tested using the Forkman et al. (Reference Forkman, Josse and Piepho2019) full parametric bootstrap method for standardized data, with 200 000 bootstrap samples.
Univariate analyses were made for each of the variables (counts of termites, earthworms, dung beetles and centipedes). Linear fixed-effects and mixed-effects models were fitted using the lm and lmer functions, respectively, of R version 3.5.2 (Kuznetsova et al., Reference Kuznetsova, Brockhoff and Christensen2017; R Core Team, 2019). Differences between sampling depths were not investigated, because there were too many zeros to model the observations effectively at different levels of this factor. Fixed-effects factors were parameterized using the contr.sum method of coding, where ‘each coefficient compares the corresponding level of the factor with the average of the levels’ (Fox and Weisberg, Reference Fox and Weisberg2011). F-tests were performed using the Anova function of the car package. Results from Type III tests were presented. However, these did not differ much from results from Type II tests. Degrees of freedom were computed using the Kenward and Roger method. The Tukey method was used for all pairwise comparisons.
For each plot and species, the average count was computed over all seasons and depths, resulting in a dataset with 40 observations (ten treatments × four replicates). To achieve homoscedasticity, counts of termites were square root transformed, and counts of earthworms, dung beetles and centipedes were log transformed before analysis. The following model was fitted:
where y ijk is the transformed average count of the ith RotTill treatment (i = 1, 2, …, 6) in the kth plot of that treatment in the jth replicate (j = 1, 2, 3, 4). For RotTill treatments MCP, MDS and MPB, there was one plot per replicate (k = 1), for RotTill treatments CM-CP and CM-DS, there were two plots per replicate (k = 1, 2) and for RotTill treatment SCM-DS, there were three plots per replicate (k = 1, 2, 3). Furthermore, in model (1), μ is an intercept, α i is a fixed effect of the ith RotTill treatment, β j is a fixed effect of the jth replicate and e ijk is a normally distributed residual error, i.e. $e_{ijk}\,\sim \,N\,(0,\sigma _e^2 )$![]() .
.
For counts of termites, models including effects of seasons were fitted. The other variables included too many zeros for the normal approximation to apply. Observations were average counts, computed by plot and season, over depths. Thus, the complete dataset comprised 120 observations (three seasons × ten treatments × four replicates). The plot structure of this experiment includes the factors replicate and plot, whereas the treatment structure includes the factors RotTill treatment and crop (Bailey, Reference Bailey2008). In addition, the experiment includes the longitudinal factor season (Brien and Demétrio, Reference Brien and Demétrio2009). A linear mixed-effects model was used with random effects of the plot structure factors and their interaction with the longitudinal factor, and fixed effects of the treatment structure factors, the longitudinal factor and their interactions. Specifically, the following model was used:
where y ijkl is the square root of the average termite count of the ith RotTill treatment (i = 1, 2, …, 6), in the jth replicate (j = 1, 2, 3, 4), with the kth crop, in the lth season (l = 1, 2, 3). For RotTill treatments MCP, MDS and MPB, there was one crop (maize) per treatment (k = 1), for RotTill treatments CM-CP and CM-DS, there were two crops (maize and cotton) per treatment (k = 1, 2) and for RotTill treatment SCM-DS, there were three crops (maize, cotton and sunn hemp) per treatment (k = 1, 2, 3). The levels of the factor crop are the phases of the RotTill treatments (Patterson, Reference Patterson1964). Furthermore, in model (2), μ is an intercept, α i is a fixed effect of the ith RotTill treatment, β ik is a fixed effect of the kth crop within the ith RotTill treatment, γ l is a fixed effect of the lth season, (αγ)il is a fixed effect of RotTill treatment-by-season interaction and (αβγ)ikl is a fixed effect of crop-by-season interaction within RotTill treatment. Moreover, a j is a normally distributed replicate effect, i.e. $a_j\,\sim \,N\,(0,\sigma _a^2 )$![]() , b ijk is a normally distributed plot effect, i.e. $b_{ijk}\,\sim \,N\,(0,\sigma _b^2 )$
, b ijk is a normally distributed plot effect, i.e. $b_{ijk}\,\sim \,N\,(0,\sigma _b^2 )$![]() , c jl is a normally distributed replicate-by-season effect, i.e. $c_{jl}\,\sim \,N\,(0,\sigma _c^2 )$
, c jl is a normally distributed replicate-by-season effect, i.e. $c_{jl}\,\sim \,N\,(0,\sigma _c^2 )$![]() , and e ijkl is a normally distributed residual error, i.e. $e_{ijkl}\,\sim \,N\,(0,\sigma _e^2 )$
, and e ijkl is a normally distributed residual error, i.e. $e_{ijkl}\,\sim \,N\,(0,\sigma _e^2 )$![]() . The random effects are independent.
. The random effects are independent.
A third model was fitted for analysis of termite counts in maize plots only. The dataset included 72 observations (three seasons × six treatments × four replicates). The model was the same as model (2), but without effects of crops and interactions with crops:
where y ijl is the square root of the average termite count of the ith RotTill treatment (i = 1, 2, …, 6), in the jth replicate (j = 1, 2, 3, 4), in the lth season (l = 1, 2, 3) in the plot with maize. Furthermore, in model (3), μ is an intercept, α i is a fixed effect of the ith RotTill treatment, γ l is a fixed effect of the lth season and (αγ)il is a fixed effect of RotTill treatment-by-season interaction. Moreover, a j is a normally distributed replicate effect, i.e. $a_j\,\sim \,N\,(0,\sigma _a^2 )$![]() , b ij is a normally distributed plot effect, i.e. $b_{ij}\,\sim \,N\,(0,\sigma _b^2 )$
, b ij is a normally distributed plot effect, i.e. $b_{ij}\,\sim \,N\,(0,\sigma _b^2 )$![]() , c jl is a normally distributed replicate-by-season effect, i.e. $c_{jl}\,\sim \,N\,(0,\sigma _c^2 )$
, c jl is a normally distributed replicate-by-season effect, i.e. $c_{jl}\,\sim \,N\,(0,\sigma _c^2 )$![]() and e ijl is a normally distributed residual error, i.e. $e_{ijkl}\,\sim \,N\,(0,\sigma _e^2 )$
and e ijl is a normally distributed residual error, i.e. $e_{ijkl}\,\sim \,N\,(0,\sigma _e^2 )$![]() . The random effects are independent.
. The random effects are independent.
Fauna richness, the Shannon–Weiner diversity (H) index and the Pielou evenness (E) index were used to assess soil macrofauna composition in each plot. Fauna richness is the total number of species recorded in an environment. In the current study, richness ranged from 0 to 4.
The Shannon–Weiner diversity index was calculated using the following equation:
where N is the sum of the densities/m2 of the four soil macrofauna organisms, and n i is the density (/m2) of the ith soil macrofauna organism, i = 1, 2, 3, 4. In the current study, n i ln n i were defined as 0 when n i = 0, since
When none of the four species were observed, H was set to 0. The Shannon–Weiner diversity index is a measure of species composition, in terms of both the number of species and their relative abundances (Legendre and Legendre, Reference Legendre and Legendre1998, p. 237). A diverse cropping system, i.e. when H is high, is considered healthier than systems with low diversity (Altieri, Reference Altieri1999).
The Pielou evenness index, which relates diversity to its maximum value, was calculated as E = H/ln4 (Legendre and Legendre, Reference Legendre and Legendre1998, p. 243), since there were a maximum of four faunal species. Greater values of E signify greater uniformity between species abundances. The Pielou evenness index measures the distribution of each biota group present in an environment, i.e. how close the concentrations of the individual species are to each other. The evenness index ranges from 0 to 1. A low evenness index may indicate domination of some species over others.
Fauna richness, the Shannon–Weiner diversity index and the Pielou evenness index were analysed using the same model (2) as used for analysis of counts of termite. However, when analysing these variables, no transformation was needed.
Maize grain yield was analysed by year using the model
where y ijk is the observed maize grain yield using the ith RotTill treatment (i = 1, 2, …, 6) in the jth replicate (j = 1, 2, 3, 4), μ is an intercept, α i is a fixed effect of the ith RotTill treatment, β j is a fixed effect of the jth replicate and e ij is a normally distributed residual error, i.e. $e_{ij}\,\sim \,N\,(0,\sigma _e^2 )$![]() .
.
Results
Rainfall distribution and soil moisture content from 2009/10 to 2012/13 cropping seasons
All cropping seasons, except for 2011/12 season which had 476 mm of rainfall, received at least 700 mm rainfall (Fig. 1). During the 2009/10 cropping season, there were 4 days that received more than 50 mm of rainfall and the highest recorded was approximately 70 mm. During the study period, CP treatments had lower soil moisture than NT direct seeding and PB treatments with a maize crop during the days with no or low rainfall (Fig. 1).
Effect of experimental factors on earthworms, termite, dung beetles and centipedes
The PCA biplot shows that large counts of species were observed for NT and CA RotTill treatment, whereas low counts were observed for conventionally tilled MCP and CM-CP RotTill treatments. Centipedes were most abundant in plots with SCM-DS RotTill treatments (Fig. 2). However, since the first principal component was significant (P < 0.05), but the second was not, patterns in the biplot along the second principal component should not be over-emphasized (Forkman et al., Reference Forkman, Josse and Piepho2019).
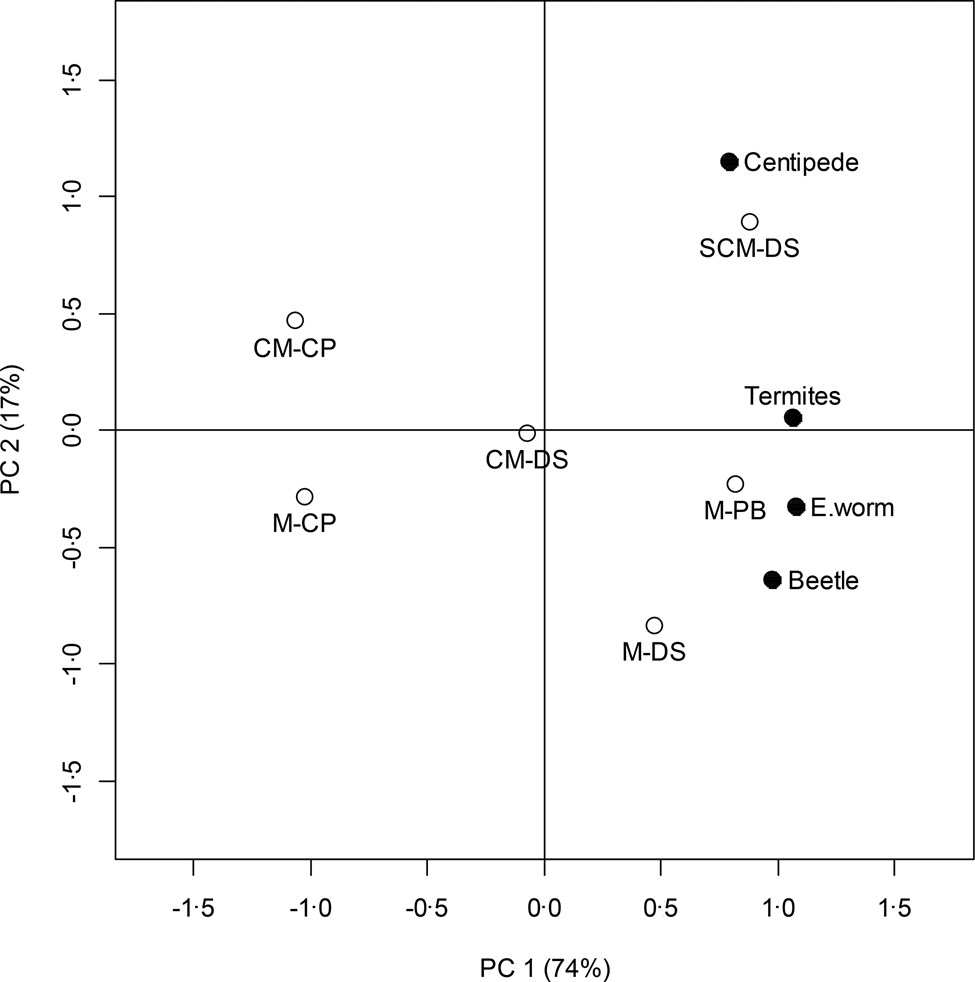
Fig. 2. PCA biplot of RotTill treatments and species. Before analysis, species were standardized to zero mean and unit standard deviation. The first principal component (PC1) accounts for 74%, and the second principal component (PC2) accounts for 17% of the total sum of squares. Empty circles indicate RotTill treatments. Letters before the dash denote the crop (C, cotton; M, maize; S, sunn hemp); those after the dash denote the tillage system (CP, conventional ploughing; DS direct seeding; PB, plant basins).
Earthworms
There were significant differences between the RotTill treatments for earthworms (P < 0.001), model 1 (Fig. 3). Earthworm density was lower in CP, with or without rotations (4 and 5 individuals/m2 respectively), than CM-DS and SCM-DS RotTill treatments (ranging between 18 and 27 earthworms/m2) throughout the study period (Fig. 3). Direct seeding and PB had significantly higher densities of earthworms and these treatments had approximately 260 and 450% higher densities, respectively, than CP treatments in the top 30 cm soil depth.
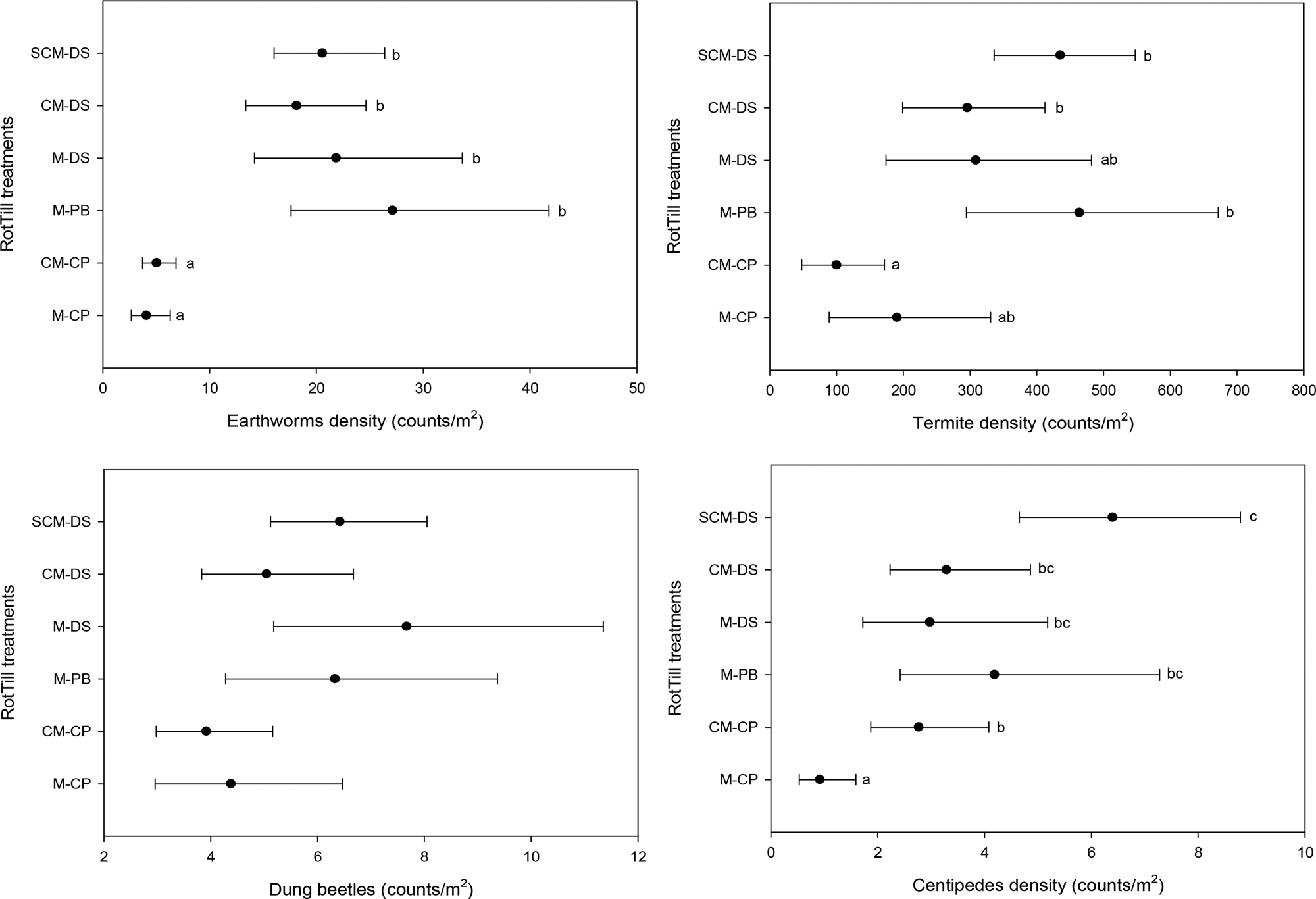
Fig. 3. Estimates of means, with 95% confidence intervals, for (a) earthworms, (b) termites, (c) dung beetles and (d) centipedes (model 1) in Monze Farmer Training Center, Zambia from 2011 to 2013 cropping season at 0–30 cm sampling depth. Letters before the dash in RotTill treatments denote the crop (C, cotton; M, maize; S, sunn hemp); those after the dash denote the tillage system (CP, conventional ploughing; DS direct seeding; PB, plant basins).
Termites
RotTill treatments had a significant effect on termites, model 1 (P < 0.001) (Fig. 3). The CP plots had lower counts of termites (190 and 100 termites/m2 with and without rotations, respectively) than plots with DS and PB tillage systems. Results from model 2 (Table 2) indicate that in SCM-DS, counts of termites were significantly higher in maize (681 termites/m2) than in sunn hemp (171 termites/m2). Also, cropping season had a significant effect on termites (Fig. 4). The 2010–11 cropping seasons had the lowest counts of termites. In maize plots only (model 3), the highest count of termites (681 termites/m2) was observed in the direct-seeding SCM rotation (Fig. 5).
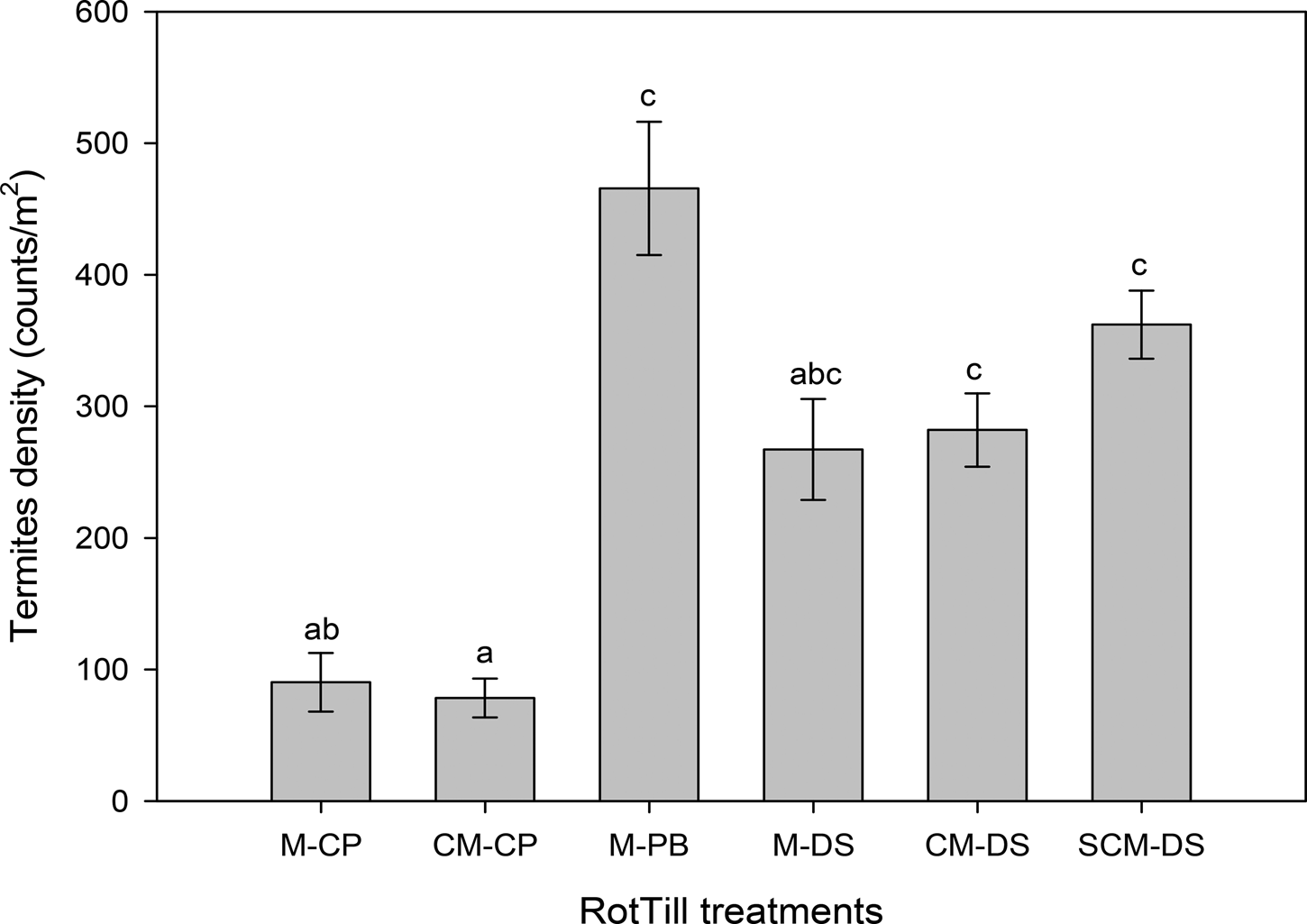
Fig. 4. Estimated marginal termite means of RotTillCrop treatments by season, in Monze Farmer Training Centre Zambia, model 2. Letters before the dash in RotTill treatments denote the crop (C, cotton; M, maize; S, sunn hemp); those after the dash denote the tillage system (CP, conventional ploughing; DS direct seeding; PB, plant basins).
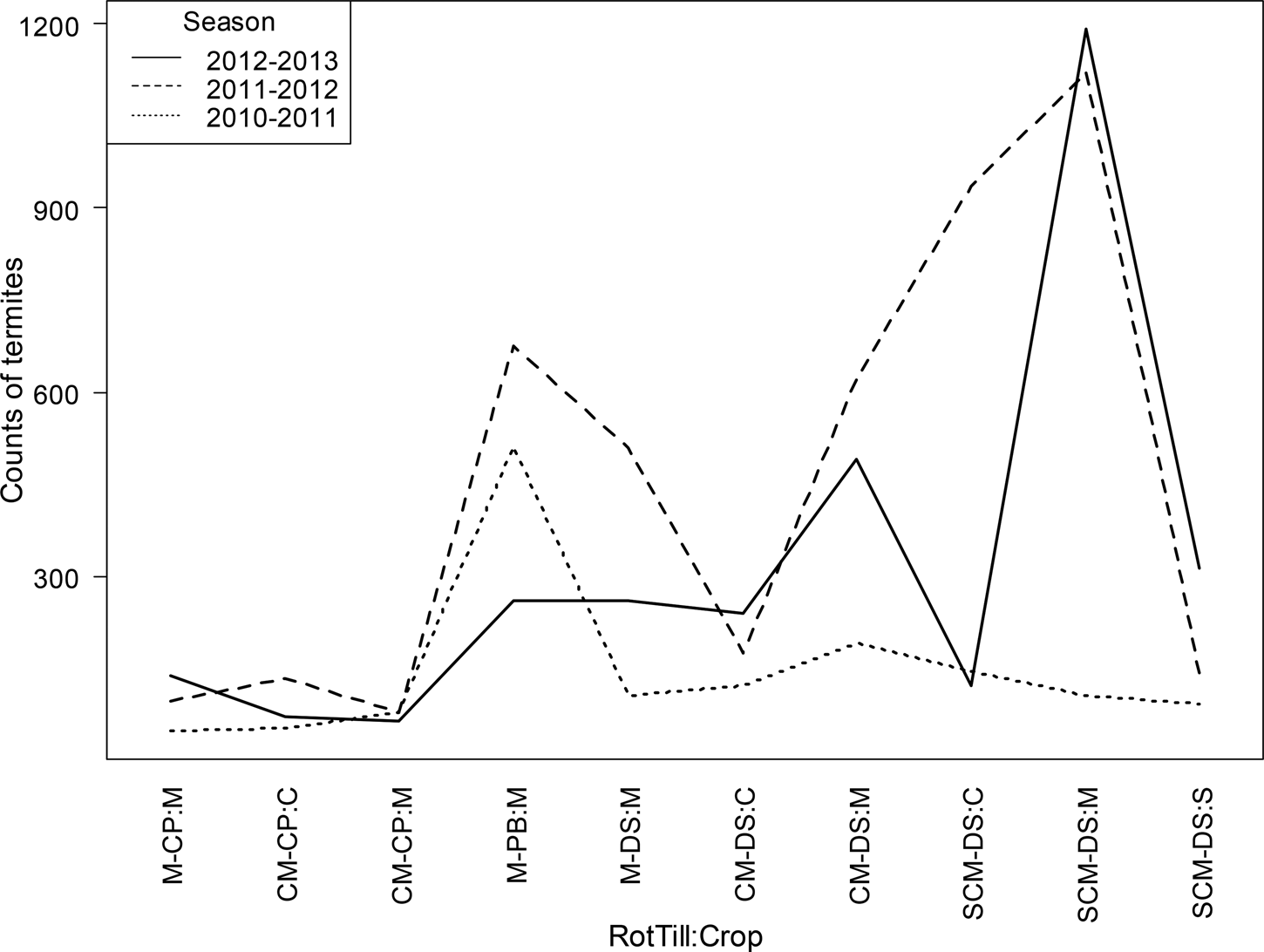
Fig. 5. Effect of RotTill on termites density in maize plots for three cropping seasons in Zambia, model 3. Means with different letters are significantly different from each other and error bars are drawn using standard error of mean. Letters before the dash in RotTill treatments denote the crop (C, cotton; M, maize; S, sunn hemp); those after the dash denote the tillage system (CP, conventional ploughing; DS direct seeding; PB, plant basins).
Table 2. Effect of crop on counts of termites/m2 at Monze Farmer Training Centre Zambia from 2011 to 2013 cropping seasons
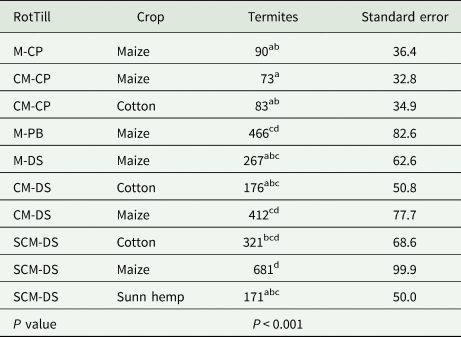
Numbers followed by a different letter are significantly different from each other. The letters before the dash in RotTill treatments denote the crop (C, cotton; M, maize; S, sunn hemp) and those after the dash denote the tillage system (CP, conventional ploughing; DS, direct seeding; PB, plant basins).
Dung beetles
The results show that RotTill had a significant effect on dung beetles, model 1 (P < 0.039) (Fig. 3). However, due to P value adjustments for pairwise comparisons, no specific treatment was significantly different from any other. The numbers observed were generally low. More dung beetles were observed in MDS, MPB, CM-DS and SCM-DS treatments, 5–7 beetles/m2, than in CP treatments (3–4 beetles/m2), with maize and cotton rotations having the lowest counts.
Centipedes
Centipede density was affected significantly by RotTill treatments, model 1 (P < 0.001) (Fig. 3). The observed numbers of centipedes during the study period were generally low. The direct seeding treatments had the highest density of centipedes (6 individuals/m2), while the CP had the lowest density of 1 individual/m2. PB were not significantly different from conventional ploughing and direct seeding (Fig. 3).
Biota diversity index, evenness and richness
RotTill treatments had no significant effect on biota diversity index and biota evenness in any season, model 2 (Table 3). Results show that RotTill treatments had a significant effect on biota richness during all cropping seasons (Table 3). The conventional ploughing treatment with continuous maize had the lowest biota richness (1.92) while CA with three crops had the highest (3.07) (Table 3). PB and direct seeding had significantly higher biota richness than CP. The results show that RotTill had no significant effect on biota evenness.
Table 3. Effect of RotTill treatments on biota diversity, evenness and richness at Monze Farmer Training Centre Zambia from 2011 to 2013 cropping seasons
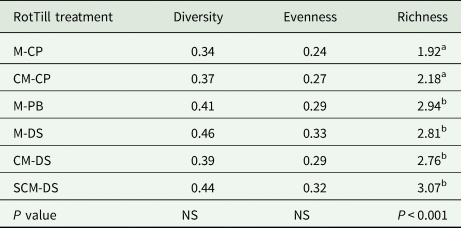
NS, not significant.
Means with different letters are significantly different from each other. The letters before the dash in RotTill treatments denote the crop (C, cotton; M, maize; S, sunn hemp) and those after the dash denote the tillage system (CP, conventional ploughing; DS, direct seeding; PB, plant basins).
Maize grain yield
RotTill treatments had a significant effect on maize grain yields in all cropping seasons, model 4. Conventional ploughing had lower grain yields (at most 4074 kg/ha) than CA and NT treatments (ranging between 3000 and 6000 kg/ha). The direct seeding treatment with three crop rotations had the highest grain yields in all seasons (Fig. 6).
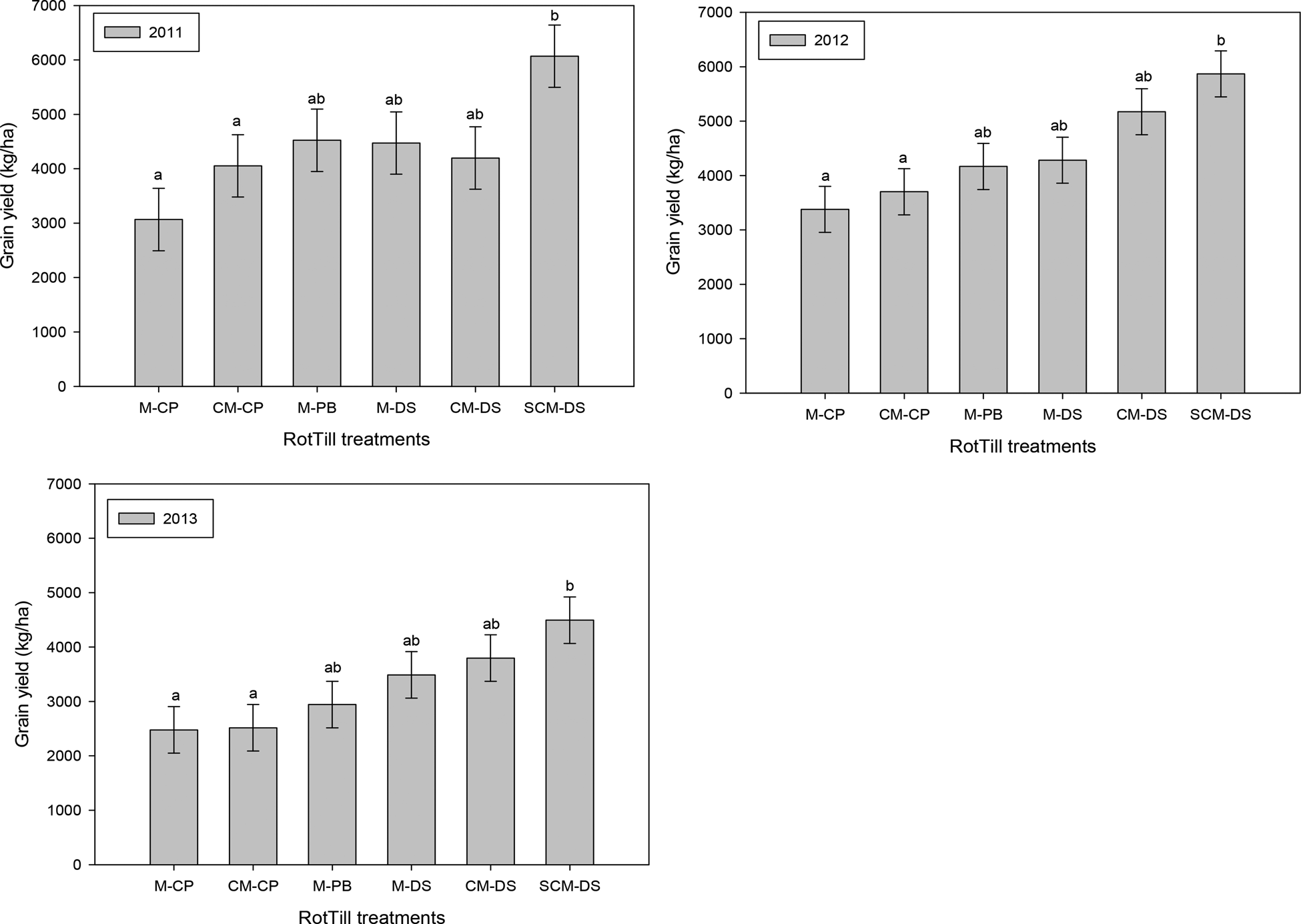
Fig. 6. Effect of treatments on maize grain yield for 2010–11 to 2012–13 cropping seasons in Zambia. Means with different letters are significantly different from each other and error bars are drawn using standard error of mean. Letters before the dash in RotTill treatments denote the crop (C, cotton; M, maize; S, sunn hemp); those after the dash denote the tillage system (CP, conventional ploughing; DS direct seeding; PB, plant basins).
Discussion
Effect of system on earthworms, termites, dung beetles and centipedes
Higher soil water retention and presence of crop residues in CA and NT treatments, with crop residue retention, led to greater earthworm densities compared with CP treatments. This result concurs with Crittenden et al. (Reference Crittenden, Eswaramurthy, de Goede, Brussaard and Pulleman2014), who reported a positive correlation between earthworm abundance, soil water and soil organic matter content. Previous studies by Thierfelder and Wall (Reference Thierfelder and Wall2009, Reference Thierfelder and Wall2010) recorded higher soil water, earthworms and soil organic carbon in the CA and NT treatments in the Monze long-term trial. Lower earthworms were observed in the rotational phase where sunn hemp was grown compared with maize and cotton in the 3-year rotation, indicating that the earthworms have a range of food preferences (Curry and Schmidt, Reference Curry and Schmidt2007) and might be affected by root exudates produced by sunn hemp (Wang et al., Reference Wang, McSorley and Gallaher2003, Reference Wang, McSorley, Gallaher and Kokalis-Burelle2008). Mouldboard ploughing reduces earthworm density through low soil moisture retention, physical damage and exposure to heat and predation (Crittenden et al., Reference Crittenden, Eswaramurthy, de Goede, Brussaard and Pulleman2014).
The least density of termites was observed in CP treatments. This is due to the absence of crop residues in conventional systems that would otherwise be available as a food source for the termites (Nyagumbo et al., Reference Nyagumbo, Munamati, Mutsamba, Thierfelder, Cumbane and Dias2015). Also, minimum soil disturbance practices under CA and NT reduce destruction of termite colonies; hence, greater densities of termites were observed in CA treatments. It is important to note that termites exhibited a preference for different types of residues. The maize crop residues were definitely preferred over sunn hemp (Yêyinou Loko et al., Reference Yêyinou Loko, Orobiyi, Agre, Dansi, Tamò and Roisin2017), which again is in response to residue quality, C : N ratio and the composition of maize stalks compared with sunn hemp residues.
The density of dung beetles and centipedes was very low across all treatments in all seasons and showed little contribution to soil fauna. Dung beetles feed on young plant bugs in situations where normal food sources are scarce (Vodka et al., Reference Vodka, Konvicka and Cizek2009). However, having a large density of beetles can be desirable within CA systems, since the adult beetles also feed on insects with smooth skins such as aphids, which have a detrimental effect on crops but a positive effect on ants. Centipedes prefer damp and dark places such as leaf litter and they feed on insects (Vodka et al., Reference Vodka, Konvicka and Cizek2009). Thus, they are likely to increase in CA systems where greater crop residue cover is promoted for a longer term.
Effect of different systems on biota composition
Biota diversity index
The current results indicated that different cropping systems had no significant effect on soil biota diversity index. Conventional ploughing had lower biota diversity than CA treatments, as tillage destroys biota habitats and exposes them to predation. Retention of crop residues under CA facilitates the build-up of food reserves, unlike in CP systems that favour burning or grazing of crop residues as common practices (Thomson and Hoffmann, Reference Thomson and Hoffmann2007). In addition, crop rotations increase the variety of crop residues available for ground cover, which can attract a wider range of macro-organisms.
Biota richness
Conventional mouldboard ploughing resulted in lower biota richness at all depths than all the other treatments, due to a shortage of food to attract different biota groups. The mixing of soil during ploughing is known to destroy the habitats of macro-organisms and reduce the availability of litter to feed on, hence it disturbs the life-cycle of macro-organisms (Blanco-Canqui and Lal, Reference Blanco-Canqui and Lal2010). Also, the moderation of soil temperature fluctuations and increased soil water on residue-covered fields promotes the occurrence of macro-organisms by simultaneously providing food and shelter via the crop residues (Kassam et al., Reference Kassam, Friedrich, Shaxson and Pretty2009). This facilitates faster breakdown of crop residues by macro-organisms, increasing the surface area for micro-organisms to act upon the remaining crop residues (Hesammi et al., Reference Hesammi, Farshidi, Sadatebrahimi and Talebi2014). High biota richness is desirable within CA systems because it may result in faster degradation of crop residues, which in turn enhances a build-up of soil fertility.
Biota evenness
Generally, the biota evenness was low across all treatments. This indicates the dominance of a few species. Termites were observed in higher numbers than other organisms. Biota evenness of the ploughed treatment with maize only was close to zero because there only termites were observed in high numbers in most of the cropping seasons. This is due mainly to the absence of crop residues that could be used as food for other faunal species. However, such a situation often results in crop damage when termites feed on the growing crops instead of residues, thus causing lodging and associated yield losses. Hence, the availability of crop residues may help to reduce the negative effect of termites on crops (Nyagumbo et al., Reference Nyagumbo, Munamati, Mutsamba, Thierfelder, Cumbane and Dias2015). Decomposition of crop residues can be elevated due to high termite activity in all seasons, which may lead to improved soil fertility within a short time under CA (Brown and Whitford, Reference Brown and Whitford2003) or more crop damage once the residues have been eaten. Therefore, the trade-offs must be considered. In a trial in Mozambique, Nyagumbo et al. (Reference Nyagumbo, Munamati, Mutsamba, Thierfelder, Cumbane and Dias2015) could not find a viable biological remedy for controlling termites and had to use commercial chemicals (e.g. Fipronil) as a termiticide, leading to good control but fewer associated benefits. Site-specific solutions need to be found where termites reach pest proportions, to control the termites on one hand without losing their beneficial effect on the other.
Maize grain yield
Conventional ploughing treatments had lower maize grain yield than CA treatments. Thierfelder and Wall (Reference Thierfelder and Wall2009, Reference Thierfelder and Wall2010) reported that this is due to low moisture retention in the absence of crop residues that improves water infiltration and reduces run-off. Also, the absence of crop rotations in CP resulted in the low soil fertility status (especially nitrogen), which affects crop productivity. Increased crop diversity in CA systems resulted in increased soil biological activity, and mobilization of nutrients that may have helped increase the maize grain yields.
Conclusions
CA and NT treatments had higher concentration of earthworms and termites than treatments under conventional tillage. Termites were observed in high concentration when maize was grown under NT. CA treatments had a higher abundance of organisms because of minimum soil disturbance and retention of crop residues. Based on the results obtained in the current study, the hypothesis tested can be accepted and the following concluded: CA and NT with crop residue retention have potential to increase biodiversity below-ground of species that participate in litter decomposition, which may increase soil organic matter content and improve soil structure. Combined with retention of crop residues, there is potential to improve the conditions for soil biota, e.g. soil moisture retention, which is essential for earthworm survival. Also, crop residues act as sources of food for micro- and mesofauna, thus attracting more macro-organisms than in CP where crop residues are removed, burnt or fed to livestock.
Inclusion of rotational crops such as sunn hemp reduced soil biota such as termites. When continuous maize sole cropping was practiced, termite abundance increased. Thus, practicing rotations with these crops have potential to moderate termite numbers in the cropping system.
Although increase in soil biota is crucial for improving crop productivity, some macro-organisms may damage the crop physically, e.g. termites. Hence, there is a need to identify threshold levels to recommend the time when control is necessary. However, the current study measured biota activity only once in the season, which could paint an incomplete picture as some macro-organisms may have been missed, or increased during the time of sampling and decreased afterwards. Thus, further studies are required where traps can be set and the sampling times spread throughout the season, which can be helpful in obtaining more information about biota activities under CA systems throughout the season.
Acknowledgements
We wish to thank the anonymous reviewer for advice on statistical modelling.
Financial support
This work was supported by CIMMYT CA projects funded by the International Fund for Agriculture Development (IFAD). It is embedded in the MAIZE CRP (www.maize.org) with special emphasis on sustainable intensification of maize cropping systems. The donors of MAIZE and their financial support are gratefully acknowledged.
Conflict of interest
None.
Ethical standards
Not applicable.











