Fe is an essential trace element in many physiological processes, and systemic Fe homeostasis plays an essential role in metabolism( Reference Andrews and Schmidt 1 ). Hepcidin is a peptide hormone that is produced and excreted by the liver. Hepcidin is encoded by the hepcidin antimicrobial peptide ( HAMP) gene and regulates systemic Fe homeostasis by binding to ferroportin, the only known mammalian non-haeme Fe export protein. This binding leads to the degradation of ferroportin, thereby controlling Fe transport and ultimately regulating several processes, including Fe absorption in the intestine, Fe mobilisation from the liver and Fe recycling from the macrophages( Reference Nemeth, Tuttle and Powelson 2 – Reference Ganz and Nemeth 4 ).
The expression of hepatic HAMP is regulated by dietary Fe, inflammation and erythropoietin activity, all of which primarily involve the bone morphogenetic protein (BMP) mothers against decapentaplegic homolog protein (SMAD) and Janus kinase–signal transducer and activator of transcription signalling pathways( Reference Ganz 5 – Reference Pinto, Ribeiro and Pontes 7 ). Impaired hepcidin secretion causes Fe overload, and hepcidin overexpression is associated with many diseases, including anaemia of chronic disease and Fe-deficiency anaemia( Reference Ganz and Nemeth 4 ). Recent studies( Reference Sun, Vaja and Babitt 8 , Reference Guan, An and Zhang 9 ) have focused on the identification of hepcidin antagonists. For example, dorsomorphin and its derivatives (which are inhibitors of the BMP/SMAD pathway)( Reference Yu, Hong and Sachidanandan 10 , Reference Theurl, Schroll and Sonnweber 11 ) and an IL-6 antagonist( Reference Song, Tomosugi and Kawabata 12 ) have a curative effect in a mouse model of chronic inflammation-associated anaemia. However, further studies are needed to test the safety of these compounds in clinical applications. The ultimate therapeutic goal is to treat Fe metabolism disorders using functionally active foods that target hepcidin and/or its regulatory proteins( Reference Wang, Trebicka and Fu 13 , Reference Fung, Sugianto and Hsu 14 ).
Since ancient times, many empirical dietary therapies for treating a wide variety of diseases have emerged in both traditional Chinese medicine and dietary culture( Reference Li, Yin and Saito 15 , Reference Koo 16 ). Food therapies for treating anaemia are abundant in the traditional Chinese pharmacopoeia, particularly foods that have unique colours( Reference Graziose, Lila and Raskin 17 ). However, because it is generally more difficult to absorb Fe from plant matter than haeme Fe from animal sources( Reference Layrisse, Cook and Martinez 18 ), we hypothesised that plant foods that treat anaemia might actually function by regulating hepcidin expression rather than serving as a significant source of dietary Fe. Traditionally, Chinese foods that are black in colour are believed to contain more nutrients and to induce erythropoiesis; in China, these foods are called ‘black foods’. In the present study, to investigate their function and molecular mechanisms, we obtained extracts of several black foods, including Glycine max (black soyabean, also known as Hei Dou), Auricularia auricula-judae (black fungus, Hei Mu Er), Sesamum indicum seeds (black sesame seeds, Hei Zhi Ma) and Diospyros lotus (date plum or persimmon, Hei Zao), and tested their effects on hepcidin expression. We found that black soyabean extract regulates Fe metabolism by inhibiting hepcidin expression both in vitro and in vivo.
Experimental methods
Preparation of experimental materials
Standard water extracts (10:1) of four foods, including G. max, A. auricula-judae, S. indicum seeds and D. lotus, were purchased from Hao Yang Biotech Company Limited for the first screening stage (Table 1). High-concentration extracts of black soyabean coat (containing at least 30 % anthocyanins) were purchased from JF-Natural Company Limited. These extracts were dissolved in sterile PBS to 50 mg/ml. Recombinant human BMP6 and recombinant human IL-6 were purchased from R&D Systems. Each aqueous extract was filtered through a 0·22 μm membrane (Millipore) before being added to the cultured cells.
Table 1 Iron concentrations and functional components of food extracts that were used in the cell line screening experiment (Mean values and standard deviations, n 3)
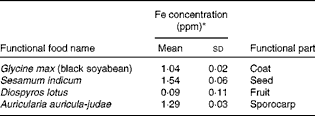
ppm, Parts per million.
* Total Fe concentration was measured using inductively coupled plasma MS.
Cell-culture assays
HepG2 cells (a human hepatocyte cell line) and human embryonic kidney (HEK293) cells were obtained from the Cell Bank of Shanghai Institutes for Biological Sciences, CAS, and were cultured in Dulbecco's modified Eagle's medium (Gibco) supplemented with 10 % heat-inactivated fetal bovine serum (Gibco) and 1 × penicillin–streptomycin (Gibco); the cells were incubated at 37°C in 5 % CO2. The Cell Counting Kit-8 (Dojindo Laboratories) was used in accordance with the manufacturer's instructions to measure black soyabean seed coat extract (BSSCE) cytotoxicity. Methods reported by Poli et al. ( Reference Poli, Girelli and Campostrini 19 ) were used to assess the phosphorylation levels of Smad1/5/8, extracellular signal-regulated kinase (Erk)1/2 and signal transducer and activator of transcription (Stat)3. For screening, HepG2 cells were treated for 12 h with 200 μg/ml of each functional food extract. To test the inhibitory effect of BSSCE on BMP6- and IL-6-induced HAMP expression, the cell-culture medium was pretreated with BSSCE for 30 min before stimulation with 20 ng/ml BMP6 or 50 ng/ml IL-6, respectively, and the cells were then incubated for 12 h( Reference Guan, An and Zhang 9 , Reference Poli, Girelli and Campostrini 19 , Reference Steinbicker, Sachidanandan and Vonner 20 ).
Luciferase reporter assay
In accordance with the manufacturer's instructions (Fugene HD Transfection Reagent; Promega), we plated HEK293 cells in twenty-four-well plates at least 1 d before transfection, and the medium was replaced when the cells reached approximately 60 % confluence. The HAMP promoter luciferase reporter gene construct pGL3-HAMP was generated, containing 2·7 kb of the 5′-flanking genomic region of the human HAMP gene plus the 5′-UTR (untranslated region, from − 2700 to +71 bp), and the pGL3-HAMP and the control Renilla reporter were co-transfected( Reference Truksa, Lee and Beutler 21 ). After 24 h, the cells were subjected to a variety of treatments and then lysed in 150 μl of luciferase cell-culture lysis reagent (Promega). The cell lysates were analysed for luciferase activity using a dual-luciferase reporter assay system (Promega). Relative luciferase activity was calculated as the ratio of firefly: Renilla luciferase. All the experiments were carried out at least three times( Reference Pandur, Sipos and Grama 22 ).
Animal experiments
Male C57BL/6 mice (SLRC Laboratory Animal Company Limited) aged 8 weeks were maintained under pathogen-free conditions and given free access to an AIN-76A standard diet (Fe concentration: 57·23 (sd 1·24) parts per million)( 23 ) or a BSSCE-containing diet (Fe concentration: 58·36 (sd 2·12) parts per million). The experimental mice were randomly assigned to the various groups and fed a diet containing 2·0 % (w/w) dried BSSCE (see Fig. 5(A)). After BSSCE treatment for 0, 1, 7, 15 or 30 d, the mice were killed under anaesthesia (5 % chloral hydrate, 10 ml/g body weight by intraperitoneal injection). Whole blood was collected, and the livers and spleens were harvested for further analysis. The protocols for measuring serum and tissue Fe concentrations have been described previously( Reference Zhang, Zhang and An 3 ); each group contained six to eight mice. All the animal experiments were approved by the Institutional Animal Care and Use Committee of Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, and Animal Care and Use Committee of Zhejiang University.
RNA extraction and quantitative PCR analysis of mRNA transcripts
RNA was extracted as described previously( Reference Zhang, Zhang and An 3 ) using the SuperfecTRI RNA Isolation Reagent (Pufei). The following primer sequences (5′–3′) were used for quantitative PCR: HAMP: forward CAGCTGGATGCCCATGTTC/reverse CAGCAGCCGCAGCAGAA; ACTIN: forward CACGGCATCGTCACCAACT/reverse CACGCAGCTCATTGTAGAAGGT; mouse Hamp1: forward GCACCACCTATCTCCATCAACA/reverse TTCTTCCCCGTGCAAAGG; mouse Actb (β-actin): forward AAATCGTGCGTGACATCAAAGA/reverse GCCATCTCCTGCTCGAAGTC; mouse inhibitor of DNA binding 1 (Id1): forward CGCAGCCACCGGACTCT/reverse AACCCCCTCCCCAAAGTC; mouse Bmp6: forward ATGGCAGGACTGGATCATTGC/reverse CCATCACAGTAGTTGGCAGCG.
Western blot analysis
The cells were lysed and analysed as described previously( Reference Zhang, Zhang and An 3 ). The following primary antibodies were used: rabbit anti-pSmad1/5/8 (1:1000; Cell Signaling Technology, no. 9511s); rabbit anti-Smad1 (1:1000; Cell Signaling Technology, no. 9743s); rabbit anti-pStat3 (1:1000; Cell Signaling Technology, no. 9131s); rabbit anti-Stat3 (1:1000; Cell Signaling Technology, no. 9132s); rabbit anti-pErk1/2 (1:1000; Cell Signaling Technology, no. 4376); rabbit anti-Erk1/2 (1:1000; Cell Signaling Technology, no. 4695); mouse anti-β-actin (1:2000; Sigma-Aldrich, no. A5316).
Inductively coupled plasma MS measurement of total iron concentrations
An Agilent 7500cx inductively coupled plasma MS system equipped with a G3160B I-AS integrated autosampler was used to measure Fe concentrations in the functional food extracts that were used for cell treatment and added to the animal diet. This procedure was carried out as described previously( Reference Sun, Yu and Huang 24 ).
Statistical analysis
Group differences were analysed using ANOVA, and Tukey's post hoc test was used to compare two specific groups. Some data were log-transformed to meet the assumption of homogeneity of variances (Bartlett's test). Where applicable, group means without a common letter differ significantly. Differences with P< 0·05 were considered to be statistically significant.
Results
Screening black foods for their effects on HAMP expression in HepG2 cells
HepG2 cells, a human hepatocyte cell line, were used for the initial screening process. We treated cells for 12 h with 200 μg/ml of the standard water extract of each food, including black soyabean, black fungus, black sesame seeds and date plum (persimmon). Of these four black foods that were tested, only BSSCE significantly inhibited hepcidin expression (Fig. 1). Importantly, the total Fe concentration of this food is relatively low (Table 1), suggesting that the effect of BSSCE on hepcidin expression is independent of the extract's Fe content. Based on this initial screening process, we used high-concentration extracts of black soyabean coat (containing at least 30 % anthocyanins) in our subsequent experiments.

Fig. 1 Black soyabean seed coat extract (BSSCE) inhibits hepcidin expression. (A) HepG2 cells were treated for 12 h with 200 μg/ml of the standard water extract of the indicated foods, after which HAMP expression was measured. Cell viability was measured using the Cell Counting Kit-8 assay. (B) HepG2 cells were treated with the indicated concentrations of BSSCE for 12 h. (C) HepG2 cells were treated with 200 μg/ml BSSCE for the indicated number of hours. Values are means and standard deviations represented by vertical bars; n 3 experiments per group. * Mean values were significantly different from that of the blank group (P< 0·05). Blank, basal control cells treated with PBS.
Black soyabean seed coat extract significantly inhibits HAMP expression in HepG2 cells
BSSCE, which contains ≥ 30 % anthocyanins, inhibited the expression of HAMP (the gene that encodes hepcidin) in the HepG2 cell line, exhibiting both dose and time dependence. At a concentration of 200 μg/ml, BSSCE reduced HAMP expression to only 6 % of the control levels (Fig. 2(A)). In the time-course experiments, HAMP expression decreased rapidly, reaching < 9 % of the control levels within 12 h (Fig. 2(B)). Moreover, the phosphorylation levels of SMAD1/5/8, a group of transcription factors that activate the HAMP gene, also decreased with similar dose and time dependence; in contrast, the phosphorylation levels of STAT3 and ERK1/2 were relatively unchanged (Fig. 2(C) and (D)).

Fig. 2 Black soyabean seed coat extract (BSSCE) inhibits HAMP expression and mothers against decapentaplegic homolog protein 1/5/8 (Smad1/5/8) and signal transducer and activator of transcription 3 (Stat3) phosphorylation in HepG2 cells. (A) HAMP expression in HepG2 cells that were treated for 12 h with the indicated concentrations of BSSCE. (B) HAMP expression in HepG2 cells that were treated with 200 μg/ml BSSCE for the indicated time points. (C) Western blot analysis of phosphorylated SMAD1/5/8 (pSMAD1/5/8), phosphorylated STAT3 (pSTAT3) and phosphorylated extracellular signal-regulated kinase 1/2 (pERK1/2) in HepG2 cells after BSSCE treatment for 12 h. (D) Western blot analysis of pSMAD1/5/8, pSTAT3 and pERK1/2 levels in HepG2 cells that were treated for the indicated time points with 200 μg/ml BSSCE. β-Actin was used as a loading control. The summary data in (A) and (B) are presented as means and standard deviations represented by vertical bars; n 3 replicates per group. a,b,c,d,eMean values with unlike letters were significantly different (P< 0·05).
Black soyabean seed coat extract potently inhibits bone morphogenetic protein 6- and IL-6-induced HAMP expression
BMP6 plays a key role in the regulation of hepcidin expression( Reference Andriopoulos, Coradini and Xia 25 ). In our experiments, treatment with BMP6 (20 ng/ml) triggered an almost 5·5-fold increase in HAMP expression relative to the basal levels, and this increase was inhibited by treating cells with increasing concentrations of BSSCE; at a concentration of 400 μg/ml, BSSCE reduced HAMP expression to 5·6 % of the basal levels (Fig. 3(A)). BSSCE had a similar inhibitory effect on HAMP expression induced by IL-6; IL-6 (50 ng/ml) increased HAMP expression 2-fold( Reference Steinbicker, Sachidanandan and Vonner 20 , Reference Nishimoto and Kishimoto 26 ), and BSSCE (50–400 μg/ml) inhibited IL-6-induced expression; HAMP expression was reduced to 2·3 % of the basal levels following treatment with 400 μg/ml BSSCE (Fig. 3(B)). Western blot analysis also revealed that BMP6-induced phosphorylation of SMAD1/5/8 was significantly reduced by BSSCE treatment, whereas the phosphorylation levels of STAT3 and ERK1/2 remained unchanged (Fig. 3(C)). Moreover, IL-6 induced the phosphorylation of STAT3 and SMAD1/5/8, and BSSCE treatment reduced the phosphorylation of SMAD1/5/8 but not of STAT3 (Fig. 3(D)).

Fig. 3 Black soyabean seed coat extract (BSSCE) inhibits bone morphogenetic protein 6 (BMP6)- and IL-6-induced HAMP expression. (A and B) HAMP expression in HepG2 cells that were cultured with the indicated concentrations of BSSCE in the presence or absence of 20 ng/ml BMP6 (A) or 50 ng/ml IL-6 (B). (C–D) Western blot analysis of phosphorylated mothers against decapentaplegic homolog proteins 1/5/8 (pSMAD1/5/8), phosphorylated signal transducer and activator of transcription 3 (pSTAT3) and phosphorylated extracellular signal-regulated kinase 1/2 (pERK1/2) in HepG2 cells after BSSCE treatment in the presence or absence of 20 ng/ml BMP6 (c) or 50 ng/ml IL-6 (d) for 12 h. β-Actin was used as a loading control. The summary data in (A) and (B) are presented as means and standard deviations represented by vertical bars; n 3 replicates per group. a,b,c,d,eMean values with unlike letters were significantly different (P< 0·05).
Black soyabean seed coat extract inhibits bone morphogenetic protein 6- and IL-6-induced HAMP expression measured using a luciferase assay
We found that treating cells for 24 h with BMP6 (20 ng/ml) or IL-6 (50 ng/ml) increased the relative activity of a HAMP reporter luciferase assay by 2·5- and 3·5-fold, respectively (Fig. 4). Moreover, BSSCE completely inhibited the up-regulation of BMP6-induced HAMP expression, decreasing the relative activity of the HAMP promoter to 10 % of the basal levels (Fig. 4(A)). Similarly, IL-6-induced HAMP expression was significantly decreased by BSSCE treatment; luciferase activity was reduced to 33 % of the basal levels following treatment with 400 μg/ml BSSCE (Fig. 4(B)).

Fig. 4 Black soyabean seed coat extract (BSSCE) inhibits bone morphogenetic protein 6 (BMP6)- and IL-6-induced HAMP transcription measured using a luciferase promoter assay. HAMP promoter activity was measured as relative luciferase activity (calculated as the ratio of firefly:Renilla luciferase) in HEK293 cells transfected with a HAMP promoter-driven luciferase reporter. The cells were treated with BSSCE in the presence or absence of 20 ng/ml BMP6 (A) or 50 ng/ml IL-6 (B) for 24 h. Data are means and standard deviations represented by vertical bars; n 3 replicates per group. a,b,c,d,eMean values with unlike letters were significantly different (P< 0·05).
Black soyabean seed coat extract inhibits Hamp1 expression and increases iron mobilisation in mice
We next carried out animal experiments to investigate the effect of BSSCE on Hamp1 expression in vivo. C57BL/6 mice were maintained on a diet containing 2·0 % BSSCE for various time periods up to 30 d, after which the mice were killed (Fig.5(A)). Even after 1 d on the BSSCE-containing diet (i.e. the day 1 group), hepatic hepcidin (Hamp1) expression in the treated mice decreased significantly to approximately 46 % of that in the standard diet-fed mice (i.e. the day 0 group), and this decrease persisted up to 30 d of treatment (Fig. 5(B)). Interestingly, the phosphorylation of Smad1/5/8 was also reduced, particularly in the mice treated for 7 d, and this reduction was transient, as the phosphorylation levels returned to approximately control levels by 30 d; in contrast, the phosphorylation of neither Stat3 nor Erk1/2 was affected by BSSCE treatment (Fig. 5(C)). In the day 7 and day 15 groups, the hepatic non-haeme Fe concentrations were approximately 75 % of those in the control diet-fed mice and increased slightly to 82 % of those in the day 30 group (Fig. 5(D)). Splenic non-haeme Fe concentrations decreased steadily, reaching 65 % of those in the control group by day 15 (Fig. 5(E)). Serum Fe concentrations increased significantly, reaching approximately 135 % of the control levels in the day 7, 15 and 30 groups (Fig. 5(F)). Similarly, transferrin saturation in the treated groups increased steadily, reaching 132 % of the control levels by day 30 (Fig. 5(G)). Furthermore, because Hamp1 expression decreased significantly in the BSSCE-fed mice, we also measured the mRNA levels of the Id1 (Fig. 5(H)) and Bmp6 (Fig. 5(I)) genes (both of which have been reported to exhibit the same regulatory changes as Hamp1 in the liver( Reference Kautz, Meynard and Monnier 27 )), and we found that the BSSCE-fed mice had similar transient reductions in the expression of these two genes. We also measured several haematological parameters in the BSSCE-fed mice and found that their erythrocyte counts, Hb concentrations and haematocrit values were elevated relative to those in the control diet-fed mice (Table 2).

Fig. 5 Black soyabean seed coat extract (BSSCE) inhibits hepcidin expression in vivo and increases iron mobilisation in mice. (A) Male C57BL/6 mice were fed a standard diet (![]() ) containing 2·0 % BSSCE (■) for 0, 1, 7, 15 or 30 d, after which they were killed for biochemical and physiological analyses. (B) Hamp1 expression normalised to Actb (β-actin) expression. (C) Western blot analysis of phosphorylated mothers against decapentaplegic homolog protein 1/5/8 (pSmad1/5/8), phosphorylated signal transducer and activator of transcription 3 (pStat3) and phosphorylated extracellular signal-regulated kinase 1/2 (pErk1/2) levels. β-Actin was used as a loading control. (d–i) Mobilised hepatic iron concentrations (D), non-haeme splenic iron concentrations (E), serum iron concentrations (F), serum transferrin saturation (G), and the expression levels of hepatic inhibitor of DNA binding 1 (Id1) (H) and bone morphogenetic protein 6 (Bmp6) (I) were measured in mice that were treated with BSSCE for the indicated number of days. The summary data in (B) and (D)–(I) are presented as means and standard deviations represented by vertical bars; n 6–8 mice/group. a,b,cMean values with unlike letters were significantly different (P< 0·05).
) containing 2·0 % BSSCE (■) for 0, 1, 7, 15 or 30 d, after which they were killed for biochemical and physiological analyses. (B) Hamp1 expression normalised to Actb (β-actin) expression. (C) Western blot analysis of phosphorylated mothers against decapentaplegic homolog protein 1/5/8 (pSmad1/5/8), phosphorylated signal transducer and activator of transcription 3 (pStat3) and phosphorylated extracellular signal-regulated kinase 1/2 (pErk1/2) levels. β-Actin was used as a loading control. (d–i) Mobilised hepatic iron concentrations (D), non-haeme splenic iron concentrations (E), serum iron concentrations (F), serum transferrin saturation (G), and the expression levels of hepatic inhibitor of DNA binding 1 (Id1) (H) and bone morphogenetic protein 6 (Bmp6) (I) were measured in mice that were treated with BSSCE for the indicated number of days. The summary data in (B) and (D)–(I) are presented as means and standard deviations represented by vertical bars; n 6–8 mice/group. a,b,cMean values with unlike letters were significantly different (P< 0·05).
Table 2 Effect of dietary black soyabean seed coat extract (BSSCE) on haematopoietic function in mice (Mean values and standard deviations, n 6–8 mice/group)
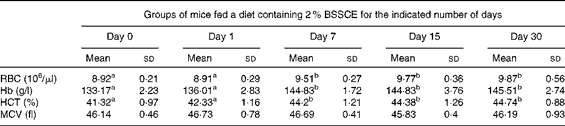
RBC, erythrocytes; HCT, haematocrit; MCV, mean corpuscular volume.
a,bMean values with unlike superscript letters were significantly different (P< 0·05).
Discussion
Hepcidin, the major regulator of Fe homeostasis, binds to and induces the degradation of ferroportin, the only known cellular Fe exporter, thereby decreasing both Fe absorption from the duodenum and Fe release from the reticuloendothelial macrophages( Reference Nemeth, Tuttle and Powelson 2 ). Therefore, controlling Fe absorption in the intestine by up-regulating the levels of ferroportin is a potentially feasible approach for boosting Fe concentrations to treat inflammatory anaemia, chronic inflammatory anaemia and Fe-deficiency anaemia. Recent studies( Reference Theurl, Schroll and Sonnweber 11 , Reference Song, Tomosugi and Kawabata 12 , Reference Nishimoto and Kishimoto 26 ) have shown that an antagonist of IL-6 ameliorates inflammatory anaemia; however, further studies are needed to test this compound's safety in clinical applications. Moreover, soluble haemojuvelin (HJV) and dorsomorphin (two inhibitors of BMP in the BMP/SMAD-targeting pathway) have promising curative effects in mouse models of chronic inflammation( Reference Sun, Vaja and Babitt 8 , Reference Yu, Hong and Sachidanandan 10 , Reference Theurl, Schroll and Sonnweber 11 , Reference Wang, Trebicka and Fu 13 ). The use of hepcidin-targeted therapeutics is considered a breakthrough cure for Fe metabolic disorders and associated diseases( Reference Sun, Vaja and Babitt 8 , Reference Wang, Trebicka and Fu 13 , Reference Fung, Sugianto and Hsu 14 ).
In China, many remedies are derived from daily dietary components( Reference Li, Yin and Saito 15 , Reference Graziose, Lila and Raskin 17 ). Indeed, many documented remedies are focused on curing anaemia and enhancing physical fitness, and these remedies include daily foods that are listed in the traditional Chinese pharmacopoeia( Reference Koo 16 ). However, whether these foods cure anaemia by regulating hepcidin expression has not been investigated previously. Therefore, we obtained extracts of several ‘black foods’, including G. max (black soyabean), A. auricula-judae (black fungus), S. indicum seeds (black sesame seeds) and D. lotus (date plum or persimmon, Hei Zao), and tested their effect on hepcidin expression. Of these four extracts, only the black soyabean coat extract significantly inhibited hepcidin expression, despite the relatively low total Fe concentration in this food. We, therefore, conclude that black soyabean coat extract probably regulates Fe metabolism by down-regulating hepcidin expression.
In Asia, black soyabeans are used medicinally for detoxification, reduction of inflammation and enhancement of blood components, and the safety and pharmacokinetics of black soyabeans have been investigated( Reference Jeon, Han and Lee 28 ). Studies have shown that polysaccharides in black soyabeans promote myelopoiesis and the reconstitution of bone marrow following myelopoiesis suppression by 5-fluorouracil treatment and/or irradiation( Reference Liao, Chen and Yang 29 ), and recent research has focused on the antioxidant activity of anthocyanins, which are an abundant component of BSSCE( Reference Liao, Chou and Wu 30 ). Herein, we report for the first time that BSSCE inhibits hepcidin expression and thus its feasibility in treating hepcidin-associated diseases merits further analysis.
We also found that the phosphorylation levels of SMAD1/5/8 (a set of transcription factors that activate the HAMP gene) decreased in a dose- and time-dependent manner, whereas those of STAT3 and ERK1/2 were relatively unchanged in the BSSCE-treated cells. These data suggest that BSSCE might function by inhibiting the BMP/SMAD signalling pathway. Moreover, BSSCE treatment reduced the phosphorylation levels of SMAD1/5/8 but not of STAT3 in the context of IL-6 induction, which suggests that BSSCE inhibits IL-6-induced hepcidin expression primarily by inhibiting the SMAD pathway, but not the STAT pathway( Reference Besson-Fournier, Latour and Kautz 31 ).
Importantly, our in vitro results were supported by the results of in vivo experiments using mice that were fed a diet containing BSSCE. These mice exhibited reduced hepatic hepcidin expression, as well as reduced expression of both the Id1 and Bmp6 genes, both of which have been reported to exhibit the same regulatory changes as Hamp1 in the liver( Reference Kautz, Meynard and Monnier 27 ). Consistent with our in vitro results, BSSCE also reduced the phosphorylation levels of Smad1/5/8 in vivo. At the physiological level, the BSSCE-fed mice had reduced Fe concentrations in the spleen and elevated Fe concentrations in the serum, which would be expected based on the biological actions of hepcidin, perhaps due to decreased hepcidin expression and increased Fe mobilisation. However, hepatic Fe content was decreased, which was somewhat unexpected, given that a hallmark of hepcidin-knockout mice is increased hepatic Fe concentration( Reference Nicolas, Bennoun and Devaux 32 ). Because hepatic Fe accumulates over a long period of time in the hepcidin-knockout mice, we speculated that the reduced hepatic Fe concentrations in the BSSCE-fed mice might be caused by the relatively short treatment time and the mobilisation of hepatic Fe is a result of inhibited hepatic hepcidin expression in the early stages. Starting on the 15th day of BSSCE treatment, hepatic Fe concentrations increased, further supporting this hypothesis. Moreover, a recent report has shown that the Chinese medicinal herb Caulis Spatholobi (a novel hepcidin inhibitor) has a similar effect on hepatic Fe concentrations( Reference Guan, An and Zhang 9 ). Finally, the BSSCE-fed mice exhibited large increases in their erythrocyte counts, Hb concentrations and haematocrit values. These experiments suggest that BSSCE regulates Fe metabolism by inhibiting the expression of hepcidin, thereby boosting haematopoiesis.
In previous studies( Reference Yu, Hong and Sachidanandan 10 , Reference Poli, Girelli and Campostrini 19 ), the hepcidin antagonists dorsomorphin and LDN193189 have been found to cause adverse side effects due to their chemical structures. Based on our findings, it can be suggested that BSSCE may be a suitable substitute for these antagonists. In China, black soyabeans have been grown for several centuries and are an important daily food staple in the Chinese diet. Black soyabeans also play a functional role in traditional Chinese medicine dating back to ancient China.
In conclusion, the present results suggest that black soyabeans can be used as a daily dietary supplement and as a potential therapeutic agent to improve the Fe status in patients with anaemia of chronic disease or Fe-deficiency anaemia. Future studies should attempt to identify the precise components in BSSCE that regulate hepcidin expression and mediate these positive therapeutic effects.
Acknowledgements
The authors are grateful to Dr Pauline Lee and Dr Jaroslav Truksa (The Scripps Research Institute, La Jolla, CA, USA) for generously providing the HAMP promoter plasmid. They are also grateful to the members of the Wang Laboratory for their encouragement and helpful comments.
The present study was supported by research grants from the Chinese Ministry of Science and Technology (numbers 2011CB966200 and 2012BAD33B05 to F. W.) and Chinese National Natural Science Foundation grants (numbers 31225013, 31330036 and 31030039 to F. W.) and also by the Distinguished Professorship Program from Zhejiang University (to F. W.).
The authors' contributions are as follows: M. M. and F. W. designed the research; M. M., A. W., P. A., X. D., Q. W. and X. S. carried out the experiments; M. M. and F. W. wrote the paper.
None of the authors has a conflict of interest to declare.









