Periodontitis is the most common chronic inflammatory disease caused by an interaction of a specific bacterial flora with the host. Many modifiable and non-modifiable risk factors, among them obesity, can modify the individual predisposition to periodontal disease(Reference Van Dyke and Sheilesh1). Obesity has significant metabolic, systemic immune and inflammatory effects and may increase the host's susceptibility to periodontal disease through its impact on metabolic and immune parameters(Reference Ritchie and Kinane2). It is known that obesity is strongly linked to a raised subclinical inflammatory status and type 2 diabetes(Reference Browning and Jebb3).
The role of nutrition as a possible modifiable systemic factor in periodontal disease onset, progression and treatment has not been thoroughly examined. Only a few studies have examined relationships between diet and periodontitis and suggest that a high intake of fruits and whole-grain products is associated with a lower prevalence of periodontitis(Reference Blignaut and Grobler4, Reference Merchant, Pitiphat, Franz and Joshipura5). Individuals with poor dietary habits were found to be three times more likely to have periodontitis than individuals with good dietary habits, independent of other major risk factors for periodontitis(Reference Sakki, Knuutilla, Vimpari and Hartikainen6). An analysis of the third National Health and Nutrition Examination Survey data suggest a protective association between a good overall quality of diet and the prevalence of periodontitis(Reference Al-Zahrani, Borawski and Bissada7–Reference Saito, Shimazaki, Koga, Tsuzuki and Ohshima9).
Nutrition might influence the development of oral biofilms, inflammation and immune response, tissue metabolism, wound healing and quality and quantity of saliva(Reference Boyd and Madden10, Reference Enwonwu11). For example, the release of cytokines, such as IL-1β and IL-6, which are implicated in periodontal tissue destruction, can be modulated by nutrients(Reference Grimble12).
Furthermore, changes in granulocyte functions occur in obesity(Reference Palmblad, Hallberg and Rossner13). Polymorphonuclear granulocytes have an important role in the maintenance of gingival and periodontal health. During their interactions with micro-organisms, polymorphonuclear granulocytes develop a potential destructive role(Reference Miller, Lamster and Chasens14). The result of their activation as a reaction on the microbial attack is, among others, an increased release of elastase(Reference Drugarin, Onisei, Koreck, Negru and Drugarin15). Neutrophilic elastase has been suggested to be a potential indicator of the degree of periodontal disease and disease progression(Reference Armitage, Jeffcoat, Chadwick, Taggart, Numabe, Landis, Weaver and Sharp16).
Nutrients, such as vitamin E, vitamin C, carotenoids, polyphenols, glutathione and trace elements, can contribute directly and indirectly to the robustness of antioxidant defences of the host(Reference Aruoma17). Oxidative stress is involved in the pathogenesis of a number of diseases, including periodontitis(Reference Wei, Ho, Ho, Wu, Yang and Tsai18). An adequate intake of antioxidants and PUFA may be important for preventing oxidative stress(Reference Jenkinson, Franklin, Whale and Duthie19). However, the extent of dietary influence upon salivary antioxidant status is unclear(Reference Sculley and Langley-Evans20).
The purpose of the present study was to assess the effects of a guided nutritional intervention over 1 year on clinical, immunological and microbiological variables in patients with mild to moderate chronic periodontitis and the metabolic syndrome.
Experimental methods
Patients and nutrition intervention
Twenty women (aged 55·0 (sd 10·9) years) participated in the present prospective clinical study. After being informed on the purpose of the study, written informed consent was obtained from all patients. The study has been independently reviewed and approved by the local ethical committee of the University of Leipzig.
The inclusion criteria were as follows: at least fourteen teeth in occlusion; untreated mild or moderate chronic periodontitis; unmodified conventional oral hygiene and no current periodontal therapy, non-smoker; diagnosed metabolic syndrome; BMI>25 kg/m2.
Exclusion criteria were: severe adiposity with a BMI>40 kg/m2; intake of lipid metabolism-regulating drugs; diabetes mellitus with administration of insulin and/or antidiabetic therapy; intake of antibiotics during the last 3 months; special dietary patterns, such as vegetarian diet; systemic diseases other than the metabolic syndrome.
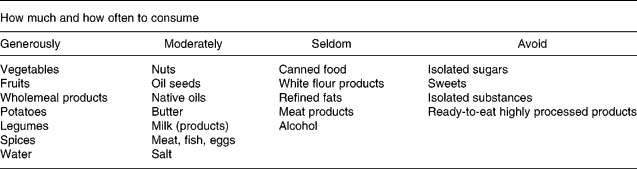
All participants were integrated in a nutritional intervention programme assisted by nutritionists. The consultations with the nutritionists included the evaluation of individual dietary habits, lectures about diet and health, dietary habits, specificity of single groups of food, instructions for wholesome nutrition, the preparation of food in groups as well as continuous individual sessions. The consultations started directly after the baseline examination and were perpetuated the first 2 months every 2 weeks and then every 2 months. Participants were deemed to be highly compliant if they regularly attended and cooperated with the consultations. During an intervention period of 1 year, the participants changed their dietary habits from an average German mixed diet towards wholesome nutrition. Wholesome nutrition has the following general features: (1) preference of food of plant origin; (2) preference of food processed as little as possible; (3) plentiful consumption of unheated fresh food; (4) careful preparation of meals from fresh foods; (5) the sparse use of fat(Reference Koerber, Maennle and Leitzmann21). This diet is mainly composed of vegetables, fruits, whole-grain products, potatoes, legumes and dairy products. The consumption of meat, fish and eggs is limited to one or two portions per week. The recommendations of wholesome nutrition are given in Table 1.
Table 1 Recommendations of wholesome nutrition for food consumption(Reference Koerber, Maennle and Leitzmann21)
Clinical periodontal measurements
The clinical data and samplings were obtained before nutritional intervention and 2 weeks, 3, 6 and 12 months later. At all visits, gingival crevicular fluid and stimulated whole saliva were collected first and then clinical variables were recorded. Clinical probing depth was determined at four sites per tooth. The extent of gingival inflammation was determined by the criteria of the gingival index according to Löe & Silness(Reference Löe and Silness22). The gingival index differentiates the gingival inflammation into the score 0 (normal), 1 (slight inflammation), 2 (moderate inflammation) and 3 (severe inflammation). Oral hygiene status was assessed using the modified Quigley–Hein plaque index(Reference Turesky, Gilmore and Glickman23). This index assessed the plaque formation on the teeth into the score 0 (no plaque), 1 (separate spots of plaque at the cervical margin of the tooth), 2 (thin continuous band of plaque up to 1 mm at the cervical margin of the tooth), 3 (band of plaque wider than 1 mm but covering less than one-third of the tooth), 4 (plaque covering at least one-third but less than two-thirds of the crown) and 5 (plaque covering two-thirds or more of the crown). The clinical assessments were carried out with a manual periodontal probe with 1 mm graduations (UNC 15; Hu-Friedy, Chicago, IL, USA). All measurements and sample collections were made by a calibrated examiner.
Determination of microflora and immunological variables in gingival crevicular fluid
Before each clinical examination, gingival crevicular fluids were obtained from two selected sites with a probing depth ≥ 3 mm for each subject. For the following examinations the same sites were chosen for sampling. Supragingival plaque was carefully removed with a curette and the site was gently dried with an air stream. Periopaper (Pro flow™, Amityville, NY, USA) was inserted into the gingival crevice and kept there for 2 min. Contamination with blood or saliva was avoided. Thereafter, strips were stored in 100 μl of 0·5 m-NaCl at − 18°C until analysis. Immediately before the analysis, all samples were eluted for 2 h at room temperature in 500 μl of 0·5 m-NaCl.
Elastase activity was determined by means of a chromogenic substrate specific for granulocyte elastase (1 mm-N-methoxysuccinyl-Ala-Ala-Pro-Val-pNa). In brief, to 90 μl eluate of gingival crevicular fluid sample, 10 μl substrate was added. The absorbance at 405 nm was measured immediately in a microplate reader. After incubation for 30 min at 37°C the measurement was repeated. The readings were subtracted from the 0 h value (difference of optical density at 405 nm) and activity was expressed as arbitrary units per site.
IL-1β and IL-6 were analysed from 100 μl eluate by using a commercially available ELISA (Human IL-1beta CytoSets™, Human IL-6 CytoSets™; BioSource International Inc., Camarillo, CA, USA). These assays were used according to the manufacturer's instructions using recombinant human standards. Through an antibody–antigen–antibody reaction with a direct coupled enzymic reaction, small amounts of cytokines were detectable. Results for IL-1β and IL-6 are reported as pg/ml per site.
For the microbial analysis, 100 μl of the eluate from the paper strips were used for DNA extraction and purification with a commercial kit (Genomic Mini; A&A Biotechnology, Gdynia, Poland). After isolation, DNA was stored at − 20°C until analysis by quantitative real-time PCR. The real-time PCR amplification was performed in a total reaction mixture volume of 20 μl. Each sample was quantitatively analysed for Tannerella forsythia, Treponema denticola, Porphyromonas gingivalis, Aggregatibacter actinomycetemcomitans, Prevotella intermedia and Fusobacterium nucleatum. The reaction mixture contained 7·9 μl distilled water, 2 μl PCR puffer, 2 μl dNTPs, 2·1 μl MgCl2, 1 μl SYBR Green, 1 μl Taq polymerase, 2 μl of the pathogen-specific primer and 2 μl of purified DNA from gingival crevicular fluid samples. The primers used for Porphyromonas gingivalis, Tannerella forsythia, Prevotella intermedia and Treponema denticola have been described by Ashimoto et al. (Reference Ashimoto, Chen, Bakker and Slots24), for A. actinomycetemcomitans by Rudney et al. (Reference Rudney, Chen and Pan25) and for F. nucleatum by Fouad et al. (Reference Fouad, Barry, Caimano, Clawson, Zhu, Carver, Hazlett and Radolf26). The amplification was performed in the Rotor-Gene™ 2000 real-time cycler (Corbett Research, Sydney, Australia) and the complete PCR sample data of each periodontopathogen were quantitatively analysed using the real-time PCR software of the Rotor-Gene™ 2000 system.
Saliva collection and assays for oxidative and antioxidative variables
The stimulated saliva samples were obtained at each date of examination under standardised conditions while chewing paraffin wax for 5 min. The samples were centrifuged at 3000 g and 2–4°C for 15 min; the supernatant fractions were stored at − 18°C until analysis.
The assay used for determining the activity of myeloperoxidase has been described by de Mendez et al. (Reference de Mendez, Young, Bignon and Lambre27). The substrate includes Triton-X-100, o-dianisidine and H2O2 in sodium citrate buffer. The absorbance at 450 nm was measured immediately in a microplate reader. After incubation for 30 min at 37°C the measurement was repeated. These measurements were also performed including sodium azide as an inhibitor of myeloperoxidase(Reference Davies and Edwards28). The readings of substrate and sample were subtracted from the values including additional inhibitor.
Lipid peroxidation products were assayed using TCA, thiobarbituric acid and deoxylsulfate as substrate(Reference Esterbauer and Cheeseman29). A standard was also compounded. After incubation for 25 min at 95°C and cooling down in ice, the reaction was stopped with a mix of water and butanol. After shaking for 20 min and centrifugation at 1000 g for 7 min the absorbance was measured at a wavelength of 586 nm.
Glutathione peroxidase activity was measured according to Lawrence & Burk(Reference Lawrence and Burk30). PBS solution including EDTA, glutathione, NADPH and glutathione reductase was used as substrate. The change in absorbance at 304 nm was monitored spectrophotometrically for 1 min and the mean substrate blank was subtracted from the sample readings.
The activity of catalase was assayed using the method of Beers & Sizer(Reference Beers and Sizer31). The decomposition of H2O2 was monitored spectrophotometrically at 240 nm for 1 min. The values of substrate without sample were subtracted from the sample readings. For all variables the rate of activity is expressed as arbitrary units per site.
Statistical analysis
Due to normal distribution tested by the Kolmogorov–Smirnov test, the values of the clinical variables are expressed as means and standard deviation. Overall differences were tested using general linear model variance analysis; differences between two appointments were tested using the t test. The differences of the microbiological and immunological variables during the intervention period were tested using the non-parametric Friedman test, because no normal distribution was calculated. In the case of a significant Friedman test, differences between two appointments were assessed using the Wilcoxon test. Significance was set at P ≤ 0·05.
Results
Twenty female subjects (mean age: 55·0 (sd 10·9) years) completed all evaluations during the nutritional intervention period of 12 months. Only compliant subjects were included in the analysis. Non-compliant subjects were a priori excluded from the study. The baseline characteristics of the subjects are shown in Table 2. The clinical measurements are given in Table 3. Regarding the whole oral cavity, the mean probing depth was significantly reduced from 2·40 mm at baseline to 2·20 mm after 12 months (P < 0·001). The probing depth of the collected sites showed no significant changes during the nutritional intervention period. Oral hygiene status, expressed by the Quigley–Hein index, showed no changes during the intervention period. We observed a significant reduction of the gingival index from 1·13 at baseline to 0·90 after 12 months, respectively (P < 0·001). In accordance with the gingival index the concentration of IL-1β in gingival crevicular fluid decreased continuously over the nutritional intervention period from 4·63 pgml per site at baseline to 1·10 pgml per site after 12 months (P < 0·001) (Fig. 1). Furthermore, the concentration of IL-6 was also significantly reduced (P = 0·022). A decrease from 1·85 pgml per site at baseline to 0·34 pgml per site after 12 months could be observed (Fig. 2). No significant changes could be found for the activity of granulocyte elastase in gingival crevicular fluid (data not shown).
Table 2 Baseline characteristics of the study participants
(Mean values and standard deviations)
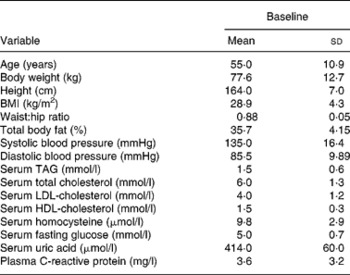
Table 3 Clinical variables at baseline and during the nutritional intervention period
(Mean values and standard deviations)
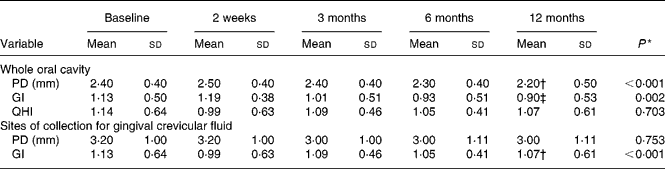
PD, probing depth; GI, gingival index; QHI, modified Quigley–Hein index for oral hygiene status.
* General linear model variance analysis.
Mean value was significantly different from that at baseline: † P < 0·001, ‡ P = 0·029 (paired t test).
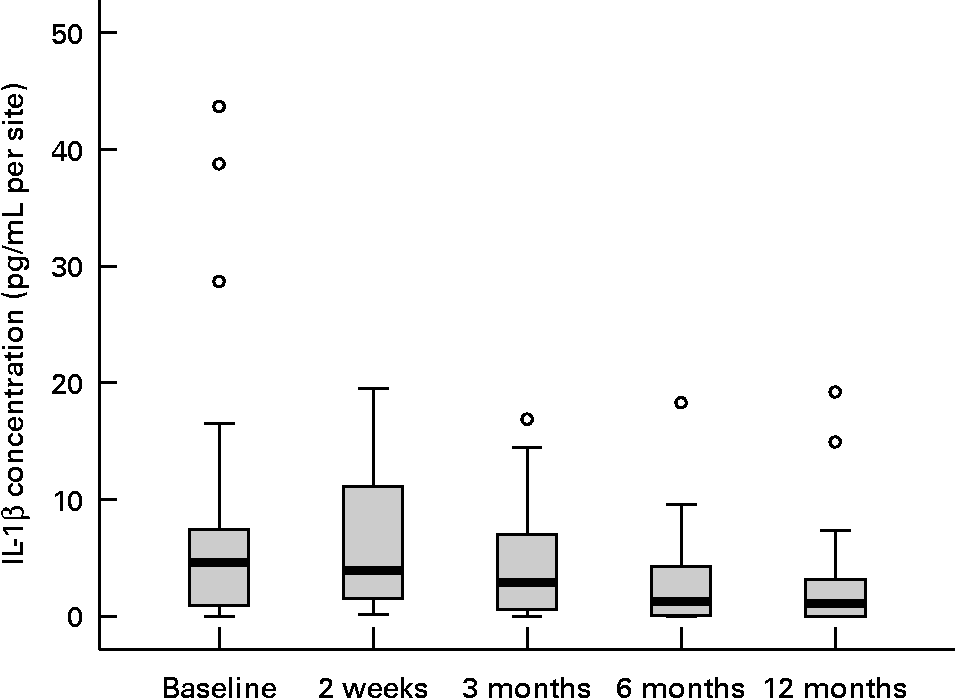
Fig. 1 Concentration of IL-1β in gingival crevicular fluid during the nutritional intervention programme. The central line is the mean; the box represents the lower and upper quartiles; the whisker shows the maximum and minimum values; ○, outliers. The concentration of IL-1β was significantly reduced after the nutritional intervention (P < 0·001; Friedman's test). Mean value at 12 months was significantly different from that at baseline (P = 0·004; Wilcoxon's test).
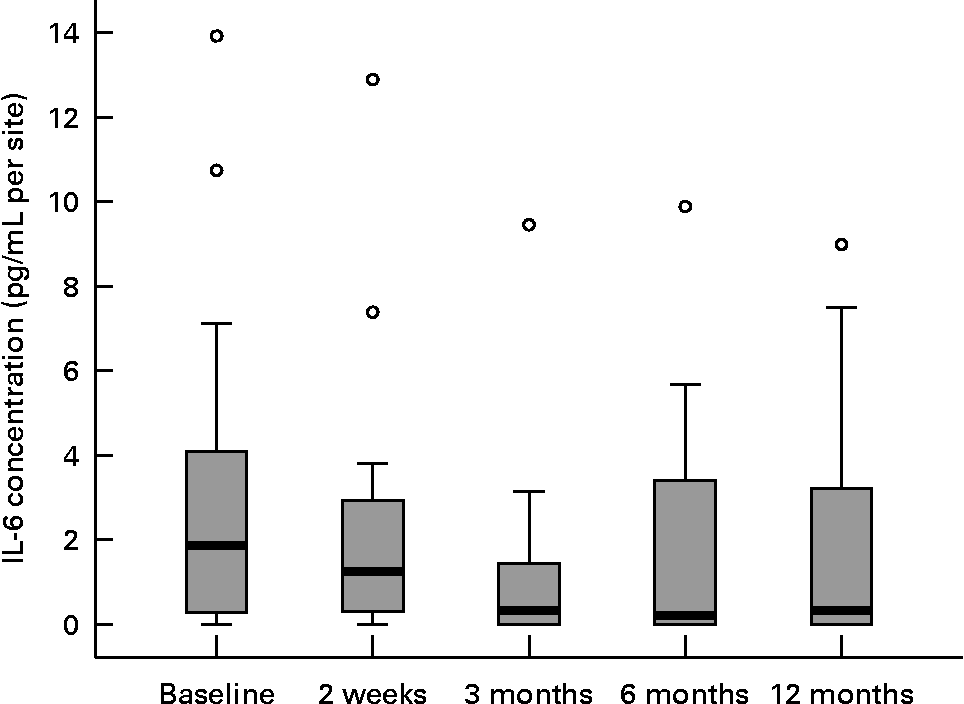
Fig. 2 Concentration of IL-6 in gingival crevicular fluid during the nutritional intervention programme. The central line is the mean; the box represents the lower and upper quartiles; the whisker shows the maximum and minimum values; ○, outliers. The concentration of IL-6 was significantly reduced after the nutritional intervention (P = 0·022; Friedman's test).
In stimulated whole saliva the oxidative variables (activity of myeloperoxidase and level of lipid peroxidation products) were determined. The result for myeloperoxidase was 0·118 arbitrary units and for lipid peroxidation 0·024 arbitrary units at baseline. Any change of myeloperoxidase activity and of lipid peroxidation was not observed during the study period; analogous results were also noted for the recorded antioxidative variable glutathione peroxidase in stimulated whole saliva (data not shown). After 12 months of the nutritional intervention the activity of catalase showed a tendency to lower values (Fig. 3).
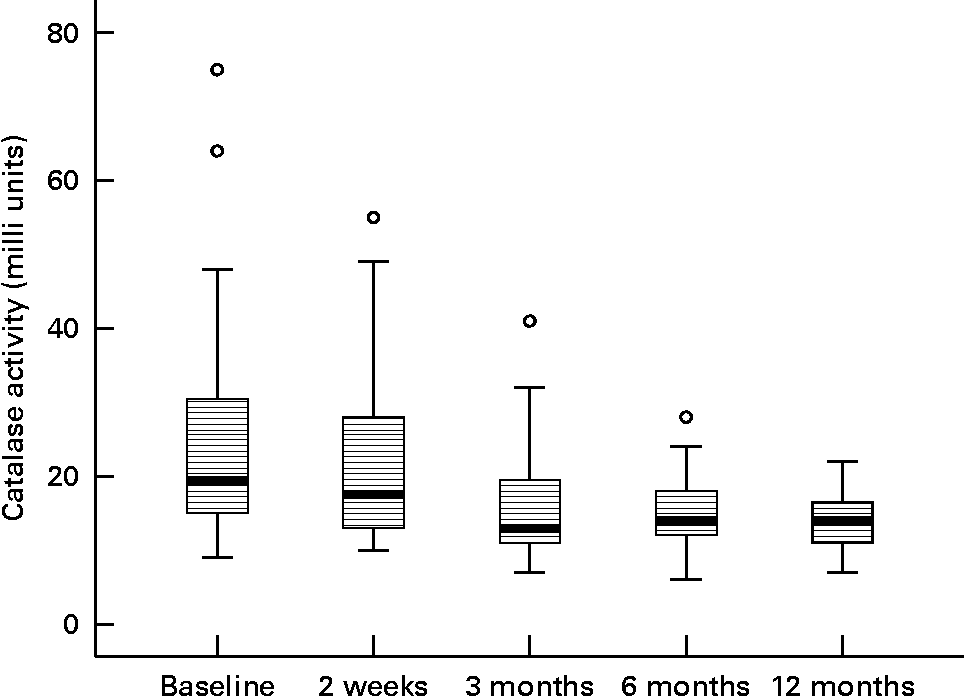
Fig. 3 Activity of catalase during the nutritional intervention programme. The central line is the mean; the box represents the lower and upper quartiles; the whisker shows the maximum and minimum values; ○, outliers. The reduction during the intervention period is not statistically significant (P = 0·937).
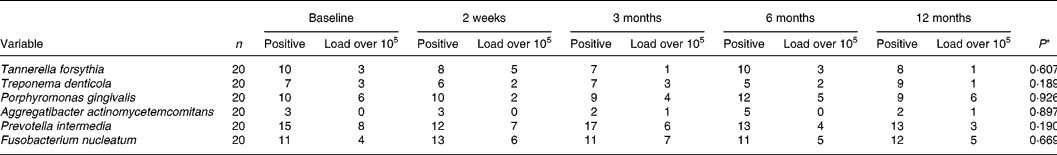
The results of the microbiological analysis of gingival crevicular fluid by real-time PCR are given in Table 4. In 50 % of the patients, Porphyromonas gingivalis was detectable at baseline and only three subjects had a load of more than 105 per site. A. actinomycetemcomitans was present in three of twenty subjects. The differences between the dates of examination of the percentages of bacterial counts from the recorded six periodontal pathogens were not statistically significant during the nutritional intervention period.
Table 4 Numbers of subjects positive and bacterial load over 105 per site for different periodontopathogens in gingival crevicular fluid at baseline and during the nutritional intervention period analysed by real-time PCR
* Significance of different values between the several dates of examination (Friedman's test).
Discussion
This is the first study to have focused on the effects of a nutritional intervention programme on clinical, immunological and microbiological variables in patients with chronic periodontitis and the metabolic syndrome, respecting the concept of wholesome nutrition. With professional support of nutritionists, the participants of the study changed their dietary patterns to a low-fat diet, so-called wholesome nutrition. Wholesome nutrition is gaining increasing acceptance as a health-promoting, sustainable and equitable diet(Reference Leitzmann32). This form of diet is described by Koerber et al. (Reference Koerber, Maennle and Leitzmann21) and is mainly composed of vegetables, fruits, whole-grain products, potatoes, legumes and dairy products as well as a limited consumption of meat, fish and eggs. The regular participation at the consultations with the nutritionists demonstrates the participants' high compliance.
Vegetarian-based diets offer a number of nutritional benefits, including lower levels of saturated fat, cholesterol and animal protein, as well as higher levels of carbohydrates, dietary fibre, Mg, K, folate and antioxidants such as vitamins C and E and phytochemicals. Substantial evidence indicates that diets using non-hydrogenated unsaturated fats as the predominant form of dietary fat, whole grains as the main form of carbohydrates, an abundance of fruits and vegetables and adequate n-3 fatty acids can offer significant protection against CVD(Reference Hu and Willet33). The effects of diet on the pathogenesis of CVD can be mediated through multiple biological pathways other than serum lipids, including oxidative stress, subclinical inflammation, endothelial dysfunction, insulin sensitivity, blood pressure and thrombotic tendency(Reference Giugliano, Ceriello and Esposito34). It is plausible, therefore, that especially through its impact on lipid profile, oxidative stress and inflammation, diet may develop effects on the onset and progression of periodontal diseases.
At 12 months after the nutritional intervention period, the patients showed a significant reduction of probing pocket depth, gingival inflammation and, in accordance with these clinical variables, decreased concentrations of the cytokines IL-1β in gingival crevicular fluid. IL-1β and IL-6 are important proinflammatory cytokines, which mediate the tissue destruction in periodontal disease. The levels of IL-1β and IL-6 are closely related to severity of the periodontitis(Reference Ishihara, Nishihara, Kuroyanagi, Shirozu, Yamagishi, Ohguchi, Koide, Ueda, Amano and Noguchi35). Expression and release of these cytokines might be also influenced by nutrients(Reference Grimble12). Among the nutrients, fats have a large potential for modulating cytokine metabolism. For example, fats rich in n-6 PUFA enhance IL-1 production and tissue responsiveness to cytokines, fats rich in n-3 PUFA have the opposite effect, MUFA decrease tissue responsiveness to cytokines and IL-6 production is enhanced by unsaturated fatty acid intake(Reference Grimble and Tappia36). In contrast to Western dietary patterns, the wholesome diet contains a higher intake of PUFA and non-hydrogenated unsaturated fats. n-3 Fatty acids are found in high concentrations in certain plant products, for example, linseed and walnut(Reference Leitzmann32). Fish oils, also rich in n-3 fatty acids, have been found to inhibit the production of IL-1β, IL-6 and TNF-α(37) and decrease alveolar bone resorption in animal experiments(Reference Kesavalu, Vasudevan and Raghu38). On the basis of these facts the present study suggests that the modulation of cytokine expression may be a biological mechanism responsible for the observed changes in the clinical variables of periodontal disease since other factors with influence on periodontal disease had not been modified.
The inflammatory response, leading to destruction of periodontal tissues, is initiated by micro-organisms, organised in the biofilm. The role of nutrition in the development and composition of the supragingival plaque biofilm is well established. Investigations that suggest an influence on the periodontopathogenic subgingival microflora are still lacking. Nutrient inadequacies may influence oral microbiological ecology(Reference Enwonwu11). In the present study we observed no significant changes in quantity of the periodontal pathogens Tannerella forsythia, Treponema denticola, Porphyromonas gingivalis, A. actinomycetemcomitans, Prevotella intermedia and F. nucleatum in gingival crevicular fluid during the nutritional intervention, suggesting that the nutritional intervention has no significant effect on the quantity of the determined periodontopathogens in the subgingival area. It should be mentioned that patients in this nutrition intervention programme had a mild to moderate form of periodontitis, so we found only about 50 % positive for Porphyromonas gingivalis and about 20 % positive for A. actinomycetemcomitans.
As a result of the microbiological challenge, polymorphonuclear granulocytes are activated and release proteolytic enzymes, including elastase(Reference Drugarin, Onisei, Koreck, Negru and Drugarin15). Elastase activity correlated with attachment loss and probing depth(Reference Alpagot, Silverman, Lundergan, Bell and Chambers39, Reference Gustafsson, Asam, Bergström and Söder40). In the present study including only patients with mild to moderate periodontitis, we detected low values of elastase activity and no changes occurred over the intervention period. The mean probing depth from the collection sites in the present study was 3·2 mm at baseline and 3·0 mm after 12 months; this may explain the low values.
Another possible link between nutrition, inflammation and periodontitis is the influence of nutrients on the homeostatic balance between reactive oxygen species and the antioxidant defence system. Damage of tissues in inflammatory periodontal disease can be mediated by reactive oxygen species resulting from the physiological activity of polymorphonuclear granulocytes during the phagocytosis of periodontopathogens. Increased gingival crevicular fluid flow in inflammation is related to higher polymorphonuclear granulocyte levels, and gingival crevicular fluid is constantly mixed with saliva. Polymorphonuclear granulocytes release myeloperoxidase from the azurophil granules into oral fluids; this enzyme catalyses the oxidation of chloride and reduction of H2O2 to form hypochlorous acid(Reference Miyasaki41). This reaction is specifically considered to be relevant in inflammatory conditions.
Increased levels of lipid peroxidation products, caused by reactive oxygen species, may play a role in the inflammation and tissue destruction in periodontitis. In comparison with periodontal healthy subjects, higher levels of lipid peroxidation products were found in whole saliva from patients with periodontitis. After periodontal therapy, the levels of lipid peroxidation products were reduced(Reference Tsai, Chen, Chen, Ho, Ho, Wu and Hung42).
Catalase and glutathione peroxidase facilitate the processing of oxidant molecules to harmless by-products. Glutathione peroxidase and catalase are antioxidant enzymes, which are important for the detoxification of H2O2(Reference Ho, Magnenat, Gargano and Cao43), but little information relevant to periodontal disease is available. Wei et al. (Reference Wei, Ho, Ho, Wu, Yang and Tsai18) observed no significant differences in whole saliva glutathione peroxidase activities between periodontal-diseased and healthy subjects.
An adequate intake of antioxidants and PUFA may be important for preventing oxidative stress(Reference Jenkinson, Franklin, Whale and Duthie19). In the present study we found very low values of oxidative and antioxidative enzyme activities and lipid peroxidation products in stimulated whole saliva and between the several dates of examination no changes could be found. So changes in dietary patterns might be developing no effects on the determined variables in whole saliva.
During the nutritional intervention period the aetiological conditions of periodontitis remained constant. The oral hygiene status did not change. The observed slight reduction of the extent of gingival inflammation may be explained through a modulation of the disposition of the host, because alterations by the microbiological exposition could be as far as possible excluded in the present study. So the most probable mechanism responsible for the slight reduction of inflammation with the attendant slight decrease of probing depth and gingival index over the nutritional intervention period could be a reduced production of IL-1β and IL-6 in consequence of the diet-related modulation of cytokine expression. The present study gives evidence about the causal association between nutritional factors and periodontal diseases. There are some limitations in the present study. Only patients with mild or moderate periodontitis were included and no controls could be considered. Furthermore it should be mentioned that only women with a mean age of 55·0 (sd 10·9) years participated in the present study. Therefore population-level conclusions should be made with caution. It may be concluded from this prospective clinical study that wholesome nutrition in patients with the metabolic syndrome might reduce inflammatory variables in chronic periodontitis and may improve periodontal health. Further studies in a larger population are needed to find out the mechanisms underlying the associations between several properties of the metabolic syndrome, nutrition and periodontitis.
Acknowledgements
No external funding, apart from the support of the authors' institutions, was available for the present study. The authors declare that they have no conflict of interest.
We thank all those who volunteered for the study. The authors thank Claudia Ranke for her excellent technical assistance. R. P. performed the clinical examinations and collected the samples. A. J. carried out the laboratory work, analysed the data and wrote the manuscript. S. E. supported the laboratory work, collaborated on the interpretation of the results and performed the statistical analyses. H. J. designed the study, participated in the interpretation of the results and provided statistical support. All authors participated in critically revising the manuscript.









