Introduction
Ascaris lumbricoides infects about 820 million people and is prevalent in at least 103 of the 218 countries of the world (WHO, 2017, 2020). In general, preschool-age and school-age children are at higher risk of infection, because they are more likely to ingest soil, food or water contaminated with infectious stages (egg with 3rd stage larva) (Jourdan et al., Reference Jourdan, Lamberton, Fenwick and Addiss2018). Diagnosis of A. lumbricoides infections is based on the microscopic detection of eggs in stool. However, the morphological identification of A. lumbricoides eggs by stool microscopy is not always straightforward and requires specially trained laboratory personnel (Montresor et al., Reference Montresor, Mupfasoni, Mikhailov, Mwinzi, Lucianez, Jamsheed, Gasimov, Warusavithana, Yajima, Bisoffi, Buonfrate, Steinmann, Utzinger, Levecke, Vlaminck, Cools, Vercruysse, Cringoli, Rinaldi, Blouin and Gyorkos2020). Interestingly, A. lumbricoides eggs may appear in three different forms: unfertilized, fertilized corticated and fertilized decorticated (WHO, 2019). Unfertilized eggs are elongated and larger than fertile eggs (~90 μm in length), their shell is thinner and the mammillated layer is more variable, either with large protuberances or practically none (Fig. 1A) (WHO, 2019). Fertilized corticated eggs are round-shaped, 45–75 μm in diameter (Fig. 1B) and have a thick shell with an external mammillated layer (indicated in Fig. 1B with a green line). In some cases, the outer layer is absent (fertilized decorticated eggs) (Fig. 1C). Due to this polymorphism, non-parasitic elements (artefacts) can be sometimes misidentified as A. lumbricoides eggs (Colmer-Hamood, Reference Colmer-Hamood2001; Ash and Orihel, Reference Ash and Orihel2007; Speich et al., Reference Speich, Ali, Ame, Albonico, Utzinger and Keiser2015; WHO, 2016; Benjamin-Chung et al., Reference Benjamin-Chung, Pilotte, Ercumen, Grant, Maasch, Gonzalez, Ester, Arnold, Rahman, Haque, Hubbard, Luby, Williams and Colford2020). Identification of artefacts (e.g. pollen, plant cells, psocid insects, etc.) is an integral part of the diagnosis process to avoid common misdiagnosis in the laboratory (Podhorsky, Reference Podhorsky2011; Szwabe and Kurnatowski, Reference Szwabe and Kurnatowski2012; Lanocha et al., Reference Lanocha, Zdziarska, Lanocha-Arendarczyk, Kosik-Bogacka, Guzicka-Kazimierczak and Marzec-Lewenstein2016). In order to distinguish between parasitic and non-parasitic elements, technicians should be well trained and experienced on the complex characteristics of parasite eggs (e.g. size, shape, shell structure and internal features in the case of A. lumbricoides eggs) (Colmer-Hamood, Reference Colmer-Hamood2001; Ash and Orihel, Reference Ash and Orihel2007; Garcia, Reference Garcia2007; Garcia et al., Reference Garcia, Arrowood, Kokoskin, Paltridge, Pillai, Procop, Ryan, Shimizu and Visvesvara2018; WHO, 2019).
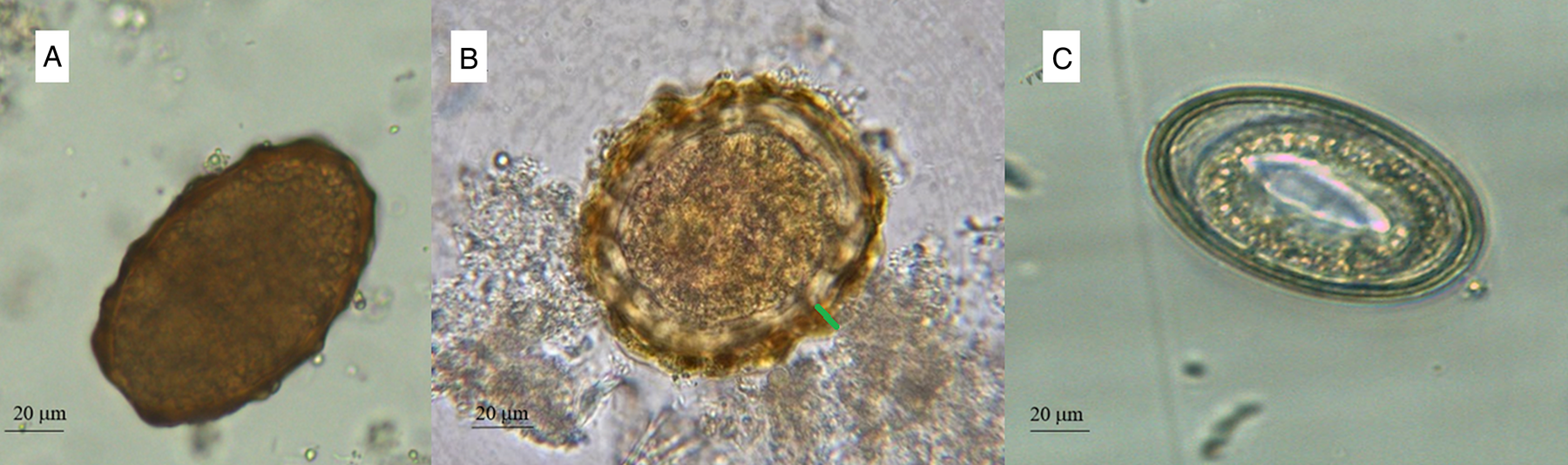
Fig. 1. Unfertilized (A), fertilized mammillated (B) and fertilized decorticated (C) eggs of Ascaris lumbricoides. Mammillated layer is indicated with a green line.
To date, there are a variety of laboratory methods used to detect A. lumbricoides, but some are more prone to misdiagnosis than others. For example, when applying methods based on a stool smear (e.g. Kato-Katz thick smear and direct smear), the microscopic view is often troubled by debris, increasing the risk of misclassification of artefacts as A. lumbricoides (Speich et al., Reference Speich, Ali, Ame, Albonico, Utzinger and Keiser2015). This is in contrast to flotation-based methods (e.g. (Mini-)FLOTAC, McMaster and FECPAKG2), where the microscopic view is clear by allowing eggs to float to the surface of the device (debris will not float and will be separated from the eggs) (Barda et al., Reference Barda, Cajal, Villagran, Cimino, Juarez, Krolewiecki, Rinaldi, Cringoli, Burioni and Albonico2014; Cringoli et al., Reference Cringoli, Maurelli, Levecke, Bosco, Vercruysse, Utzinger and Rinaldi2017; Ayana et al., Reference Ayana, Cools, Mekonnen, Biruksew, Dana, Rashwan, Prichard, Vlaminck, Verweij and Levecke2019). The Mini-FLOTAC technique (Cringoli et al., Reference Cringoli, Maurelli, Levecke, Bosco, Vercruysse, Utzinger and Rinaldi2017) has proven to be a reliable method for diagnosis of A. lumbricoides and other soil-transmitted helminths (STHs) (Barda et al., Reference Barda, Cajal, Villagran, Cimino, Juarez, Krolewiecki, Rinaldi, Cringoli, Burioni and Albonico2014; Benjamin-Chung et al., Reference Benjamin-Chung, Nazneen, Halder, Haque, Siddique, Uddin, Koporc, Arnold, Hubbard, Unicomb, Luby, Addiss and Colford2015; Lamberton and Jourdan, Reference Lamberton and Jourdan2015; Lim et al., Reference Lim, Brooker, Belizario, Gay-Andrieu, Gilleard, Levecke, van Lieshout, Medley, Mekonnen, Mirams, Njenga, Odiere, Rudge, Stuyver, Vercruysse, Vlaminck and Walson2018; Dukpa et al., Reference Dukpa, Dorji, Thinley, Wangchuk, Tshering, Gyem, Wangmo, Sherpa, Dorji and Montresor2020). Comparisons performed between Mini-FLOTAC technique and Kato-Katz thick smear showed a higher specificity of the first method (Assefa et al., Reference Assefa, Crellen, Kepha, Kihara, Njenga, Pullan and Brooker2014) and a similar sensitivity (Assefa et al., Reference Assefa, Crellen, Kepha, Kihara, Njenga, Pullan and Brooker2014; Barda et al., Reference Barda, Cajal, Villagran, Cimino, Juarez, Krolewiecki, Rinaldi, Cringoli, Burioni and Albonico2014, Reference Barda, Albonico, Ianniello, Ame, Keiser, Speich, Rinaldi, Cringoli, Burioni, Montresor and Utzinger2015; Nikolay et al., Reference Nikolay, Brooker and Pullan2014) with Kato-Katz direct smear. This paper describes the findings of either A. lumbricoides eggs or artefacts in stool samples from two cohorts analysed by both the Kato-Katz thick smear and Mini-FLOTAC, using additional methods (i.e. coprocultures and molecular techniques) to validate microscopical identification of A. lumbricoides.
Materials and methods
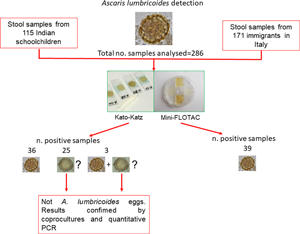
Stool samples (n = 286) used for this study were obtained from (i) a survey conducted in November 2019 in 115 schoolchildren (6–10 years old) at a primary school in Delhi, India and (ii) a survey conducted in November 2020 in 171 adult (>18 years) immigrants in Naples, Italy. These immigrants were mainly from Bangladesh, Pakistan and western and southern Africa. The stool samples were analysed by applying both Kato-Katz thick smear and Mini-FLOTAC (Fig. 2). The Kato-Katz was performed using the 41.7 mg template, after filtration of stool samples. A piece of cellophane (which has been soaked overnight in glycerol malachite green solution) was placed over the stool sample for 1 h before reading. For the Mini-FLOTAC, 2 g of stool were placed in the Fill-FLOTAC and then diluted and homogenized with 38 mL of zinc sulphate flotation solution (specific gravity = 1.35; dilution ratio 1:20). After a careful homogenization (by pumping the conical collector of the Fill-FLOTAC up and down ten times, while turning to the right and left), Mini-FLOTAC chambers were filled and translated after 10 min. The standard operating procedures described in the WHO Bench Aids for the diagnosis of intestinal parasites were used for both techniques (WHO, 2019). Ascaris lumbricoides eggs were identified according to the WHO guidelines (which describe their characteristics to recognize them) (WHO, 2012, 2017), photographed and measured using a light microscope (Leica DM 1000, Leica Microsystems, Wetzlar, Germany) and LAS ver. 4.13 software (Leica Microsystems, Wetzlar, Germany), at 20× and 40× magnifications. In order to ensure the quality of parasitological examination, the operator that prepared samples to analyse provided randomized Kato-Katz thick smears and Mini-FLOTACs to the reader to obtain blinded results, without influences on comparison between the two techniques. Moreover, to avoid possible bias in reading (i.e. misdiagnosis), all slides were analysed by an experienced microscopist on eggs recognizing.
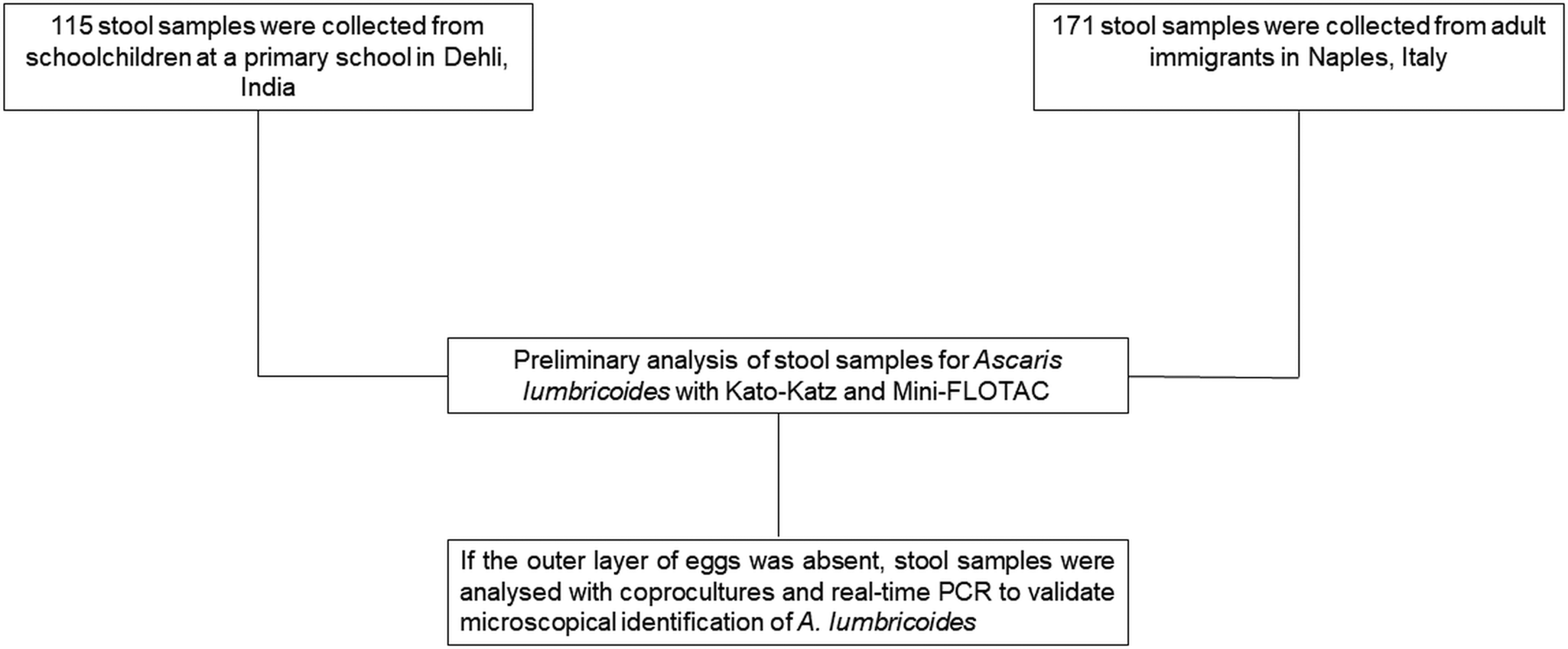
Fig. 2. Study design.
The prevalence and the 95% confidence interval (95% CI) of A. lumbricoides eggs and artefacts was calculated for both populations (i.e. from Indian children and from immigrants in Italy) using free online software ‘Sample Size Calculator’ (Creative Research Systems, CA, USA). The non-parametric Mann–Whitney U test was used to compare the difference in eggs per gram of faeces detected by the two methods (Mini-FLOTAC and Kato-Katz) using SPSS Statistics v.23 (IBM, Armonk, NY, USA). The test was considered statistically significant at P < 0.05.
If the outer layer of A. lumbricoides eggs was absent, two aliquots of each stool sample were preserved for further analysis as follows: one at +4°C for coprocultures and another at −20°C for molecular analysis. For the coprocultures, an aliquot of each sample was diluted in tap water and the suspension was filtered through a wire mesh (aperture of 250 μm). The suspension obtained was centrifuged at 170 × g for 3 min. The sediment containing the eggs was cultured in culture flasks at +25°C for 20 days (WHO, 2004; Kim et al., Reference Kim, Pyo, Hwang, Park, Hwang, Chai and Shin2012). Then, the samples were analysed under a microscope, to evaluate the presence of developed larvae inside the eggs.
For molecular analysis, two pooled stool samples (one from Indian samples and one from immigrants in Italy) were prepared taking 0.5 g of faeces from each sample only with dubious fertilized decorticated A. lumbricoides eggs (15 samples for the pool of Indian children's stools and 10 samples for the pool of immigrants' stools), then after a careful homogenization, 0.25 g of faeces were used for DNA extraction by the DNeasy Blood & Tissue kit (Qiagen, Hilden, Germany). The quantitative polymerase chain reaction (qPCR) was performed as described by Cools et al. (Reference Cools, Vlaminck, Albonico, Ame, Ayana, José Antonio, Cringoli, Dana, Keiser, Maurelli, Maya, Matoso, Montresor, Mekonnen, Mirams, Corrêa-Oliveira, Pinto, Rinaldi, Sayasone, Thomas, Verweij, Vercruysse and Levecke2019) with minor modifications. The reactions were performed in a final reaction mixture of 20 μL, containing 10 μL of FastStart PCR Master Mix (Roche, Rotkreuz, Switzerland), 1.2 μL of both forward and reverse primers (both at 10 μ m), 0.95 μL of probe (10 μ m) and 5 μL of DNA template. The primers and probe used were 5′-GTAATAGCAGTCGGCGGTTTCTT-3′ (forward) (Liu et al., Reference Liu, Gratz, Amour, Nshama, Walongo, Maro, Mduma, Platts-Mills, Boisen, Nataro, Haverstick, Kabir, Lertsethtakarn, Silapong, Jeamwattanalert, Bodhidatta, Mason, Begum, Haque, Praharaj and Houpt2016), 5′-GCCCAACATGCCACCTATTC-3′ (reverse) (Liu et al., Reference Liu, Gratz, Amour, Nshama, Walongo, Maro, Mduma, Platts-Mills, Boisen, Nataro, Haverstick, Kabir, Lertsethtakarn, Silapong, Jeamwattanalert, Bodhidatta, Mason, Begum, Haque, Praharaj and Houpt2016) and Texas Red-TTGGCGGACAATTGCATGCGAT-BHQ2 (probe) (Wiria et al., Reference Wiria, Prasetyani, Hamid, Wammes, Lell, Ariawan, Uh, Wibowo, Djuardi, Wahyuni, Sutanto, May, Luty, Verweij, Sartono, Yazdanbakhsh and Supali2010). The PCR amplification was performed in a StepOnePlus real-time PCR system (Applied Biosystems, Foster City, CA, USA), using the following thermal profile: 50°C for 2 min, 95°C for 10 min, 45 cycles of 10 s at 95°C, 30 s at 60°C. The results were expressed in genome equivalents per mL of stool DNA extract (GE/mL).
Results
A total of 64/286 (22.4%; 95% CI = 17.8–27.7) stool samples (37/115 = 32.2%, 95% CI = 23.9–41.6 for Indian children and 27/171 = 15.8%, 95% CI = 10.8–22.3 for immigrants in Italy) were classified as positive for A. lumbricoides using the Kato-Katz technique. Of all the positive stool samples, 36 samples showed mammillated (Fig. 1A and B) A. lumbricoides eggs (56.3%; 95% CI = 43.3–68.4), 25 elements ascribable to fertilized decorticated eggs (39.1%; 95% CI = 27.4–52.1) (Fig. 3), while three samples showed mammillated and fertilized decorticated eggs (4.7%; 95% CI = 1.2–14.0).
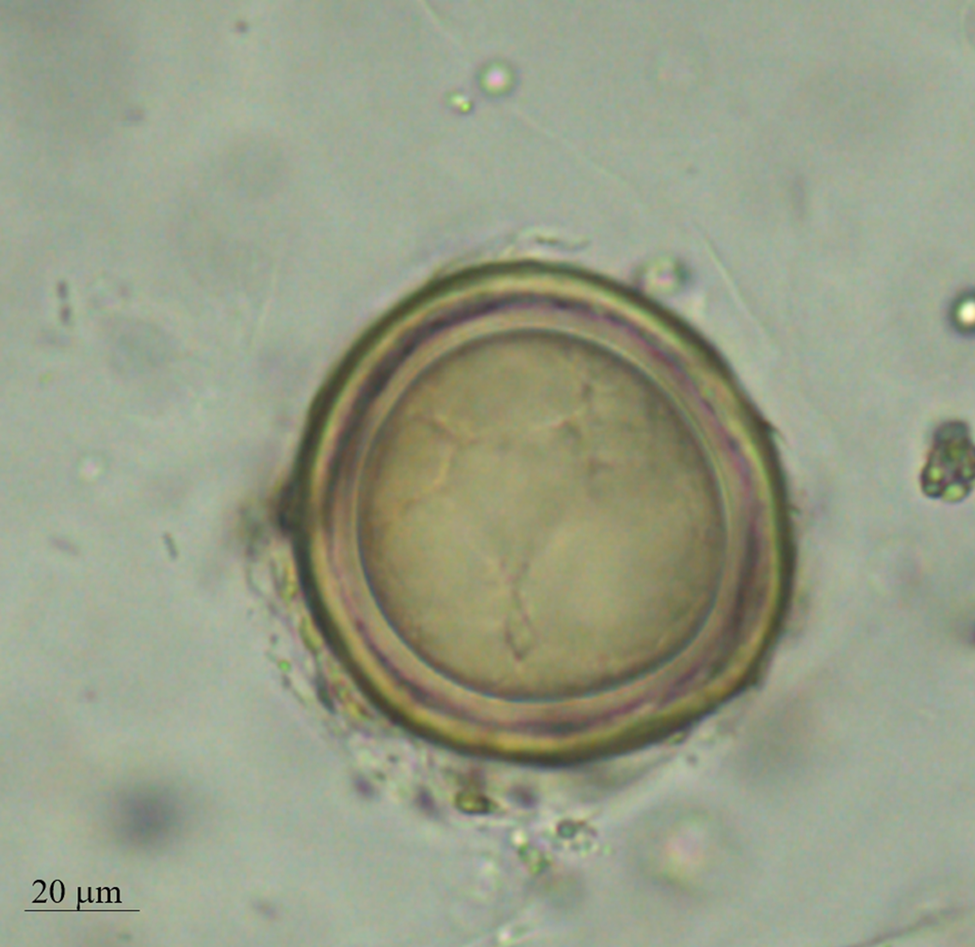
Fig. 3. Elements ascribable to fertilized decorticated A. lumbricoides eggs founded in Kato-Katz.
A total of 39/286 stool samples (13.6%; 95% CI = 10.0–18.3; 22/115 = 19.1%, 95% CI = 12.6–27.8 for Indian children and 17/171 = 9.9%, 95% CI = 6.1–15.7 for immigrants in Italy) were classified as positive for A. lumbricoides by Mini-FLOTAC. The elements identified as fertilized decorticated A. lumbricoides eggs in 28 samples by Kato-Katz were identified as artefacts by Mini-FLOTAC, because they were different in size and in morphology (i.e. size often was larger than 65 μm and internal granular nature was not present) from fertilized decorticated eggs of A. lumbricoides. Mini-FLOTAC provided statistically significant (P < 0.05) higher mean A. lumbricoides egg counts than the Kato-Katz technique (285 vs 174 eggs per gram of stool) for the 39 positive samples for both techniques. All the results obtained for each technique are summarized in Table 1. Coprocultures performed on all the 28 dubious stool samples, with fertilized decorticated eggs, confirmed that no larvated eggs were identified after incubation. Moreover, negative results were obtained also by qPCR performed on the same dubious samples, grouped in two pools: one for Indian samples and another for immigrant samples. For these reasons, these elements were identified as artefacts, probably referable to pollen grains.
Table 1. Summary of results obtained with Kato-Katz thick smear and Mini-FLOTAC for the diagnosis of Ascaris lumbricoides in stool samples from two cohorts (school-age children from India and adult immigrants in Italy)

Discussion
The current global strategy by the WHO is to achieve and maintain the STH moderate-to-heavy intensity to less than 2% reducing the preventive chemotherapy (PC) deworming programmes based on albendazole or mebendazole treatment of pre-school and school age children living in endemic areas. However, an accurate diagnosis is necessary for an appropriate strategy of intervention, as well as for monitoring the impact of PC programmes.
The clinical diagnosis of STH is not possible, because infected people might be asymptomatic or showing unspecific signs (Shalaby and Shalaby, Reference Shalaby and Shalaby2016). For these reasons, currently, the diagnosis of A. lumbricoides, as for the other STHs, relies on the microscopic demonstration of eggs in stool (Cools et al., Reference Cools, Vlaminck, Albonico, Ame, Ayana, José Antonio, Cringoli, Dana, Keiser, Maurelli, Maya, Matoso, Montresor, Mekonnen, Mirams, Corrêa-Oliveira, Pinto, Rinaldi, Sayasone, Thomas, Verweij, Vercruysse and Levecke2019; Momčilović et al., Reference Momcilovic, Cantacessi, Arsic-Arsenijevic, Otranto and Tasić-Otašević2019). However, the main problem in parasite identification is distinguishing the parasitic structures from artefacts that can be present in stool samples, especially for A. lumbricoides that can be easier misclassified (Colmer-Hamood, Reference Colmer-Hamood2001; Garcia et al., Reference Garcia, Arrowood, Kokoskin, Paltridge, Pillai, Procop, Ryan, Shimizu and Visvesvara2018). Moreover, the presence of artefacts can perturb the eggs per gram of faeces (EPG) evaluation especially when the level of infection is low (<5000 EPG for A. lumbricoides, Cools et al., Reference Cools, Vlaminck, Albonico, Ame, Ayana, José Antonio, Cringoli, Dana, Keiser, Maurelli, Maya, Matoso, Montresor, Mekonnen, Mirams, Corrêa-Oliveira, Pinto, Rinaldi, Sayasone, Thomas, Verweij, Vercruysse and Levecke2019). This error can diminish when the infections are moderate-heavy and the fraction of artefacts is lower than ‘true’ eggs value.
In this study, the Mini-FLOTAC discriminated correctly between parasitic elements and artefacts, as reported in ‘Results’.
The Mini-FLOTAC, indeed, thanks to the flotation (using a flotation solution with a specific gravity able to evidence precise parasitic elements) and translation features, allows the complete separation of parasitic elements and debris in counting chambers, with a subsequent clearer view that facilitates a correct differential diagnosis between parasitic eggs and artefacts. This technique was used previously and compared with Kato-Katz for A. lumbricoides detection with similar sensitivity and higher specificity. However, using the appropriate flotation solution higher sensitivity can be obtained (Barda et al., Reference Barda, Cajal, Villagran, Cimino, Juarez, Krolewiecki, Rinaldi, Cringoli, Burioni and Albonico2014; Lamberton and Jourdan, Reference Lamberton and Jourdan2015). Indeed, the choice of the flotation solution is of utmost importance to increase the sensitivity to detect parasitic elements and to easily distinguish the artefacts from eggs (Cringoli et al., Reference Cringoli, Rinaldi, Maurelli and Utzinger2010). Based on the above-mentioned advantages, in 2019, the Mini-FLOTAC was included in the WHO guidelines among the suggested techniques for STH diagnosis (WHO, 2019).
One limitation of our study was that we could not confirm by molecular techniques if the artefacts that we found were vegetable material, pollen grains or other elements as food residues, because we did not have enough quantity of stool. The use of innovative tools such as fluorescent dyes for a rapid discrimination between A. lumbricoides eggs and artefacts could be very useful to facilitate egg identification. This approach was used previously to evaluate viability of some eggs, e.g. Schistosoma haematobium and Ascaris suum (Włodarczyk et al., Reference Włodarczyk, Zdybel, Próchniak, Osiński, Karamon, Kłapeć and Cencek2017; Forson et al., Reference Forson, Tetteh-Quarcoo, Ahenkorah, Aryee, Okine, Afutu, Djameh, Agyapong, Anang and Ayeh-Kumi2019). The permeable fluorescent dyes penetrate in the intact structures of cells, emitting light of different colours which permit to distinguish between live and dead eggs.
Moreover, recent advances in parasitological diagnosis are based on the development of semi-automated and automated systems (e.g. smartphone-based technologies, digital microscopes, etc.) combined with image analysis (e.g. FECPAKG2, Lab-on Disk Platform, Kubic FLOTAC Microscope, etc.) for the detection of helminth eggs. These systems are currently improving to increase the sensitivity and accuracy or to reduce the costs or to validate the tools in the lab and/or in the field (Cringoli et al., Reference Cringoli, Amadesi, Maurelli, Celano, Piantadosi, Bosco, Ciuca, Cesarelli, Bifulco, Montresor and Rinaldi2021). However, automated identification and counting of STH eggs, based on artificial intelligence will be very useful in order to reduce human errors and time of reading, increasing diagnostic efficiency (Lu et al., Reference Lu, Liu, Xiao, Hu, Zhang, Xu, Chu, Xu and Smith2018; Moser et al., Reference Moser, Bärenbold, Mirams, Cools, Vlaminck, Ali, Ame, Hattendorf, Vounatsou, Levecke and Keiser2018; Sukas et al., Reference Sukas, Van Dorst, Kryj, Lagatie, De Malsche and Stuyver2019; Cringoli et al., Reference Cringoli, Amadesi, Maurelli, Celano, Piantadosi, Bosco, Ciuca, Cesarelli, Bifulco, Montresor and Rinaldi2021). Moreover, often these tools permit also to transfer via internet the captured pictures to other laboratories, supporting the technicians also directly in the field (Tele-Parasitology) (Cringoli et al., Reference Cringoli, Amadesi, Maurelli, Celano, Piantadosi, Bosco, Ciuca, Cesarelli, Bifulco, Montresor and Rinaldi2021). Therefore, in this way, it will be possible also to achieve one of the main goals of the WHO NTD 2021-2030 roadmap to control and eliminate STH in endemic regions, using standardized and advanced diagnostic techniques (WHO, 2020).
Data
All data generated or analysed during this study are included in this published article. The datasets used and/or analysed during the present study available from the corresponding author upon reasonable request.
Acknowledgements
The authors thank all the staff of National Centre for Disease Control in New Delhi (India) for their collaboration to collect and process the stool samples at the NCDC laboratory.
Author contributions
MPM, LCA, PC, BL, PC, DI and LG: performed sampling and laboratory analyses. AM, GC, CSA and LR: conceived the study. All authors contributed to data analysis and interpretation, and preparation of the manuscript. All authors read and approved the final manuscript.
Financial support
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Conflict of interest
The Mini-FLOTAC technique was developed and is patented by GC, but the patent has been handed over to the University of Naples Federico II. The fact that GC is the current patent holder of the Mini-FLOTAC and Fill-FLOTAC had no role in the preparation and submission of the protocols reported or the design and implementation of ongoing and future studies. To obtain Mini- FLOTAC or Fill-FLOTAC devices, a contribution is required that is used only to cover costs of production and packaging, and to contribute to the ongoing FLOTAC research. The remaining authors declare that they have no competing interests.
Ethical standards
This research was included in the monitoring activities on STHs by the WHO.