Reversals, reductions or absence of normal cerebral asymmetries have been described in schizophrenia in several structures such as the planum temporale (Reference Rossi, Stratta and MatteiRossi et al, 1992; Reference Barta, Pearlson and BrillBarta et al, 1997), the Sylvian fissure (Reference Crow, Brown and BurtonCrow et al, 1992; Reference Falkaï, Bogerts and GreveFalkaï et al, 1995), both occipital and frontal lobes (Reference Bilder, Houwei and BogertsBilder et al, 1994), and the cerebral ventricles (Reference Crow, Ball and BloomCrow et al, 1989a ). Moreover, people with early-onset schizophrenia might be more likely to exhibit reduced brain asymmetries (Reference Crow, Colter and FrithCrow et al, 1989b ). Cerebral sulcal and gyral patterns and their asymmetries may provide a robust marker of the contribution of neurodevelopmental factors to the aetiology of schizophrenia. Indeed, cerebral sulci are formed during the second and third trimesters (Reference Chi, Dooling and GillesChi et al, 1977; Reference HuangHuang, 1991) and remain relatively stable after birth (Reference Armstrong, Schleicher and OmranArmstrong et al, 1995; Reference Magnotta, Andreasen and SchultMagnotta et al, 1999), whereas other brain measurements such as cerebral volumes can vary with ageing, life experiences, nutrition (Reference Dalman and CullbergDalman & Cullberg, 1999), substance misuse (Reference Pfefferbaum, Sullivan and MathalonPfefferbaum et al, 1997; Reference Wilson, Mathew and TurkingtonWilson et al, 2000) and even neuroleptic medication (Reference Chakos, Lieberman and BilderChakos et al, 1994). Yücel et al (Reference Yücel, Stuart and Maruff2002a ) reported a lack of leftward paracingulate sulcus asymmetry among right-handed men with schizophrenia compared with a control group. Since these findings are relevant to the study of the neurobiological aspects of schizophrenia, they need replication in independent samples. Our hypothesis was that the asymmetric patterns of the paracingulate sulcus observed in healthy individuals would be disrupted in men with early-onset schizophrenia. Such abnormalities could provide evidence of abnormal neurodevelopment of paralimbic areas in schizophrenia.
METHOD
Study participants
The study included 40 right-handed male patients (mean age 27.2 years, s.d=6.6) fulfilling DSM-IV criteria for schizophrenia (American Psychiatric Association, 1994), with clinical onset before age 25 years (Reference Crow, Colter and FrithCrow et al, 1989b ; Reference Corrigal and MurrayCorrigal & Murray, 1994). Patients were recruited from the psychiatric departments of several hospitals in the Paris area of France, and from Barcelona in Spain. Clinical ratings and review of research and medical records were performed by senior psychiatrists (M.B., I.B., M.-L.P.-M., C.R. and J.-L.M.). Clinical symptoms were assessed by means of the Scale for the Assessment of Positive Symptoms (SAPS; Reference AndreasenAndreasen, 1984) (mean score 27.5, s.d.=17.6) and the Scale for the Assessment of Negative Symptoms (SANS; Reference AndreasenAndreasen, 1982) (mean score 53, s.d.=25.6). The comparison group included 100 right-handed healthy male volunteers (mean age 28.5 years, s.d.=7.7), with no family history of psychiatric disorders. All participants were examined to exclude medical conditions, including substance misuse, and all were found to be right handed according to Annett's questionnaire (Reference AnnettAnnett, 1970).
Magnetic resonance imaging
Whole-brain T 1-weighted images were acquired using a 1.5 T magnetic resonance imaging (MRI) scanner. A three-dimensional inversion-recovery-prepared fast-spoiled gradient echo sequence was used with the following scanning parameters: 256 × 256 matrix, 124 or 248 contiguous slices of 1.5-mm or 0.6-mm thickness, field of view 24 cm × 24 cm, flip angle 10°, echo time 2.2 ms, T 1 600 ms, repetition time 12.5 ms. Everyone who was scanned first gave written informed consent, according to the local ethics committee requirements.
Paracingulate sulcus rating
The paracingulate sulcus extends dorsally and parallel to the cingulate sulcus, lying in the medial walls of the frontal lobes. Measurements were made using the method of describing paracingulate sulcus patterns defined by Yücel et al (Reference Yücel, Stuart and Maruff2001) in healthy adults. The origin of the paracingulate sulcus was defined as the point where the sulcus extends posteriorly, from a coronal plane parallel to the line through the anterior commissure, and perpendicular to the line through the anterior and posterior commissures (Reference Yücel, Stuart and MaruffYücel et al, 2001). The paracingulate sulcus was classified as ‘prominent’ if the sulcus extended at least 40 mm and exhibited no more than 20 mm of interruptions between its origin and a coronal plane passing through the anterior commissure (Fig. 1c ). If interruptions exceeded 20 mm and the length was at least 20 mm, the paracingulate sulcus was classified as ‘present’ (Fig. 1b ). Finally, when no clearly horizontal sulcus parallel to the cingulate sulcus could be found or was less than 20 mm in length, it was classified as ‘absent’ (Fig. 1a ). Leftward asymmetry was defined as a ‘prominent’ pattern in the left hemisphere with a ‘present’ or ‘absent’ pattern in the right hemisphere, or as ‘present’ left and ‘absent’ right patterns. Conversely, rightward asymmetry was defined as a right ‘prominent’ pattern occurring with a left ‘present’ or ‘absent’ pattern, or as a right ‘present’ pattern and a left ‘absent’ pattern. Symmetry of the paracingulate sulcus was rated when the same pattern was observed in both hemispheres. Two independent raters, masked to participant status, examined the images. Intrarater reliability was assessed by one examiner (J.-B.L.P.), who examined all cases (κ=0.92). Interrater reliability was assessed by using a second rater (D.B.-F.) to evaluate 70 randomly chosen participants (κ=0.90).
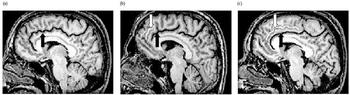
Fig. 1 Magnetic resonance images of the cingulate sulcus (black arrow) and paracingulate sulcus (white arrow): T 1-weighted sagittal views showing the distinct paracingulate sulcus patterns evaluated in this study; (a) absent, (b) present, (c) prominent.
Intragroup asymmetry was assessed using McNemar's test for symmetry. Hemispheric differences for paracingulate sulcus presence were assessed within each group using χ2 tests. Afterwards, between-group differences for rightward/leftward asymmetry rates were also assessed using χ2. Statistical significance was set at P=0.05. Correlations between clinical scores and paracingulate sulcus patterns were searched for in the patient group, using the Spearman rank order statistic.
RESULTS
Within-group comparisons
Healthy volunteers had a significant paracingulate sulcus asymmetry (McNemar's test χ2=31.47, P<0.00001, d.f.=3). The presence of a paracingulate sulcus (‘prominent’ or ‘present’) was more frequent in the left hemisphere than in the right (χ2=30.5, P<0.001) and it was more often defined as ‘prominent’ than ‘present’ in the left hemisphere (χ2=6.7, P=0.009). In participants with schizophrenia however, no significant asymmetry was detected (McNemar's test χ2=2.33, P=0.51, d.f.=3). The frequency of a paracingulate sulcus (‘prominent’ or ‘present’) did not differ between left and right hemispheres (χ2=0.05, P=0.82). When a ‘prominent’ paracingulate sulcus was found, it was equally frequent on both sides (χ2=1.13, P=0.29).
Between-group comparisons
Paracingulate sulcus patterns (Table 1) were more often leftwardly asymmetric in healthy participants than in patients (χ2=7.48, P=0.006). In contrast, patients had more rightward asymmetric patterns (χ2=4.84, P=0.03). The incidence rates of a symmetrical pattern were similar in both groups (χ2=1.12, P=0.29).
Table 1 Hemispheric distribution of the morphological patterns of the paracingulate sulcus in healthy men and men with schizophrenia. Values are percentages of cases presenting distinct patterns of paracingulate sulcus morphology in both left and right hemispheres

Clinical correlates
The presence or absence of paracingulate sulcus, either in the right or left hemisphere, was not related to any clinical measure (SANS and SAPS scores). Spearman correlation tests were applied to search for relationships between asymmetry or symmetry of the paracingulate sulcus (leftward, rightward or symmetrical) and clinical measures. No significant correlation was observed.
DISCUSSION
The main finding of this study was a lack of paracingulate sulcus asymmetry among male patients with early-onset schizophrenia; this was due to both the less-frequent leftward asymmetry and the more-frequent rightward asymmetry of paracingulate sulcus patterns than in healthy participants.
Patient characteristics
The characteristics of our patient sample (all men, with disease onset before 25 years of age) may have influenced the findings. These patients were chosen because previous studies have reported more-frequent brain anomalies in early-onset cases (Reference Crow, Colter and FrithCrow et al, 1989b ) and an interaction between diagnosis and gender on frontal lobe measurements in patients with schizophrenia (Reference Highley, Esiri and McDonaldHighley et al, 1998). Moreover, previous investigations conducted in normal individuals have found gender differences in paracingulate sulcus patterns, as well as in intrasulcal paracingulate sulcus grey matter volumes (Reference Paus, Tomaiuolo and OtakyPaus et al, 1996a ; Reference Yücel, Stuart and MaruffYücel et al, 2001). Therefore, it is possible that different findings would be observed in older or female patients. Thus, it should be stated that our results pertain to a homogeneous category of patients (right-handed, male, with early-onset disease) and may not be generalisable to other types of patient with schizophrenia.
Consistent replication
The finding of an asymmetric pattern of the paracingulate sulcus in healthy individuals is consistent with previous anatomical MRI reports (Paus et al, Reference Paus, Tomaiuolo and Otaky1996a , Reference Paus, Otaky and Caramanos b ; Reference Yücel, Stuart and MaruffYücel et al, 2001). Furthermore, our results replicate those reported by Yücel et al (Reference Yücel, Stuart and Maruff2002a ) and extend to an independent sample of earlyonset cases, indicating that the reduction of leftward paracingulate sulcus asymmetry might be a robust finding. They are also complementary to reports of grey matter volume reductions in the cingulate, suggesting an involvement of the cingulate and paracingulate region in the pathophysiology of schizophrenic disorders (Reference Albanese, Merlo and MascittiAlbanese et al, 1995; Reference Wright, Ellison and SharmaWright et al, 1999; Reference Paillère-Martinot, Caclin and ArtigesPaillère-Martinot et al, 2001; Reference Sigmundsson, Suckling and MaierSigmundsson et al, 2001). Further evidence implicating these limbic or paralimbic regions in schizophrenia comes from functional findings demonstrating abnormal brain activity in these regions in response to cognitive demands (e.g. Reference Carter, Mintun and NicholsCarter et al, 1997; Reference Artiges, Martinot and VerdysArtiges et al, 2000) and from a report showing that brain activity patterns during a cognitive task depend on the underlying morphology of the paracingulate sulcus (Reference Yücel, Pantelis and StuartYücel et al, 2002b ).
Folding and connectivity
Functional neuroimaging studies indicate that schizophrenia is characterised by an alteration of brain connectivity (e.g. Reference Fletcher, McKenna and FristonFletcher et al, 1999; Reference Spence, Grasby and LiddleSpence et al, 2000). Notably, it has been suggested that gyral-shape studies might be an interesting alternative method of searching for disturbances of brain connectivity in the disorder (Reference Highley, Walker and EsiriHighley et al, 2001). Indeed, brain gyrification indexes in humans would reflect the density of intrinsic connectivity (Reference Welker, Jones and PetersWelker, 1990). A proposed mechanism derived from the tension-based morphogenesis theory explains cortical folding as depending on differences in mechanical tension along axons, dendrites or glial processes connecting different brain regions (Reference van Essenvan Essen, 1997). Thus, the presence of a prominent paracingulate sulcus could indicate a marked local connectivity within the paralimbic cortex (Brodmann's area 32) and adjacent regions (Brodmann's areas 6, 8 and 9). In contrast, the reduction in paracingulate sulcus folding, more frequently observed in the left hemisphere in our patients, could be the consequence of weaker local connectivity in these areas. According to this model, people with sulcogyral anomalies would be more likely to exhibit dysfunctional cingulate or paracingulate connectivity.
Folding during the third trimester
It has been historically proposed that losses, absences or reversals of hemispheric asymmetries could denote indexes of brain dysmaturation (Reference Crichton-BrowneCrichton-Browne, 1879) in mental disturbances (Reference SouthardSouthard, 1915). A corpus of theories postulate that the absence of right shift (Reference AnnettAnnett, 1999) or the loss of the physiological asymmetry in the ontogenetically recent heteromodal cortices (Reference Pearlson, Petty and RossPearlson et al, 1996; Reference CrowCrow, 1999) might result from genetic factors that would enhance the vulnerability to schizophrenia. An anomaly in the paracingulate sulcus pattern in patients supports these theories. Indeed, the paracingulate sulcus develops by 36 weeks of gestation, when major cerebral asymmetry has already been established. Thus, as a tertiary sulcus, it depends on the pattern of regional gyrification previously established by primary and secondary sulci (Reference Armstrong, Schleicher and OmranArmstrong et al, 1995). Consequently, evidence of altered paracingulate development in people with schizophrenia may reflect abnormalities in the course of neurodevelopment occurring, at the earliest, during week 32 of gestation, when secondary sulci are forming — i.e. during the third trimester. Folding peculiarities in this paralimbic region during the third trimester do not preclude more wide-spread and earlier anomalies in folding symmetry, which remain to be investigated (Vogeley et al, Reference Vogeley, Scheider-Axmann and Pfeiffer2000, Reference Vogeley, Tepest and Pfeiffer2001). Consequently, sulcogyral measurements can be used to explore hypotheses (e.g. Reference Crow, Ball and BloomCrow et al, 1989a ; Reference Bilder, Houwei and BogertsBilder et al, 1994) that disturbances in brain development during the second and third trimesters are related to vulnerability to schizophrenic disorders.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ Abnormal maturation of the paralimbic area may occur during the third trimester of gestation.
-
▪ The study provides further evidence of abnormal development of the cerebral hemispheres in schizophrenia.
-
▪ Reduced cerebral asymmetry could be a vulnerability factor for schizophrenia.
LIMITATIONS
-
▪ The investigation was restricted to male patients with early-onset disease.
-
▪ Continuous measures of the sulcus length were not available, and there was no interrater assessment of schizophrenia symptom rating scales.
-
▪ The findings cannot address the issue of anatomic specificity of the paracingulate sulcus since the characteristics of other sulci were not assessed.
Acknowledgement
The authors thank M. C. Bourdel for thoughtful statistical comments.





eLetters
No eLetters have been published for this article.