Hand, foot and mouth disease remains a major cause of morbidity and mortality in children in developing countries and the main causative pathogen is enterovirus 71 (EV71). In mainland China, large outbreaks of EV71-caused hand, foot and mouth disease, resulting in millions of cases and thousands of deaths in children, have been reported since 2008( Reference Yang, Ren and Xiong 1 ). Furthermore, the cumulative data indicate an increased incidence of hand, foot and mouth disease during 2009 and 2011; at that time, EV71 was still the predominant pathogen( Reference Ni, Yi and Yin 2 – Reference Wang, Feng and Yang 4 ). As yet, there is no effective vaccine to prevent EV71 infection. Without an effective antiviral treatment, patients must rely on their own immune system to overcome the infection.
Vitamin A (VA) is one of the most important nutrients for childhood nutrition. Many epidemiological and clinical investigations have confirmed that VA can boost immunity against infectious diseases in children( Reference Mullin 5 ). Previous studies carried out by our group have reported that most of the EV71-infected children present with VA insufficiency, which is associated with decreased immunity and more severe pathogenic conditions( Reference Chen, Yang and Yan 6 ). Specifically, we found serum interferon-α (IFN-α) levels to be markedly reduced and positively related to the lack of VA in EV71-infected children( Reference Chen, Yang and Yan 6 ). These clinical findings stimulated us to establish an in vitro model to investigate the potential mechanism for the effect of VA on antiviral immunity to EV71 infection. Retinoid has been reported to inhibit the replication of various viruses, including the measles virus, the herpes virus, the immunodeficiency virus type 1 and the Epstein–Barr virus, in vitro ( Reference Trottier, Chabot and Mann 7 – Reference Pomponi, Cariati and Zancai 10 ). However, there is no report regarding the antiviral effect of retinoid on EV71 in vitro.
The active VA metabolite all-trans-retinoic acid (ATRA) is the natural ligand for the retinoic acid receptors (RAR). There is a rapidly expanding body of literature that supports a role for ATRA in the IFN signalling pathway. In various in vitro systems, ATRA has been shown to regulate the expression of a number of IFN-stimulated genes, including retinoid-induced gene I (RIG-I)( Reference Soye, Trottier and Richardson 11 – Reference Dimberg, Nilsson and Oberg 13 ). RIG-I is a pattern-recognition receptor involved in the innate immune response of the host. The inhibition of RIG-I-mediated type I IFN responses may contribute to the pathogenesis of EV71 infection( Reference Lei, Liu and Ma 14 ).
In the present study, we established an in vitro model to test our hypothesis that ATRA can act against EV71 through an IFN-inducing effect, specifically by regulating RIG-I mRNA expression. We used U937 cells, a monocytic cell line, as our model. U937 cells were chosen because previous studies have demonstrated that EV71 can infect human monocytes( Reference Wang, Chen and Su 15 ). In addition, U937 cells have also been used to demonstrate that ATRA can influence elements of the type I IFN response( Reference Gianni, Terao and Fortino 16 ).
Experimental methods
Cell culture and reagents
The human monocytic cell line U937 (Chinese Academy of Sciences) was maintained in Roswell Park Memorial Institute (RPMI) 1640 (GIBCO/BRL Life Technologies) containing 2 mm-l-glutamine and 10 % fetal bovine serum (FBS) (GIBCO/BRL Life Technologies). African green monkey kidney (Vero) cells (Chinese Academy of Sciences) were maintained in modified Eagle's medium (GIBCO/BRL Life Technologies) with 10 % FBS. All cultures were maintained at 37°C in a 5 % CO2 humidified incubator (Thermo Scientific). ATRA (Sigma-Aldrich) and RAR-α antagonist (Ro 41-5253) (Enzo Life Sciences) were kept as stock solutions at 10− 2 m in 100 % dimethyl sulphoxide (Invitrogen), and further dilutions were made using RPMI 1640.
Virus preparation and titration
EV71 isolated from a patient's throat swab was provided by the virology laboratory of Children's Hospital of Fudan University. The virus was identified by reverse-transcription PCR and sequencing( Reference Yang, Zhu and Li 17 ). The virus was propagated in Vero cells in modified Eagle's medium containing 2 % FBS. Briefly, monolayers of Vero cells were inoculated with the virus at a multiplicity of infection of 1, and the virus culture medium was harvested after incubation for 24 h. The virus titre was determined by measuring the tissue culture infectious dose (TCID50) values. Serially diluted virus samples (from 10− 1 to 10− 9) were added to Vero cells in ninety-six well plates, and four replicate samples were used at each dilution. The ninety-six-well plates were incubated for 7 d at 37°C, and the TCID50 values were obtained by determining the cytopathic effects of infected Vero cells. A mock control was prepared in the same manner as the EV71 preparation, except that the Vero cells were not infected with EV71.
U937 cell virus infection
U937 cells were infected with EV71 at a multiplicity of infection of 10 for 2 h at 37°C. Following infection, the cells were resuspended at a density of 1 × 106 cells/ml in RPMI 1640 containing a reduced concentration of FBS (2 %) in a twenty-four-well plate (1 ml/well). EV71-infected cells were incubated for 24 h or 48 h in the presence of ATRA and/or Ro 41-5253. U937 cells treated with 0·1 % dimethyl sulphoxide in RPMI with 2 % FBS were used as the mock treatment cells in all the experiments.
Immunofluorescence staining of enterovirus 71-infected cells
EV71-infected and control cells were placed on glass slides, fixed in 4 % paraformaldehyde for 15 min and then blocked in 10 % normal goat serum (Vector Laboratories) in PBS (GIBCO/BRL Life Technologies) containing 0·1 % Triton X-100 (Sigma-Aldrich) for 25 min at room temperature. After washing twice with 1 % normal goat serum in PBS, the cells were stained with a 1:500 dilution of a monoclonal antibody against EV71 (Millipore Corporation) overnight at 4°C. The cells were then stained with a 1:200 dilution of fluorescein isothiocyanate-conjugated secondary antibodies (Santa Cruz Biotechnology) and counterstained with 4,6-diamidino-2-phenylindole dihydrochloride (Vector Laboratories), after which they were observed under a fluorescent microscope (Leica Microsystems).
Enterovirus 71-infected cell count by flow cytometry
A minimum of 1 × 106 cells were collected by centrifugation at 1200 g for 5 min and fixed in 4 % paraformaldehyde (Sigma-Aldrich) for 15 min. Next, 50 μl of permeabilisation medium (Invitrogen) and a 1:200 dilution of a monoclonal antibody against EV71 (Millipore Corporation) were added to each cell pellet, followed by incubation for 1 h at room temperature. Fluorescein isothiocyanate-conjugated secondary antibodies (Santa Cruz Biotechnology) at a 1:100 dilution were added, followed by incubation for 30 min in the dark at room temperature. Finally, after three washes with PBS, the cells were resuspended in 0·5 ml of PBS for flow cytometry (BD FACSCalibur; BD Biosciences).
Apoptosis analysis
The Annexin V–fluorescein isothiocyanate/Propidium Iodide Apoptosis Detection Kit (Merck) was used in accordance with the manufacturer's protocol. After 24 or 48 h of EV71 and/or ATRA treatment, the cells were harvested and resuspended in binding buffer. Fluorescence signals were detected using a flow cytometer (BD FACSCalibur) within 1 h.
Detection of interferon-α in supernatants
The level of IFN-α in the supernatants of the cultures was detected after 24 and 48 h of incubation with each treatment agent, using an ELISA kit (eBioscience). The optical density was detected at a wavelength of 450 nm on a microplate reader (Wellscan MK3; Labsystems). A standard curve ranging from 7·8 to 500 pg/ml was constructed using serial dilutions of a human IFN-α standard provided with the kit. Samples with values greater than the negative control but less than 7·8 pg/ml were assigned values based on the extrapolation of the standard curve.
RNA isolation and real-time PCR
The total RNA of the U937 cells was isolated using TRIzol (Invitrogen) according to the manufacturer's instructions. The concentration of RNA was measured by absorbance at 260 nm in a spectrophotometer (NanoVue; GE Healthcare Bio-Sciences AB). Reverse transcriptions were conducted using 1 μg of total RNA in a 20 μl final volume using the Prime Script™ RT Reagent Kit (TaKaRa Biotechnology). Real-time PCR was carried out with a Mastercycler realplex 4 PCR system (Eppendorf) using the RT2 SYBR Green qPCR MasterMix (Toyobo) under the following conditions: 95°C for 1 min, followed by forty cycles of 95°C for 15 s, 60°C for 15 s and 72°C for 45 s. The relative expression levels of the genes were normalised to those of the housekeeping gene β-actin using the comparative threshold cycle (C t) method, and data were analysed using the ΔΔC t method. Primer sequences, PCR product sizes and annealing temperatures are summarised in Table 1.
Table 1 Primer sequences, annealing temperatures and product sizes in real-time PCR

RAR-α, retinoic acid receptor α; IFN-α1, interferon-α1; IFNAR1, type I IFN receptor; IPS-1, IFN promoter-stimulating factor 1; RIG-I, retinoid-induced gene I; TRAF3, TNF receptor-associated factor 3; TBK1, TRAF family member-associated NF-κB activator-binding kinase 1; IRF3, interferon regulatory factor 3.
Statistical analysis
The results are presented as means and standard deviations. Statistical operations were conducted with the SPSS Statistical Package, version 13.0. The statistical analysis was conducted using Student's t test for the comparison of means. A probability value < 0·05 was considered significant.
Results
All-trans-retinoic acid decreased the percentage of enterovirus 71-infected cells
The EV71 viral capsid protein 1 (VP1)+ capsid proteins were detected with an anti-VP1 antibody using immunofluorescence microscopy. VP1+ proteins were observed in the cytoplasmic region of U937 cells inoculated with EV71, but not in the mock-infected control cells (Fig. 1). These findings demonstrate that U937 cells can be infected with EV71.
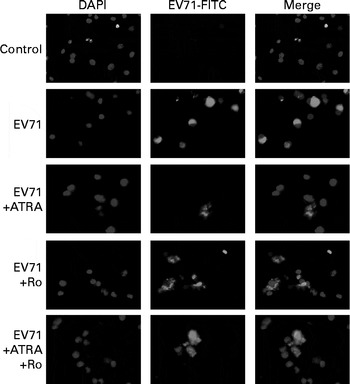
Fig. 1 Immunofluorescent staining of enterovirus 71 (EV71)-infected U937 cells with an anti viral capsid protein 1 (VP1) antibody. U937 (1 × 106 per ml) cells were infected with EV71 at a multiplicity of infection of 10 in the presence of 1 μm- all-trans-retinoic acid (ATRA) and/or 10 μm-Ro 41-5253 (retinoic acid receptor-α antagonist) for 24 h. EV71 antigen (green fluorescence) was observed in the cytoplasmic region of the EV71-infected cells. DAPI, 4,6-diamidino-2-phenylindole dihydrochloride; FITC, fluorescein isothiocyanate.
To determine whether ATRA could inhibit EV71 infection in our model, the percentage of infected cells was determined by flow cytometry after staining the cells with an anti-EV71 monoclonal antibody. Following 24 h of incubation, the percentage of EV71-infected cells in the ATRA+EV71 group was lower than that of cells in the EV71 group, but this difference did not reach statistical significance. Importantly, ATRA treatment markedly decreased the percentage of EV71-infected cells 48 h after infection (P< 0·01; Fig. 2). Because our previous studies( Reference Wei, Yang and Wang 18 – Reference Zhou, Wang and Yang 20 ) indicated that RAR-α is a major mediator of ATRA action, we sought to determine whether the antiviral effect of ATRA requires RAR-α signalling. The addition of Ro 41-5253 to the EV71-infected U937 cell culture significantly reduced EV71 inhibition mediated by ATRA (Fig. 2). These findings provide evidence that ATRA contributes to the defence against EV71 infection in U937 cells and RAR-α is likely to play an important role in this antiviral effect.
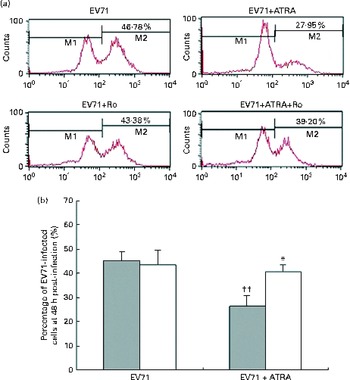
Fig. 2 Enterovirus 71 (EV71)-infected cell count using viral capsid protein 1 (VP1) antibody and flow cytometry. (a) Quantification of EV71-infected cells using flow cytometry. M1 indicates the distribution of fluorescence on cells without EV71 infection and M2 indicates the distribution of fluorescence on EV71-infected cells. (b) Percentage of EV71-infected U937 cells treated with all-trans-retinoic acid (ATRA) 48 h after infection. ![]() , without Ro; □, with Ro. Values are means, with standard deviations represented by vertical bars. * Mean value was significantly different from that of the EV71+ATRA group incubated in the absence of Ro (P< 0·05). †† Mean value was significantly different from that of the EV71 group incubated in the absence of Ro (P< 0·01).
, without Ro; □, with Ro. Values are means, with standard deviations represented by vertical bars. * Mean value was significantly different from that of the EV71+ATRA group incubated in the absence of Ro (P< 0·05). †† Mean value was significantly different from that of the EV71 group incubated in the absence of Ro (P< 0·01).
All-trans-retinoic acid reduced the cell apoptosis induced by enterovirus 71 infection
Cell apoptosis induced by EV71 infection was examined by Annexin V/propidium iodide staining 24 h after infection. The results of three independent experiments are shown in Fig. 3. A very low percentage of apoptotic cells were detected by flow cytometry in the control group, and we did not find any changes in cell apoptosis when U937 cells were treated with ATRA alone. Notably, EV71 infection induced a significantly increased percentage of apoptotic cells than of the control cells (P< 0·001). More importantly, when ATRA was present in the culture, the percentage of apoptotic cells induced by EV71 declined. However, Ro 41-5253 partly counteracted the action of ATRA. Taken together, these observations suggest that ATRA may protect cells against EV71-induced apoptosis and that RAR-α is involved in this action.
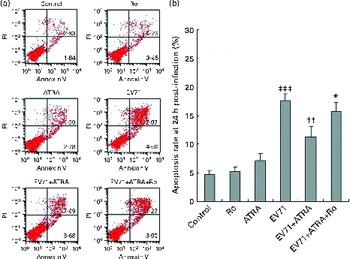
Fig. 3 Effect of all-trans-retinoic acid (ATRA) on apoptosis induced by enterovirus 71 (EV71) infection. Cells were infected with EV71 at a multiplicity of infection of 10. (a) Apoptosis ratio detected by flow cytometry in U937 cells 24 h after infection. Viable cells were Annexin V negative and propidium iodide (PI) negative (lower left quadrant) and necrotic cells were Annexin V negative and PI positive (upper left quadrant). Apoptotic cells were differentiated as those in early apoptosis (Annexin V+/PI − , lower right quadrant) and late apoptosis (Annexin V+/PI+, upper right quadrant). (b) Apoptosis rate 24 h after infection. Total cell apoptosis was calculated by including cells that were in stages of early and late apoptosis. ATRA treatment significantly decreased the percentage of apoptotic cells induced by EV71. Ro, retinoic acid receptor-α antagonist. Values are means, with standard deviations represented by vertical bars. * Mean value was significantly different from that of the EV71+ATRA group (P< 0·05). †† Mean value was significantly different from that of the EV71 group (P< 0·01). ‡‡‡ Mean value was significantly different from that of the control group (P< 0·001).
All-trans-retinoic acid increased the production of interferon-α induced by enterovirus 71 infection
Type I IFN are potent antiviral cytokines that can be regulated by retinoids in vitro in some cell models( Reference Dimberg, Nilsson and Oberg 13 , Reference Luo and Ross 21 ). To determine the effect of ATRA on the production of type I IFN induced by EV71, we measured the production of IFN-α at both mRNA and protein levels in our cell model. As shown in Fig. 4(a), ATRA treatment alone resulted in a marked up-regulation of IFN-α1 mRNA expression at 24 h. By contrast, EV71 infection alone decreased the expression of IFN-α1 mRNA. Moreover, when ATRA was added to the EV71-infected cell culture, the expression of IFN-α1 mRNA was significantly increased compared with that in cells treated with ATRA treatment (P< 0·05). A similar pattern of IFNAR1 (a component of type I IFN receptor) mRNA expression was also observed (Fig. 4(b)). These observations were further confirmed by ELISA. After treatment of EV71-infected cells with ATRA for 48 h, the production of IFN-α at the protein level was significantly higher than that in the control cells (P< 0·001; Fig. 4(c)). However, Ro 41-5253 treatment successfully blocked the induction of IFN-α mRNA and IFN-α protein expression by ATRA and EV71 infection. These results indicated that ATRA could induce the production of IFN-α in EV71-infected cells, which may contribute to the antiviral effect of ATRA, and that this effect is likely to be mediated by RAR-α activity.
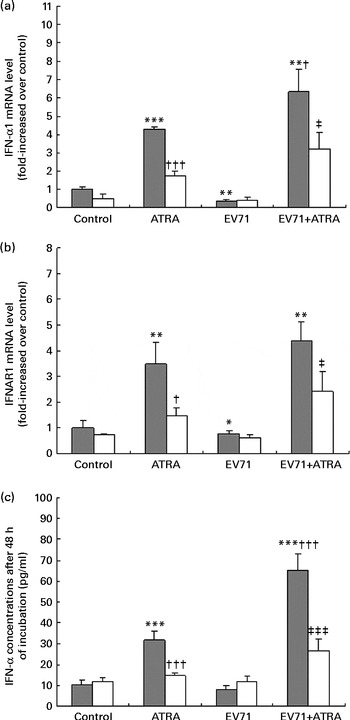
Fig. 4 Induction of interferon (IFN)-α expression. U937 cells were infected with enterovirus 71 (EV71) at a multiplicity of infection of 10 in the presence of 1 μm-all-trans-retinoic acid (ATRA) with (□) or without (![]() ) 10 μm-Ro 41-5253 (retinoic acid receptor-α antagonist). (a) IFN-α1 gene expression assessed using quantitative real-time PCR 24 h after treatment. (b) IFN-α receptor 1 (IFNAR1) gene expression assessed using quantitative real-time PCR 24 h after treatment. (c) IFN-α levels were detected after 48 h of incubation using an ELISA. Values are means, with standard deviations represented by vertical bars. Mean value was significantly different from that of the control group incubated in the absence of Ro 41-5253: * P< 0·05, ** P< 0·01, *** P< 0·001. Mean value was significantly different from that of the ATRA group incubated in the absence of Ro 41-5253: † P< 0·05, ††† P< 0·001. Mean value was significantly different from that of the EV71+ATRA group incubated in the absence of Ro 41-5253: ‡ P< 0·05, ‡‡‡ P< 0·001.
) 10 μm-Ro 41-5253 (retinoic acid receptor-α antagonist). (a) IFN-α1 gene expression assessed using quantitative real-time PCR 24 h after treatment. (b) IFN-α receptor 1 (IFNAR1) gene expression assessed using quantitative real-time PCR 24 h after treatment. (c) IFN-α levels were detected after 48 h of incubation using an ELISA. Values are means, with standard deviations represented by vertical bars. Mean value was significantly different from that of the control group incubated in the absence of Ro 41-5253: * P< 0·05, ** P< 0·01, *** P< 0·001. Mean value was significantly different from that of the ATRA group incubated in the absence of Ro 41-5253: † P< 0·05, ††† P< 0·001. Mean value was significantly different from that of the EV71+ATRA group incubated in the absence of Ro 41-5253: ‡ P< 0·05, ‡‡‡ P< 0·001.
All-trans-retinoic acid regulated the expression of retinoic acid receptor-α and retinoid-induced gene I signalling genes in enterovirus 71-infected cells
Because the present results indicated that RAR-α was crucial for the antiviral effect of ATRA in vitro, we sought to investigate the modulation of RAR-α mRNA expression by ATRA in EV71-infected cells. The results of fluorescence quantitative PCR revealed that ATRA induced a significant up-regulation of RAR-α mRNA expression in both EV71-infected and non-infected U937 cells. However, when Ro 41-5253 was added to the cell culture, ATRA was unable to increase the expression of RAR-α mRNA. Furthermore, EV71 infection itself had no visible effect on the expression of RAR-α mRNA (Fig. 5(a)).
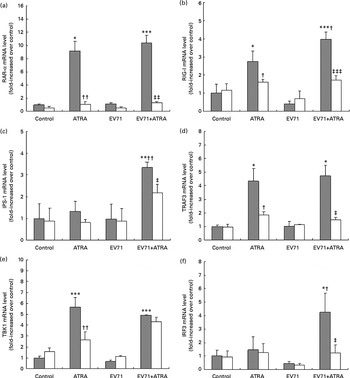
Fig. 5 Gene expression regulated by all-trans-retinoic acid (ATRA) in enterovirus 71 (EV71)-infected cells. U937 cells were infected with EV71 at a multiplicity of infection of 10 in the presence of 1 μm-ATRA with (□) or without (![]() ) 10 μm-Ro 41-5253 (retinoic acid receptor-α antagonist). (a) IFN-α1 gene expression was assessed using quantitative real-time PCR 24 h after treatment. (b) IFN-α receptor 1 (IFNAR1) gene expression was assessed using quantitative real-time PCR 24 h after treatment. (c) IFN-α levels were detected after 48 h of incubation using an ELISA. Values are means, with standard deviations represented by vertical bars. Mean value was significantly different from that of the control group incubated in the absence of Ro 41-5253: * P< 0·05, ** P< 0·01, *** P< 0·001. Mean value was significantly different from that of the ATRA group incubated in the absence of Ro 41-5253: † P< 0·05, †† P< 0·01. Mean value was significantly different from that of the EV71+ATRA group incubated in the absence of Ro 41-5253: ‡ P< 0·05, ‡‡ P< 0·01, ‡‡‡ P< 0·001.
) 10 μm-Ro 41-5253 (retinoic acid receptor-α antagonist). (a) IFN-α1 gene expression was assessed using quantitative real-time PCR 24 h after treatment. (b) IFN-α receptor 1 (IFNAR1) gene expression was assessed using quantitative real-time PCR 24 h after treatment. (c) IFN-α levels were detected after 48 h of incubation using an ELISA. Values are means, with standard deviations represented by vertical bars. Mean value was significantly different from that of the control group incubated in the absence of Ro 41-5253: * P< 0·05, ** P< 0·01, *** P< 0·001. Mean value was significantly different from that of the ATRA group incubated in the absence of Ro 41-5253: † P< 0·05, †† P< 0·01. Mean value was significantly different from that of the EV71+ATRA group incubated in the absence of Ro 41-5253: ‡ P< 0·05, ‡‡ P< 0·01, ‡‡‡ P< 0·001.
RIG-I is a classical IFN-stimulated gene involved in the induction of IFN-α production in response to EV71( Reference Lu, Yi and Zhao 22 , Reference Huang, Weng and Shih 23 ). To further investigate the antiviral effect of ATRA on IFN-α signalling, we measured the expression of RIG-I mRNA and genes involved in the downstream signalling pathway. In untreated U937 cells, RIG-I mRNA was expressed at low levels. ATRA treatment alone resulted in a moderate increase in the expression of RIG-I mRNA, while EV71 infection alone had no discernible effect on the expression of RIG-I mRNA in this cell line. Importantly, the U937 cells infected with EV71 and treated with ATRA exhibited higher levels of RIG-I mRNA expression than those treated with ATRA alone (P< 0·05; Fig. 5(b)). Additionally, in the ATRA-treated and EV71-infected U937 cell model, we also observed the up-regulation of the expression of several downstream genes (IFN promoter-stimulating factor 1 (IPS-1), TNF receptor-associated factor 3 (TRAF3), TRAF family member-associated NF-κB activator-binding kinase 1 (TBK1) and interferon regulatory factor 3 (IRF3)) in the RIG-I signalling pathway (Fig. 5(c)–(f)). These findings demonstrate that ATRA treatment can activate RIG-I signalling in EV71-infected cells. The induction of RIG-I mRNA expression by treatment of EV71-infected cells with ATRA was blocked by exposure to Ro 41-5253, suggesting that the up-regulation of the expression of this gene is also mediated via RAR-α.
Discussion
Since 1988, the WHO( 24 ) has recommended high-dose VA treatment for acute measles virus infection in many resource-poor settings. In most of the developing countries, VA is also routinely given as a nutritional supplement to reduce the overall risk of morbidity and mortality( Reference Imdad, Herzer and Mayo-Wilson 25 , Reference Benn, Martins and Rodrigues 26 ). Although non-specific effects on immune function and/or epithelial repair have been suggested, the mechanism by which this vitamin acts to prevent infection is not yet fully understood( Reference Mora, Iwata and von Andrian 27 ).
The potential impact of ATRA on viral replication in vitro has been examined previously by several groups( Reference Trottier, Chabot and Mann 7 , Reference Isaacs, Kascsak and Pullarkat 9 , Reference Ghazal, DeMattei and Giulietti 28 ). However, there has been no study to date reporting the antiviral effect of ATRA on EV71 infection. In the present study, we found that ATRA can protect cells from EV71 infection and can partially rescue infected cells from EV71-induced apoptosis. These observations indicate that ATRA has an antiviral action against EV71. However, as only one strain isolated from a patient was used in the present study, as to whether the antiviral effect of ATRA on EV71 is strain specific deserves further study.
In addition, data obtained from the in vitro study also demonstrated that ATRA could significantly increase the production of IFN-α in EV71-infected cells. IFN-α is one of the most important antiviral cytokines in innate immune response. The early induction and action of type I IFN result in cellular resistance to viral infection and inhibition of viral replication and dissemination( Reference Alsharifi, Mullbacher and Regner 29 ). A previous study has shown that IFN has a protective role in EV71 infection. In a mouse model of EV71 infection, the induction of type I IFN by polyinosinic-polycytidylic acid (poly(I:C)) was found to reduce viral loads. Conversely, the administration of IFN-neutralising antibody facilitated EV71 infection and exacerbated disease severity( Reference Liu, Lee and Wang 30 ). In our cell model, EV71 failed to induce the production of IFN-α, which may contribute to the pathogenesis of EV71 infection. Notably, ATRA exhibits a significant IFN-inducing effect in EV71-infected cells, effectively counteracting EV71's strategy for escaping the innate immune response of the host.
ATRA has been shown to induce certain elements of IFN signalling in some in vitro models, including RIG-I( Reference Soye, Trottier and Richardson 11 , Reference Dimberg, Nilsson and Oberg 13 , Reference Luo and Ross 21 ). RIG-I is an important IFN-stimulated gene, and it can induce the expression of IFN via IPS-1. IPS-1 is an adaptor involved in RIG-I-mediated antiviral immune responses( Reference Kawai, Takahashi and Sato 31 ). The interaction between activated RIG-I and IPS-1 induces the recruitment of downstream signalling molecules, including TRAF3, followed by the activation of TBK1 and inhibitor of NF-κB kinase ɛ. These kinases then activate IRF3, an essential transcription factor regulating the expression of type I IFN during virus infection; finally, these molecules induce the production of type I IFN( Reference Takeuchi and Akira 32 ). In the present study, we found that the expression of RIG-I mRNA and its downstream signalling genes was up-regulated in EV71-infected cells treated with ATRA. Therefore, it can be suggested that the effect of ATRA on the anti-EV71 immune response is modulated by the RIG-I signalling pathway.
Retinoid is known to exert most of its biological effects via nuclear retinoid receptor signalling( Reference Schug, Berry and Shaw 33 ). There are two subfamilies of retinoid receptors, the RAR and the retinoid X receptors, and each has three members: α; β; γ( Reference de Lera, Bourguet and Altucci 34 ). These receptors can form heterodimers with retinoid X receptor or homodimers with themselves and then bind to retinoic acid-responsive elements or retinoid X receptor elements to control the expression of retinoic acid-responsive genes in the presence of retinoids( Reference Pfahl 35 ). The results of our preliminary experiment indicated that RAR-α was a main receptor for ATRA in U937 cells; thus, a selective RAR-α antagonist, Ro 41-5253, was used to investigate the pathway of ATRA action in EV71-infected cells. The results of the in vitro study indicated that all the effects of ATRA on EV71-infected cells (including the reduction of the percentage of infected and apoptotic cells, induction of the production of IFN-α and up-regulation of the expression of RIG-I and its downstream signalling genes) were inhibited at different levels by Ro 41-5253. These findings indicate that the effects of ATRA on EV71 infection were likely to be mediated via the RAR-α pathway. However, Ro 41-5253 did not completely reverse all the effects of ATRA, which raises the possibility that other retinoid receptors may also contribute modestly to the antiviral effect. Further studies are needed to elucidate these findings.
In conclusion, we have demonstrated that ATRA is a potent IFN inducer that effectively inhibits EV71 and significantly regulates the RIG-I signalling pathway in the human monocytic cell line. The antiviral effect of ATRA appears to occur through a RAR-α pathway. The present results raise the possibility that ATRA may directly contribute to anti-EV71 infection by reinforcing innate immunity.
Acknowledgements
The present study was supported by a grant from the Ming Dao project of Fudan University (MDJH2012020).
The authors' contributions are as follows: Y. Y. and W. W. were responsible for study conception and design; S. C., L. S. and J. X. conducted the research; S. C. analysed the data and wrote the manuscript; Y. Y. and W. W. revised the manuscript; W. W. had primary responsibility for the final content; all the authors read and approved the final manuscript.
None of the authors has any financial and personal relationships with other people or organisations or any other conflicts of interest that could inappropriately influence the present study.








