Because minerals interact with other nutrients as well as with themselves, their study is complex, but essential for understanding and solving problems involving animal nutrition. Cu supplementation is essential for cattle as soils are deficient in Cu in most regions of Brazil and due to its important roles. Therefore, the study of Cu sources is critical. Moreover, it has been found that organic Cu has a higher availability than inorganic sources, does not interact with other elements, and may be absorbed by different metabolic processes( Reference Nockels, Debonis and Torrent 1 ). Due to the greater availability of Cu in organic form as copper proteinate, it is possible to decrease its levels in the diet as well as in the faeces and, consequently, environmental contamination, which has become a growing concern.
Cu absorption mostly occurs by active transport and is saturable and sometimes by simple diffusion. Cu absorption is very low in adult ruminants (from 1 to 5 %). Most of the absorbed Cu enters the blood bound to ceruloplasmin, and some by binding to albumin, and enters the tissues. Cu is stored as metallothionein in the liver and can be used in case of dietary Cu inadequacy. As the absorption is very low, most of the ingested Cu is excreted in faeces and is actively excreted via the bile and urine( Reference Ewing and Charlton 2 ).
Lipids are susceptible to free radical attack, and lipid oxidation can be extremely harmful due to its propensity for causing a chain reaction. Lipid oxidation can be described as a cascade of biochemical events resulting from the action of free radicals on the lipids of cell membranes and mainly generating peroxyl and alkoxy radicals. Lipids in meat products oxidise easily, leading to the development of unpleasant odour and flavour, known as ‘warmed-over-flavour’( Reference Curi, Pompéia and Myasaka 3 ), which reduces their shelf life. Lipid oxidation in tissues can be affected by cattle diet and by the presence of antioxidants or pro-oxidants in cells( Reference Rey and Lopez-Bote 4 ).
From a biological perspective, antioxidants may be defined as compounds that protect biological systems against the potentially harmful effects of processes or reactions that promote the oxidation of macromolecules or cellular structures( Reference Abdalla 5 ). Superoxide dismutase (SOD) enzyme, which is Cu-dependent, together with glutathione peroxidase (GSH-Px) enzyme and catalase, protects the cells against damage caused by free radicals, inhibiting lipid oxidation and increasing meat stability( Reference Nuernberg, Wegner and Ender 6 , Reference Lauridsen, Nielsen and Henckel 7 ), which can be measured by the thiobarbituric acid-reactive substance (TBARS) test. Therefore, as Cu is important for the activity of several enzymes, cattle study is interesting to better understand its effect on meat quality.
Some studies have shown that depending on the form in which Cu is found, it can have pro-oxidant or antioxidant effects. In the ionic form, it acts as a pro-oxidant and can stimulate lipid oxidation, but this effect is reduced due to its high ability to bind to proteins( Reference Luza and Speisky 8 ). On the other hand, as it is chelated in the organic form, it can have a different mode of action. Contrasting results have been reported in the literature regarding the effects on lipid metabolism according to Cu source (i.e. organic or inorganic). Johnson & Engle( Reference Johnson and Engle 9 ) reported that steers supplemented with 10 mg Cu/kg DM from Availa Cu (organic source) had higher (P< 0·02) total plasma cholesterol concentrations than those supplemented with 10 mg Cu/kg DM from CuSO4. Steers supplemented with 20 mg Cu/kg DM from Availa Cu had lower (P< 0·03) plasma TAG concentrations than those supplemented with 20 mg Cu/kg DM from CuSO4. On the other hand, Engle et al. ( Reference Engle, Spears and Armstrong 10 ) did not find any difference in the effects due to sources.
According to the National Research Council( 11 ), Cu requirement of beef cattle is 8 mg/kg DM. However, levels above this requirement are required to observe significant effects of this mineral on lipid metabolism( Reference Engle 12 ).
Therefore, the present study was conducted to evaluate the effects of Cu level and source and a possible interaction between these factors on meat lipid oxidation, meat colour and hepatic SOD and GSH-Px activities in Nellore beef cattle.
Materials and methods
Animal management and feeding
The experiment was carried out at the Faculty of Animal Science and Food Engineering, University of Sao Paulo, at the Pirassununga Campus. A total of thirty-five non-castrated Nellore bulls, averaging 491 (sem 37·7) kg of shrunk body weight and 30 months old, were placed in individual pens. Institutional and national guidelines for the care and use of animals were followed, and all experimental procedures involving animals were approved by the College of Animal Science and Food Engineering, University of Sao Paulo.
Before the experiment, the animals were allowed to adapt to the pens for 30 d, during which they were fed a molybdenum diet (50 mg/kg) to decrease their Cu status. After this period, a liver biopsy was performed in accordance with the technique described by Chapman et al. ( Reference Chapman, Cox and Evans 13 ) to determine the initial Cu status.
The cattle were then randomly divided into five groups, resulting in seven animals per treatment. The following treatments were used: (1) control (C) – basal diet without supplementation of Cu (7 mg Cu/kg DM); (2) I10 – basal diet supplemented with 10 mg Cu/kg DM in the form of copper sulphate (inorganic form); (3) I40 – basal diet supplemented with 40 mg Cu/kg DM in the form of copper sulphate; (4) O10 – basal diet supplemented with 10 mg Cu/kg DM in the form of copper proteinate (organic form); (5) O40 – basal diet supplemented with 40 mg Cu/kg DM in the form of copper proteinate (Table 1). Diets were formulated in accordance with the requirements of National Research Council( 14 ).
Table 1 Ingredients and chemical analysis of the basal diet* on a dry basis

NDF, neutral-detergent fibre; ADF, acid-detergent fibre; NFC, non-fibre carbohydrates.
* The basal diet contained 7 mg Cu/kg DM.
† Composition: Ca, 115 g/kg; P, 65 g/kg; S, 30 g/kg; Mg, 11 g/kg; Na, 188 g/kg; Co, 80 mg/kg; I, 83 mg/kg; Mn, 1400 mg/kg; Se, 20 mg/kg; Zn, 400 mg/kg.
‡ NFC = 100 − (crude protein+NDF+ash+fat).
Feed was provided ad libitum once a day in the morning and orts were collected every 3 d. The amount of feed provided was adjusted every 3 d to guarantee about 10 % of orts.
Slaughter and sample collection
After 84 d of feeding, the animals were slaughtered (603 kg of body weight) at the abattoir of the University of Sao Paulo, in accordance with Humanitarian Slaughter Guidelines as required by Brazilian laws( Reference Roça 15 ). During slaughter, samples were obtained from the liver of each animal and immediately frozen in liquid N2 ( − 196°C) for posterior TBARS, SOD, GSH-Px and Cu analyses.
After 24 h of cooling (0–4°C), nine samples (2·5 cm thick) of the longissimus dorsi muscle between the eighth and twelfth ribs were collected from the left side of each carcass for display life (DL), modified atmosphere (MA) and ageing period evaluations.
The first sample (caudal–cranial direction) was exposed to air for 10 min and then the colour was measured with a portable colorimeter (model MiniScan XE; Hunter Associates Laboratory), using the scale L*, a*, b*, a CIE Lab system with light source D65, an observation angle of 10° and a cell opening of 30 mm. The device was calibrated with white (L* = 93·80; a* = − 0·89; b* = 0·95) and black (L* = 1·19; a* = 1·27; b* = 1·92) standards. Measurements were taken at three different sites on the sample surface, and the colour value was considered as the mean of the three measurements. Subsequently, three subsamples (5 g each) were taken and immediately frozen in liquid N2 for TBARS analysis (day 0).
Storage
Display life
Three samples of each animal were individually placed on an absorbent polystyrene tray and wrapped with a film of polyvinyl chloride and placed in a refrigerated display counter (model vertical, 125 LX; Auden) at 4°C for 1, 2 or 3 d. On specific days, samples of each animal placed in trays were removed from the refrigerated display counter and evaluated for colour, and then three subsamples (5 g each) were collected and frozen in liquid N2 for TBARS analysis.
Modified atmosphere
Three samples of each animal were individually placed on an absorbent polystyrene tray and wrapped with a film of polyvinyl chloride and packaged in masterpacks (78·5 cm × 48·5 cm, 0·35 m2; Cryovac), using a gas composition of 75 % O2:25 % CO2. The composition of the gases inside the masterpacks was verified using a gas analyser (model CheckPoint O2/CO2; PBI Dansensor).
The masterpacks were stored in a refrigerator (2·0 ± 1·0°C) for 3, 6 or 9 d. Later, the samples were processed as described for storage in display.
Vacuum packaging
Two samples of each animal were vacuum packed (200 mm × 310 mm, code B530FZ; Cryovac) and stored in a refrigerator (2·0 ± 1·0°C) for 7 or 14 d. Later, the colour was measured and samples were processed as described for storage in display.
Oxidative stability measurement
The oxidative stability of lipids in meat samples was measured by the TBARS test, according to the method of Buege & Aust( Reference Buege and Aust 16 ). After homogenisation in sodium phosphate buffer (10 mm; pH 7·0) and 55 μl of 1 % butylated hydroxytoluene, the tissue samples were transferred to 2 ml microtubes and centrifuged at 8000 rpm for 10 min, and the supernatant was removed and stored on ice. Later, 400 μl of the supernatant and 800 μl of thiobarbituric acid (TBA)–TCA–HCl solution were transferred into a tube and 400 μl of sodium phosphate buffer (10 mm; pH 7·0) and 800 μl of TBA–TCA–HCl solution were transferred into another tube. These tubes were vortexed and kept in a boiling water-bath for 15 min and then kept on ice for 10 min. The samples were immediately transferred to 2 ml microtubes and centrifuged at 11 500 rpm for 5 min. Finally, the absorbance was read in a spectrophotometer at 535 nm by resetting the basal value with reagents used.
Copper analysis
Cu concentrations in liver samples collected in vivo or postmortem were determined by atomic absorption spectrophotometry. For this analysis, liver samples were thawed, washed with distilled deionised water, dried using disposable absorbent paper and weighed with a precision scale. This analysis was carried out at the Laboratory of Mineral at the Department of Animal Science, FZEA-USP.
Enzyme activity determination
Hepatic SOD enzyme activity was determined with improved assays and an assay applicable to acrylamide gels, according to the method of Beauchamp & Fridovich( Reference Beauchamp and Fridovich 17 ), and hepatic GSH-Px enzyme activity was determined using studies based on the quantitative and qualitative characterisation of glutathione peroxidase, according to the method of Paglia & Valentine( Reference Paglia and Valentine 18 ).
Statistical analysis
Statistical analysis was carried out using a completely randomised design with five treatments and seven replications per treatment. The effect of treatment on the variables was evaluated by variance analysis using the mixed procedure of SAS (SAS Institute, Inc.)( 19 ).
Treatments were considered as fixed effects for SOD activity, GSH-Px activity and Cu concentrations. Colour variables and TBARS concentrations were analysed as repeated measures, considering treatments, time of storage and treatment × time of storage interaction as fixed effects. Covariance structures were modelled and the best-fitting one (unstructured) was applied.
When significant treatment effects were found, means were compared through orthogonal contrasts: (1) control v. all; (2) organic v. inorganic Cu source; (3) 10 v. 40 mg Cu/kg DM; (4) source × level interaction. For colour and TBARS variables, when a significant effect of time of storage was detected, linear and quadratic effects were evaluated using polynomial regression. Animal was considered as the experimental unit for all traits.
Results
There was no difference in the initial hepatic Cu concentrations among the treatment groups (Table 2). Cu source had no effect on the final Cu concentrations. Animals supplemented with 40 mg Cu/kg DM had higher Cu concentrations (P= 0·01) than those supplemented with 10 mg Cu/kg DM. Cu-supplemented animals exhibited an increase in Cu concentrations over the feeding period according to both Cu level and source, differing from control animals (P< 0·01), which had the lowest concentrations.
Table 2 Hepatic copper concentrations (dry basis) and accumulation, according to the period of sampling and copper source and level (Mean values with their standard errors)

C, basal diet without supplementation of Cu (control); I10, basal diet supplemented with 10 mg Cu/kg DM in the form of copper sulphate; I40, basal diet supplemented with 40 mg Cu/kg DM in the form of copper sulphate; O10, basal diet supplemented with 10 mg Cu/kg DM in the form of copper proteinate; O40, basal diet supplemented with 40 mg Cu/kg DM in the form of copper proteinate; Inorg, inorganic Cu source; Org, organic Cu source.
However, this increase was not homogeneous between source and level, which resulted in a significant interaction (P= 0·05) for Cu accumulation. There were no differences in the effects of Cu sources at the 10 mg Cu/kg DM level; the organic source exhibited a higher accumulation in the liver (P< 0·01) when compared with the inorganic source at the 40 mg Cu/kg DM level (Fig. 1).
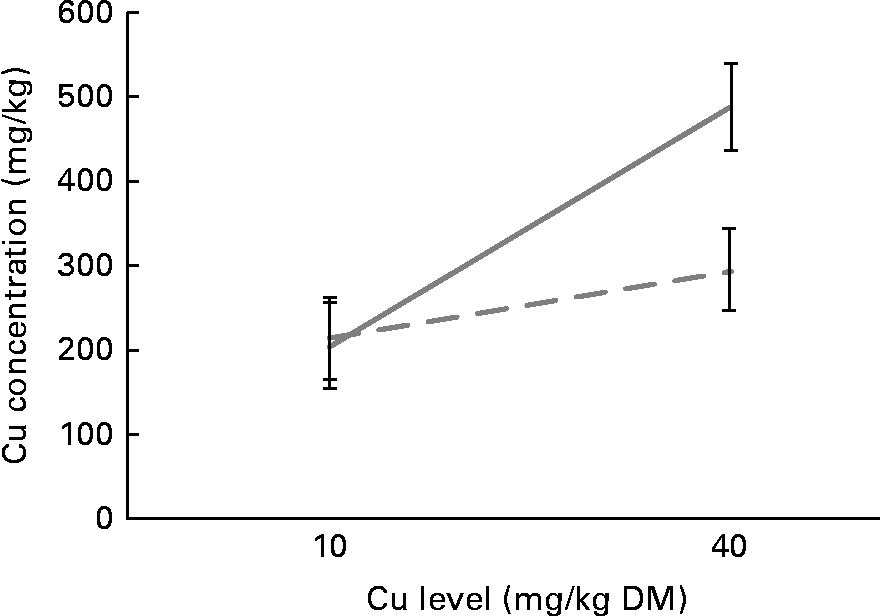
Fig. 1 Copper accumulation in the liver according to copper source and level. ![]() , Organic copper;
, Organic copper; ![]() , inorganic copper.
, inorganic copper.
There was no significant Cu source × level interaction for any trait. Hepatic TBARS concentrations were not affected by the treatments (Table 3). On the other hand, Cu supplementation reduced TBARS concentrations in meat samples exposed to display (P= 0·01), MA (P< 0·01) and vacuum packaging (VC) (P= 0·03) conditions when compared with the control treatment. There were no differences in the effects of Cu source or level on TBARS concentrations.
Table 3 Thiobarbituric acid-reactive substance (TBARS) concentrations, according to copper source and level, in liver samples and meat samples exposed to display, modified atmosphere (MA) and vacuum packaging (VC) conditions (Mean values with their standard errors)
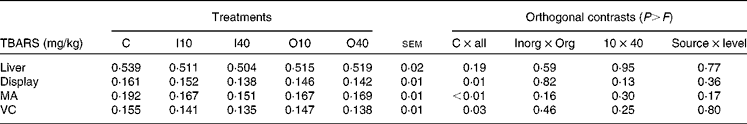
C, basal diet without supplementation of Cu (control); I10, basal diet supplemented with 10 mg Cu/kg DM in the form of copper sulphate; I40, basal diet supplemented with 40 mg Cu/kg DM in the form of copper sulphate; O10, basal diet supplemented with 10 mg Cu/kg DM in the form of copper proteinate; O40, basal diet supplemented with 40 mg Cu/kg DM in the form of copper proteinate; Inorg, inorganic Cu source; Org, organic Cu source.
TBARS values increased linearly with time of storage in display (P< 0·01; Fig. 2). Similar results were obtained for vacuum-packed samples, in which TBARS concentrations increased linearly (P< 0·01; Fig. 3) with days of ageing. A quadratic association between time of storage and TBARS concentrations was observed in samples exposed to MA conditions (P< 0·01; Fig. 4)
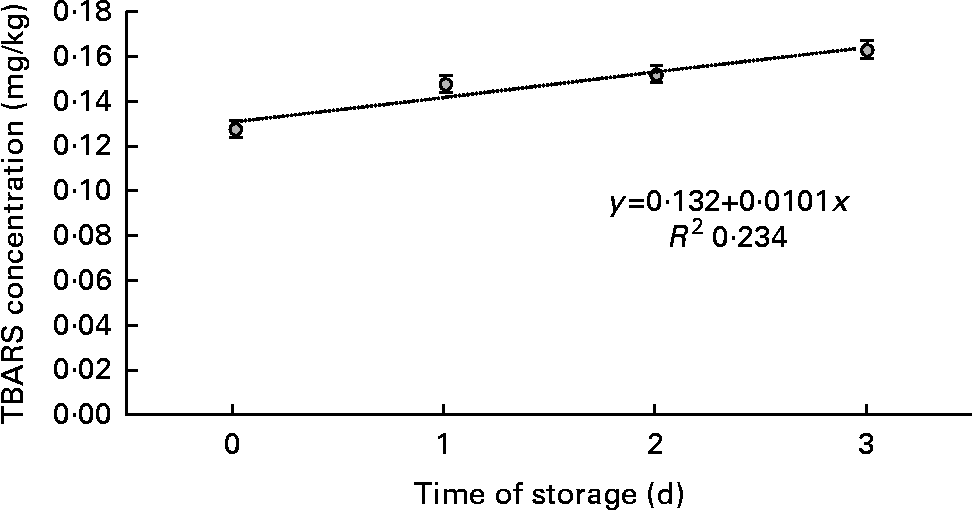
Fig. 2 Means of thiobarbituric acid-reactive substance (TBARS) concentration in meat samples, as a function of time of storage in display.
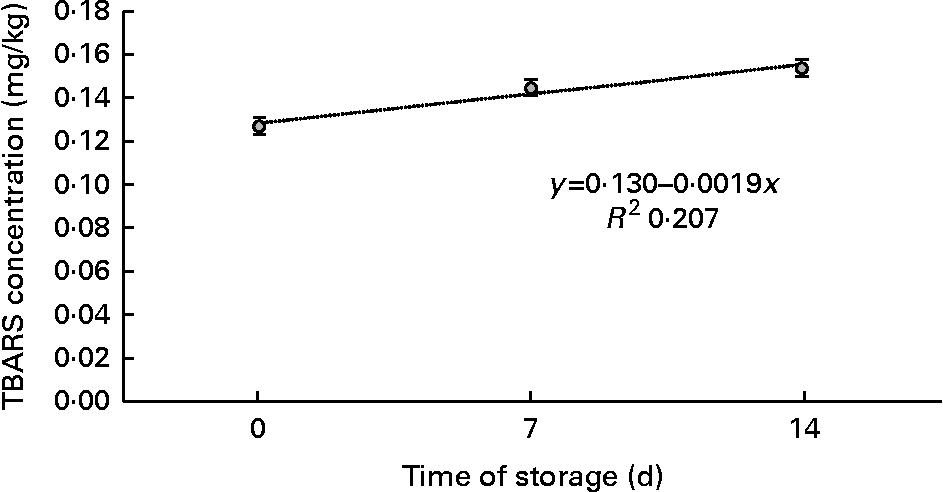
Fig. 3 Means of thiobarbituric acid-reactive substance (TBARS) concentration in meat samples, as a function of time of storage in vacuum packs.
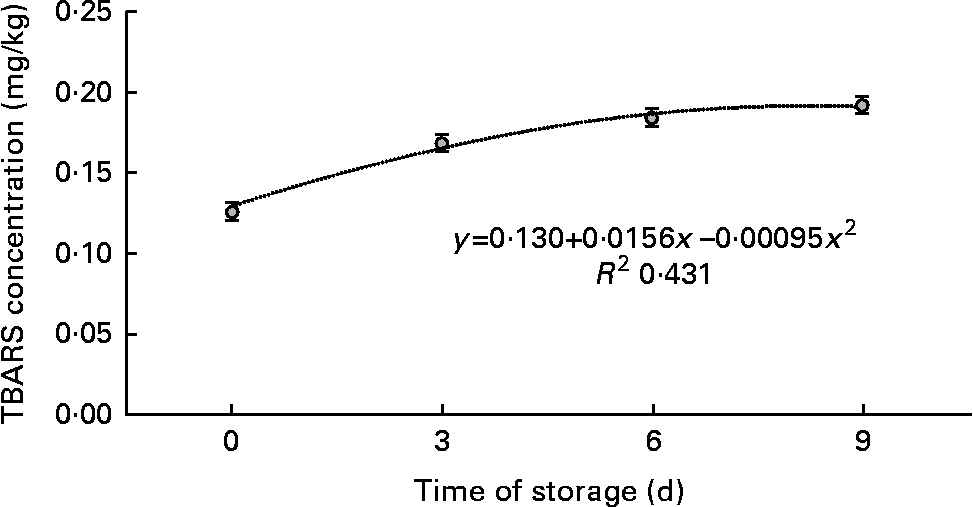
Fig. 4 Means of thiobarbituric acid-reactive substance (TBARS) concentration in meat samples, as a function of time of storage in modified atmosphere.
Cu supplementation increased the L* values of samples exposed to both display and MA conditions (Table 4) when compared with the control treatment, indicating that Cu supplementation increased the luminosity of meat. On the other hand, there were no differences in the effects of Cu source and level on the L* values of samples exposed to both display and MA conditions.
Table 4 Colour variables (L*, a*, b*), according to copper source and level, of meat samples exposed to display and modified atmosphere (MA) conditions (Mean values with their standard errors)
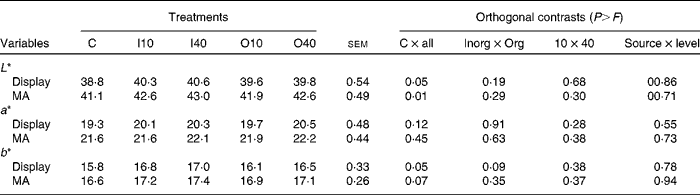
C, basal diet without supplementation of Cu (control); I10, basal diet supplemented with 10 mg Cu/kg DM in the form of copper sulphate; I40, basal diet supplemented with 40 mg Cu/kg DM in the form of copper sulphate; O10, basal diet supplemented with 10 mg Cu/kg DM in the form of copper proteinate; O40, basal diet supplemented with 40 mg Cu/kg DM in the form of copper proteinate; Inorg, inorganic Cu source; Org, organic Cu source.
There was no effect of treatment on a* values, but b* values were greater in samples exposed to both display (P= 0·05) and MA (P= 0·07) conditions. Similar to that observed for other colour attributes, there was no effect of Cu source or level on b* values.
All colour variables (L*, a*, b*) showed linear and quadratic associations with time of storage (P< 0·01) in samples exposed to both display and MA conditions (Table 5).
Table 5 Colour variables (L*, a*, b*), according to time of storage, of meat samples exposed to display and modified atmosphere (MA) conditions (Mean values with their standard errors)
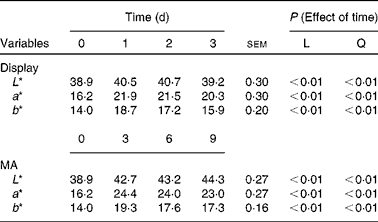
L, linear effect; Q, quadratic effect.
Cu supplementation increased SOD activity when compared with the control treatment (P= 0·01; Table 6). Cu source and level did not affect SOD activity. GSH-Px activity was not affected by any treatment. However, except for those observed upon the O10 treatment, numerical values of GSH-Px activity followed those of SOD activity.
Table 6 Superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) activities in the liver according to copper level and source (Mean values with their standard errors)
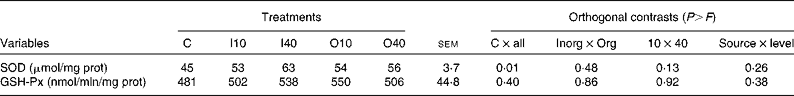
C, basal diet without supplementation of Cu (control); I10, basal diet supplemented with 10 mg Cu/kg DM in the form of copper sulphate; I40, basal diet supplemented with 40 mg Cu/kg DM in the form of copper sulphate; O10, basal diet supplemented with 10 mg Cu/kg DM in the form of copper proteinate; O40, basal diet supplemented with 40 mg Cu/kg DM in the form of copper proteinate; Inorg, inorganic Cu source; Org, organic Cu source; prot, protein.
Discussion
The lack of any significant effect on hepatic TBARS concentrations in the control group and groups supplemented with inorganic or organic Cu in the present study may have been due to the collection of samples immediately after slaughter and thereby not exposing them to any significant stress.
An increase in storage time is expected to cause an increase in TBARS values, as there are metabolic processes that continuously generate free radicals responsible for lipid oxidation independent of the presence of oxygen. Lipid oxidation can also be affected by the diet, as in the presence of antioxidants or pro-oxidants( Reference Rey and Lopez-Bote 4 ). According to Luza & Speisky( Reference Luza and Speisky 8 ), Cu is considered a pro-oxidant; however, in the liver Cu is not present in the free ionic form and is bound to proteins, which leads to its inactivation as a pro-oxidant. This may explain the absence of a pro-oxidant effect of inorganic Cu in the present study. The decrease in TBARS values in the meat samples of Nellore cattle supplemented with Cu, regardless of the source and level, exposed to display, MA and VC conditions compared with those of control animals demonstrates the antioxidant effect of this mineral.
There were no differences in the effects of Cu sources, probably because of the absence of a Cu antagonist in the diet( Reference Hansen, Schlegel and Legleiter 20 ).
TBARS values were higher in samples exposed to MA conditions than in those exposed to VC and display conditions due to a higher concentration of O2. It is known that high concentrations of O2 may adversely affect the oxidative stability of lipids in the muscle, causing a rapid development of rancidity of meat and consequently affecting quality attributes( Reference Jeremiah 21 , Reference Xiong, Decker, Faustman and López-Bote 22 ). As a result, a higher antioxidant effect of Cu on meat lipid oxidation was found in samples exposed to MA conditions than in those exposed to display and VC conditions.
There are no studies on the effect of Cu supplementation on lipid oxidation in cattle. Lauridsen et al. ( Reference Lauridsen, Hojsgaard and Sorensen 23 ) investigated the effects of different supplementation levels of Cu and vitamin E on the oxidative and antioxidative status of the liver in growing pigs and found no pro-oxidant effect of Cu (CuSO4). The increase in hepatic TBARS concentrations was reduced by the addition of vitamin E and Cu, indicating an antioxidant effect of both nutrients. In an experiment that evaluated the composition and oxidation of the longissimus dorsi muscle in pigs, Cu supplementation was found to have no effects on the susceptibility to lipid oxidation( Reference Rey and Lopez-Bote 4 ). While studying the effect of α-tocopherol and CuSO4 on the susceptibility to oxidation in pig muscle, Cava et al. ( Reference Cava, Ventanas and Tejeda 24 ) found lower TBARS values in groups supplemented with Cu and α-tocopherol compared with the control group.
In general, the values of L*, a*, b* determined in the present study as functions of DL and MA were similar. In general, higher values can be found in samples exposed to MA conditions than in those exposed to display conditions. This difference can be attributed to the high concentration of oxygen that promotes greater stability of meat colour( Reference Zakrys, Hogan and ÓSullivan 25 ). The effect of myoglobin oxidation on the valency of Fe ion influences light reflection( Reference Lima Junior, Rangel and Urbano 26 ). As Cu has antioxidant effects, and reduced meat lipid oxidation in the present study, it may have reduced myoglobin oxidation, influencing light reflection. This may be a possible explanation for the increase in the L* values of samples from animals supplemented with Cu when compared with those from control animals when exposed to display and MA conditions.
Some antioxidant enzymes can inhibit lipid oxidation. GSH-Px, along with catalase and SOD, protects cells against damage caused by free radicals( Reference Nuernberg, Wegner and Ender 6 ). SOD is a Cu-dependent enzyme and has a large role in the regulation of dependent O2 cellular metabolism( Reference Jones and Suttle 27 ) and, together with other antioxidant enzymes, it can inhibit lipid oxidation and increase the stability of living tissues in foods such as meat( Reference Lauridsen, Nielsen and Henckel 7 ). In the present study, the use of supplemental Cu did influence SOD activity, as can be seen from Table 6, and may be a possible explanation for the reduction in lipid oxidation. However, according to Lauridsen et al. ( Reference Lauridsen, Nielsen and Henckel 7 ), it is still unclear how the activity of antioxidant enzymes ‘postmortem’ is affected by the total antioxidant capacity of live muscle.
SOD is an important enzyme functionally associated with Cu in different tissues, so supplementation with this mineral may increase its activity. The reduced activity of SOD in the liver was found to be associated with Cu deficiency in rats( Reference Paynter, Moir and Underwood 28 , Reference Prohaska, Dowing and Lukasewycz 29 ).
Many authors have found an increase in SOD enzyme activity in the liver, muscle or blood in different species after Cu supplementation. Senthilkumar et al. ( Reference Senthilkumar, Nagalakshmi and Ramana Reddy 30 ), by conducting an experiment in lambs using Cu supplementation at 7 or 14 mg/kg DM in the form of sulphate or proteinate, reported higher hepatic SOD activity (P< 0·05) in animals supplemented with 14 mg/kg of CuSO4. In the study carried out by Ward & Spears( Reference Ward and Spears 31 ) in cattle, Cu supplementation was found to increase SOD activity until the 56th day of the growth phase. Sharma et al. ( Reference Sharma, Joshi and Pathak 32 ) conducted an experiment in forty Cu-deficient heifers. The animals were divided into two groups (A and B) and only group A was supplemented with a mineral mixture containing CuSO4. A significant improvement was observed in SOD activity in erythrocytes, leucocytes and complete blood from day 60 of treatment in animals in group A, while in those in group B no significant difference in the activity was observed during the 180 d experiment. In Holstein cattle supplemented with 20 mg/kg of Cu (CuSO4) or 10 mg/kg of molybdenum in the form of ammonium molybdate (to develop a Cu-deficient group), Xin et al. ( Reference Xin, Waterman and Heinken 33 ) observed that SOD activity in leucocytes and complete blood cells decreased in the Cu-deficient group.
GSH-Px is an enzyme that contains four atoms of Se( Reference Holben 34 ), with its biosynthesis being induced by Se( Reference Bengoumi, Essamadi and Tressol 35 ). However, some authors, though not using selenium supplementation, reported a correlation between GSH-Px activity and Cu concentrations. Therefore, this enzyme was assessed in the present study.
Se supplementation was not used in the present study; therefore, despite the increased activity of SOD, the increase in substrate levels was probably not enough to cause a significant increase in GSH-Px activity. It can be said that the numerical values of GSH-Px activity followed those of SOD activity. Other authors have reported results similar to those of the present study. Xin et al. ( Reference Xin, Waterman and Heinken 33 ) studied the effect of Cu supplementation on enzymes in Holstein cattle. While there was an effect on SOD activity, GSH-Px activity was not affected by Cu. Lauridsen et al. ( Reference Lauridsen, Nielsen and Henckel 7 ) studied the effect of Cu and vitamin E supplementation on the oxidative status of psoas major muscle and longissimus dorsi muscle in pigs and found no effect of treatment on GSH-Px activity in both muscles.
Conclusion
From the results of the present study, it can be concluded that Cu supplementation improved the oxidative stability of lipids in samples exposed to display, MA and VC conditions, demonstrating the antioxidant effect of this mineral. Although the antioxidant effect of Cu supplementation was verified compared with the control treatment, there were no differences in the effects of the level and source of this mineral on the parameters analysed.
The reduction in meat lipid oxidation, which can be observed from TBARS values, probably occurred due to an increased activity of SOD. Lower lipid oxidation can result in an increased shelf life of meat.
Acknowledgements
The authors are grateful to the FAPESP (Process no. 06/50898-0) for financially supporting the present study. The FAPESP had no role in the design and analysis of the study or in the writing of this article.
The authors’ contributions are as follows: L. B. C. was responsible for the conception and design of the study, acquired, analysed, and interpreted the data, drafted the manuscript, and provided final approval for the version to be published; M. A. Z. was responsible for the conception and design of the study, analysed and interpreted the data, critically revised the manuscript for important intellectual content, and provided final approval for the version to be published; G. R. D. C. acquired the data and provided final approval for the version to be published; F. A. d. P. acquired the data and provided final approval for the version to be published; S. d. L. e. S. analysed and interpreted the data, critically revised the manuscript for important intellectual content, and provided final approval for the version to be published; A. S. N. was responsible for the conception and design of the study, critically revised the manuscript for important intellectual content, and provided final approval for the version to be published.












