Severe acute respiratory syndrome coronavirus 2 (SARS CoV-2) recently emerged as a highly transmissible human pathogen that rapidly escalated into a global pandemic. Reference Jaimes, Andre, Chappie, Millet and Whittaker1 SARS CoV-2 is the causative agent of coronavirus disease 2019 (COVID-19), causing significant respiratory distress and mortality accounting for >18.1 million confirmed cases and ~691,000 deaths worldwide as of August 4, 2020. 2 Six pathogenic coronaviruses are known to infect humans. Of these, SARS CoV-1, Middle East respiratory syndrome coronavirus (MERS-CoV), and now SARS CoV-2 are considered highly pathogenic. Reference Hoffmann, Kleine-Weber and Schroeder3,Reference Channappanavar and Perlman4 Human-to-human transmission of SARS CoV-2 occurs at an elevated rate compared to SARS CoV, which shares considerable sequence homology (79%). Reference Channappanavar and Perlman4–Reference Lu, Zhao and Li6
Until there is an effective vaccine and/or therapeutic approach to treat COVID-19, SARS-CoV-2 control strategies are focused on transmission prevention, including social distancing, hand washing, and use of personal protective equipment (PPE). 7 The increased demand for and shortages of PPE in healthcare and other essential workplace settings has created a need to address decontamination strategies and reuse of PPE. The Veterans’ Health Administration (VHA) recently began developing a supplemental surgical mask. 8 The use of 3-dimensional (3D) printing technology within the healthcare industry is not new; it is currently used in applications such as drug delivery systems, Reference Norman, Madurawe, Moore, Khan and Khairuzzaman9,Reference Kjar and Huang10 surgery, Reference Klein, Lu and Wang11,Reference Chepelev, Wake and Ryan12 personalized medicine, Reference Di Prima, Coburn, Hwang, Kelly, Khairuzzaman and Ricles13–Reference Barrios-Muriel, Romero-Sanchez, Alonso-Sanchez and Rodriguez Salgado15 and biomedical engineering. Reference Murphy and Atala16,Reference Gross, Erkal, Lockwood, Chen and Spence17 In response to current needs during the COVID-19 pandemic, 3D printed technology has expanded to include the production of ventilators and other respiratory support equipment, Reference Liu, Koo and Wong18,Reference Tino, Moore and Antoline19 nasopharyngeal swabs, Reference Cox and Koepsell20 face shields, Reference Wesemann, Pieralli and Fretwurst21 and face masks. Reference Janssens, Huysmans and Swennen22,23
In 2006, the US Department of Health and Human Services (DHHS) asked the Institute of Medicine (IOM) to convene a committee to conduct an evaluation of measures that would permit the reuse of disposable N95 respirators and reusable face masks in healthcare settings. The IOM committee followed criteria to prevent transmission of the 2003 SARS-CoV. The IOM committee found no validated method of decontamination that met criteria for decontamination of N95 respirators or surgical masks. Reference Medicine and Policy24
Although reuse of PPE masks was considered before the SARS-CoV-2 pandemic, literature on potential decontamination strategies are limited and guidelines are often institution- or manufacturer specific. Reference Rowan and Laffey25,26 In this study, we assessed the ability of materials used in the 3D printing of surgical masks to be decontaminated for human viral pathogens including SARS-CoV-2 using common disinfection methods. Data showing that human coronaviruses may survive on various surfaces for up to 9 days makes identification of decontamination strategies against pathogenic viruses increasing important not only during the SARS-CoV-2 pandemic but also for future viral transmission prevention practices. Reference Kampf, Todt, Pfaender and Steinmann27 Our goal was to identify practical decontamination procedures that are easily adapted across healthcare and other workplace settings. We assessed the VHA 3D-printed mask material and 3 additional 3D-printing materials for virus inactivation in these studies.
Materials and methods
Healthy volunteers were invited to participate in the study. Following written informed consent, anticoagulated blood was obtained using heparin collection tubes. This study was approved by the University of Iowa Institutional Review Board.
We used numerous viruses in these studies. Enterovirus 68 (EV68), vaccinia virus, influenza A H1N1 and human coronavirus (HCoV-229E) were all supplied by the American Type Culture Collection (ATCC). We also tested yellow fever virus (YFV, provided by Sanofi), HIV-1 (provided by the NIH AIDS Reagent Program), mumps virus (provided by Merck), adenovirus (provided by University of Iowa Viral Vector Core), Zika virus (kindly provided by DrWendy Maury, University of Iowa), SARS CoV-2 (Seattle Washington strain MN985325), murine hepatitis virus (MHV A59 strain, kindly provided by Dr Stanley Perlman, University of Iowa), and Dengue viruses types 1–4 (DENV, kindly provided by Sarah George, St Louis University). Virus titers were determined in appropriate cell lines by median tissue culture infectious dose (TCID50) or p24 enzyme-linked immunosorbent assay (ELISA) for HIV-1. Reference Welch, Xiang, Okeoma, Schlievert and Stapleton28,Reference Mohr, Xiang and McLinden29 All SARS-CoV-2 work was performed at the University of Iowa Biosafety Level (BSL) 3 core facility, and all other virus studies were conducted at the Iowa City Veterans’ Affairs Infectious Diseases Research Laboratory under BSL2 conditions.
Vero and MDCK cells were purchased from the ATCC, and HEK293 and MT-2 cells were obtained from the NIH AIDS Reagent Program. MRC-5 was purchased from Sigma-Aldrich (St Louis, MO), and VeroE6 and 17Cl-1 were provided by Dr Stanley Perlman. Cells were maintained in media as previously described. Reference Welch, Xiang, Okeoma, Schlievert and Stapleton28,Reference Smee, Hurst and Evans30-Reference Winokur, McLinden and Stapleton32
The VHA supplemental surgical face mask 8 being developed is 3D printed using Multi-Jet Fusion (MJF) technology and a powder-based polyamide-12 (PA12) material (HP 3D HR CB PA 12 - Hewlett-Packard, Palo Alto, CA). The surgical face mask incorporates a removable filter (not tested in this study). The 3D-material is a biocompatible thermoplastic used in medical applications Reference Maitz33 and is believed to be resistant to disinfectants. Thus, it may be reused many times following standard approaches to disinfection. The surgical face mask design has undergone review in a clinical setting and was found appropriate when fabricated with the printer type and materials specified. 23
Virus titers were determined by determining the TCID50 in the cognate cell line as previously described Reference Welch, Xiang, Okeoma, Schlievert and Stapleton28,Reference McLinden, Bhattarai and Stapleton34,Reference Qing, Hantak, Perlman and Gallagher35 or by p24 ELISA (R&D Systems) for HIV-1. Reference Mohr, Xiang and McLinden29
Inactivation studies
Disks were printed in Seattle and shipped to Iowa for testing. Each virus (100 µL) was added to 2 cm diameter × 1.5-mm-thick 3-D printed circular disks (PA12 material unless otherwise noted). Disks were allowed to dry in a laminar flow safety cabinet at room temperature for 2 hours. Disks were inactivated either by thermal or chemical treatment (as described in the Results section). Viruses were recovered by placing the disks into a 12-well culture plate, adding 200 µL media to each well, pipetting 6 times, then removing the media and storing at −80°C until determining the viral titer (within 1 week in all cases). Virus infectivity following each inactivation method was compared to control disks treated only with phosphate-buffered saline (PBS), and the reduction in infectivity (log10 or p24) was calculated. Control and postinactivation virus titers were performed in a minimum of 3 biological replicate experiments.
Statistical analysis
Statistical analyses were performed using GraphPad software V8.2 (GraphPad Prism). We used 2-tailed Student t tests to compare results between treatment and control virus titers from triplicate experiments. P < .05 was considered statistically significant.
Results
To determine the effectiveness of inactivation of viruses applied to 3D printing material, we examined a diverse group of human viral pathogens that have different virion structures (envelope vs nonenveloped), different genome compositions (DNA vs RNA), and genome structures (single strand vs double strand). Reference Knipe and Howley36 In light of the COVID-19 epidemic, we included 1 human low pathogenicity coronavirus (229E), 1 nonhuman coronavirus (MHV), and SARS-CoV-2 in most inactivation experiments. We also included a variety of healthcare-relevant human pathogens (Table 1).
Table 1. Viruses Used in Inactivation Studies
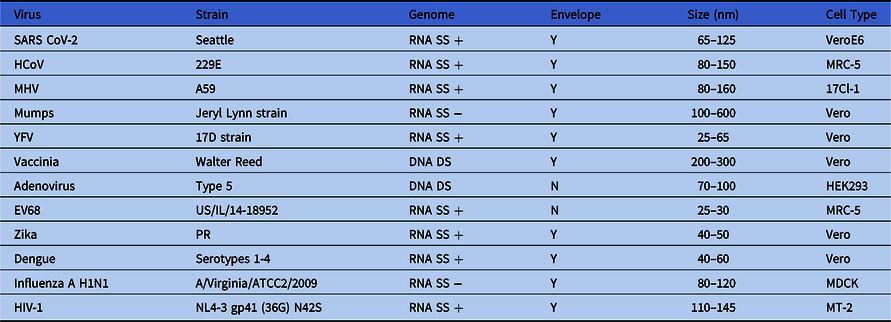
Note. SS, single strand; DS, double strand; +, positive polarity; −, negative polarity; Y, enveloped virus; N, nonenveloped virus; HCoV human coronavirus; MHV, mouse hepatitis virus; YFV, yellow fever virus; EV68, enterovirus 68; HIV-1, human immunodeficiency virus type 1.
Chemical inactivation
Chemical inactivation studies were performed by treating the virus-coated disks with a single application (by wipe) of bleach (10%; 0.6% hypochlorite), isopropyl alcohol (70% IPA), a commercial quaternary ammonium compound (Sani-Cloth germicidal disposable wipe AF3; n-Alkyl [68% C12, 32% C14] dimethyl ethylbenzyl ammonium chlorides – 0.14%; n-Alkyl [60% C14, 30% C12, 5% C18] dimethyl benzyl ammonium chlorides – 0.14%), control wipe (PBS), or a no-wipe control as indicated. After a single application, disks were allowed to dry (<5 minutes in all cases) and virus recovery was measured.
Nearly complete recovery of virus from 3D-printed surgical mask materials was observed, and wiping the disk with PBS did not significantly reduce virus titers compared to the no-wipe controls (>99% of input virus recovered) (Fig. 1A). All viruses tested were exquisitely sensitive to bleach and quaternary ammonium compounds (Fig. 1B–C, 1E), and no infectivity remained following a single wipe of these disinfectants across the 3D mask material. In contrast, 70% IPA did not eliminate viral infectivity although there was >95% (≥1.3 log) inactivation of viruses applied with the exception of HIV-1, for which IPA was ineffective (Fig. 1D and 1E). The log10 reduction in infectivity is shown in Table 2.
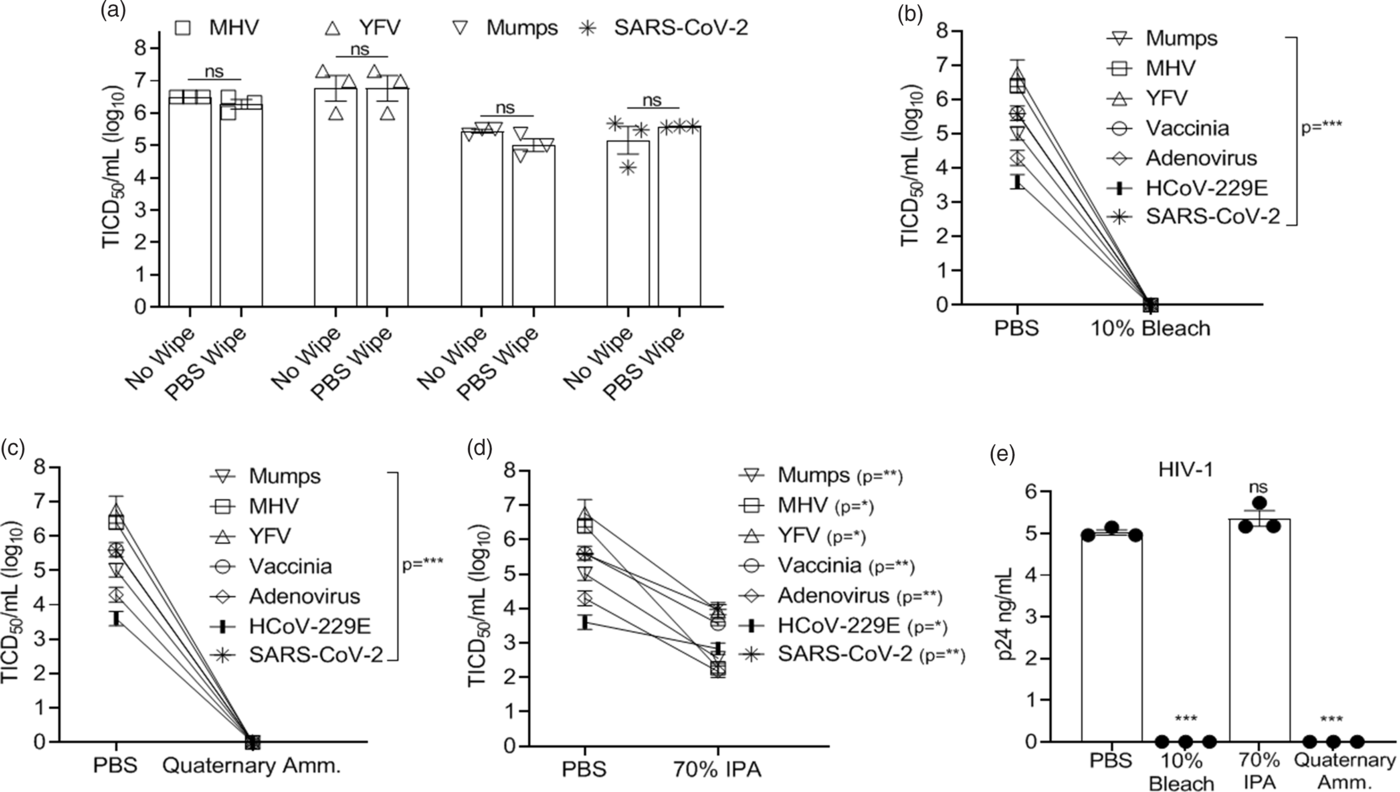
Fig. 1. Virus inactivation (log10) on 3D-printed materials by method and type of chemical disinfection. Recovery of virus from 3D-printed mask material after exposure to a single-wipe application of (A) phosphate-buffered saline (PBS), (B) 10% bleach, (C) ammonium quaternary compounds, and (D) 70% IPA. (E) Recovery of HIV-1. Virus titer was completed as described in the methods in the cell typed indicated in Table 1. Significance was determined using the Student t test. *P < .05; **P < .01; ***P < .001; ****P < .0001; ns, not significant. Error bars represent standard error of the mean (SEM) of 3 independent experiments. Note. TCID50, median tissue culture infectious dose, MHV, mouse hepatitis virus, YFV, yellow fever virus, IPA, isopropyl alcohol. HIV-1, human immunodeficiency virus type 1.
Table 2. Virus Infectivity Reduction (log10) by Treatmenta
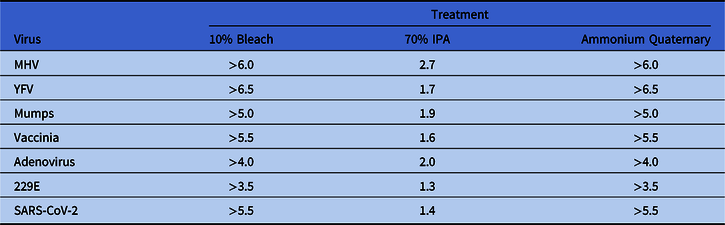
Note. IPA, isopropyl alcohol; MHV, murine hepatitis virus; YFV, yellow fever virus.
a Values represent the log10 reduction in infectivity following a single wipe application of the indicated treatment. Log reduction between viruses varied due to differences in virus inocula.
Vaporized H2O2 treatment of virus-coated disks utilized ionized H2O2 (~3% after application) for 15 minutes of vapor contact time and 15-minute air exchange using the SteraMist Binary Ionization Technology (BIT) at the University of Iowa Hospitals and Clinics Central Sterilizing Services (UIHC) in a 1.90 M L × 1.90 M H × 0.99 M W chamber. Alternatively, virus-coated disks were treated with ionized H2O2 (~3% after application) in a direct contact format at a 61-cm (24-inch) distance with a minimum contact time of 4 seconds. Ionized H2O2 delivery was generated by passing a low-concentration source liquid (7.8% H2O2) through a 17,000V cold plasma arc. Reference Cramer, Plana and Yang37 Both vaporized and direct contact H2O2 completely inactivated all viruses (Fig. 2A–C). Notably, we were not able to test inactivation by ionized H2O2 with SARS-CoV-2 in the BSL3 facility. To assess the effect of H2O2 on SARS CoV-2, we applied 3% H2O2 to the disks with a single wipe as was done with bleach, IPA, and quaternary ammonium compounds. SARS-CoV-2 was completely inactivated by H2O2 (Fig. 2D) using this approach.
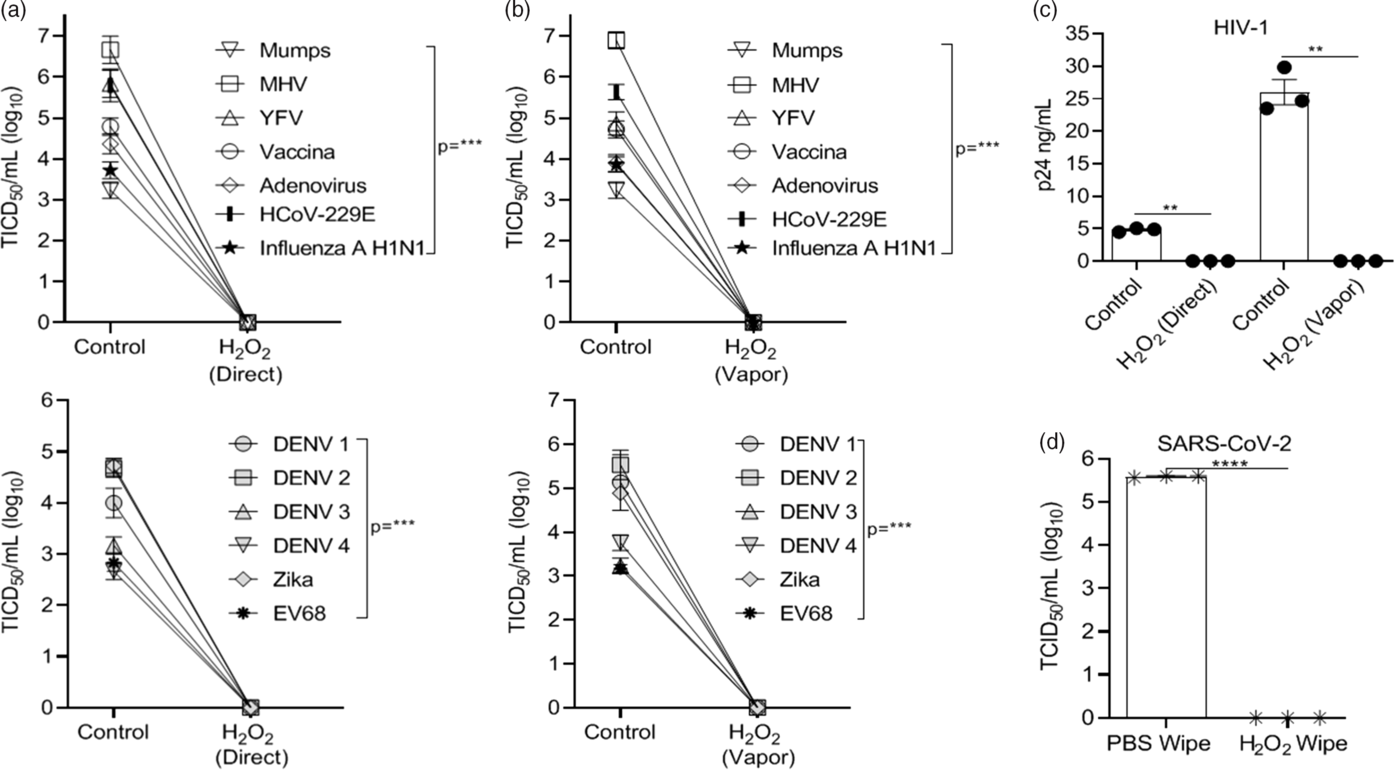
Fig. 2. Hydrogen peroxide (H2O2) inactivation on 3D-print material by application method versus controls. Virus recovery from 3D printed mask material after exposure to ionized H2O2 (3%) in (A) direct contact or (B) vaporized application format. (C) Recovery of HIV-1. Virus applied to 3D material without exposure to H2O2 acted as control. (D) Recovery of SARS-CoV-2 virus from 3D-printed mask material after exposure to a single-wipe application of phosphate-buffered saline (PBS) or H2O2 (3%). Virus titer was completed as described in the Methods section in the cell type indicated in Table 1. Significance was determined using the Student t test. *P < .05; **P< .01; ***P < .001; ****P < .0001; ns, not significant. Error bars represent standard error of the mean (SEM) of triplicate experiments. Note. DENV, dengue virus.
Thermal inactivation
The effect of thermal inactivation on select viruses was examined by incubating the disks at room temperature and measuring virus recovery over time for the murine coronavirus serving as a SARS CoV-2 surrogate (MHV) and other virus controls (YFV). As seen in Figure 3A and B, MHV and YFV both required 6 days before infectivity was completely inactivated in these high-titer virus preparations. We tested select viruses (HCoV-229E, MHV, YFV, and mumps) for inactivation at 50°C and 70°C incubation for 30 minutes. Complete inactivation occurred for all viruses incubated at 70°C. HCoV-229E, MHV, YFV, and mumps viruses incubated at 50°C were not completely inactivated, although infectivity was reduced by >97% (1.6 log) (Fig. 3C–D). We did not have the equipment to allow testing of SARS-CoV-2 in the BSL3 at these temperatures.
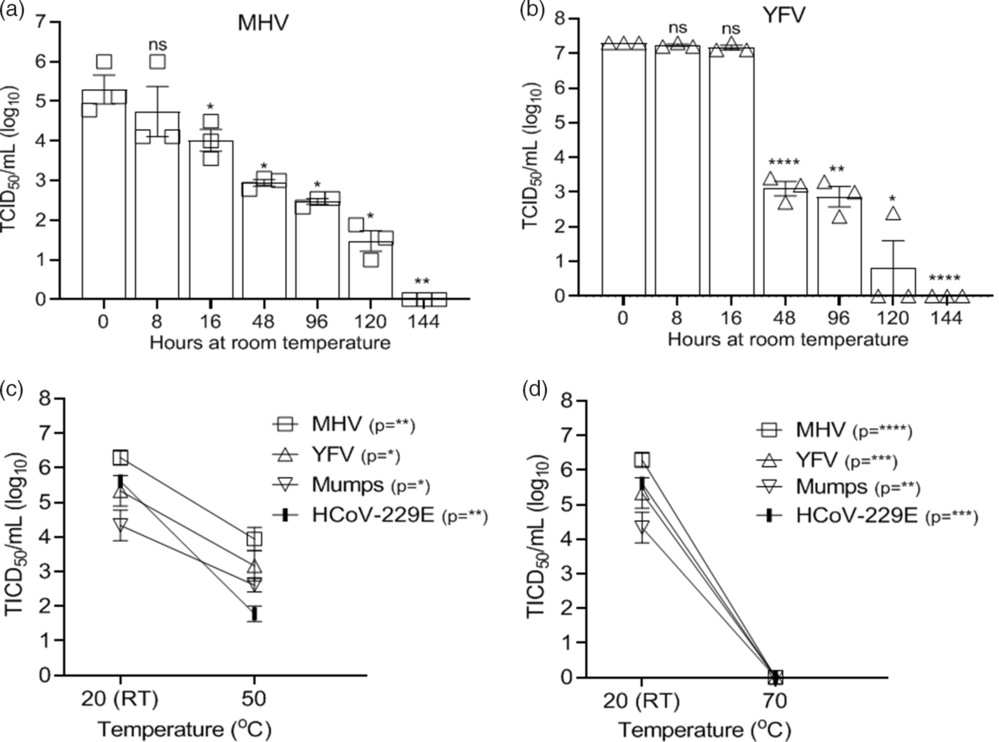
Fig. 3. Effectiveness of virus thermal inactivation. Recovery of virus over time from 3D-printed mask material incubated at room temperature (RT, 20°C) for (A) MHV and (B) YFV. Recovery of virus from 3D material after thermal inactivation for 30 minutes at (C) 50°C or (D) 70°C. Virus incubated at 20°C (RT) acted as the control. Virus titer was completed as described in the Methods section in the cell type indicated in Table 1. Significance was determined using the Student t test. *P < .05; **P< .01; ***P < .001; ****P < .0001; ns, not significant. Error bars represent standard error of the mean (SEM) of triplicate experiments.
Effects of blood and repetitive disinfection on chemical inactivation
Previous studies found that the presence of human blood may interfere with the viricidal activity of disinfectants. Reference Weber, Barbee, Sobsey and Rutala38 The effects of blood on chemical inactivation were studied by adding virus to whole blood (50% final concentration) and testing inactivation as described above. Although disinfectant sensitivity varied between viruses in the presence of blood, viruses remained highly sensitive to bleach and quaternary ammonium (Fig. 4A–B) with infectivity reduced by >93% (>1.2 log). IPA (70%) did not completely inactivate the virus preparations but did reduce infectivity by at least 92% (1.1 log) (Fig. 4C). Notably, for the MHV–blood mixture, one operator had complete inactivation, while a different operator detected a 99.9% (3 log10) reduction in inactivation with bleach application, highlighting the importance of maximizing inactivation.

Fig. 4. Effectiveness of chemical inactivation of viruses (log10) in the presence of blood and after repetitive disinfection of material. Virus recovery from 3D printed mask material after virus addition to whole blood (50% final concentration) and exposure to a single-wipe application of (A) 10% bleach, (B) ammonium quaternary, and (C) 70% IPA. Wipe application of PBS acted as control. Virus recovery from 3D material after the material was exposed to disinfectant 100 times prior to application of virus and a single-wipe application of (D) 10% bleach, (E) quaternary ammonium, and (F) 70% IPA. Virus titer was completed as described in the Methods section in the cell type indicated in Table 1. Significance was determined using the Student t test. *P < .05; **P< .01; ***P < .001; ****P < .0001; ns, not significant. Error bars represent standard error of the mean (SEM) of triplicate experiments.
An important concern for the reuse of PPE is the PA12 material’s stability and resistance to disinfectants. To address the effect of repeat exposure to disinfectants on efficacy of viral inactivation, the PA12 material was exposed to 100 applications of disinfectant before virus was applied and virus inactivation assessed using a single-wipe application as described above. Following repetitive inactivation, there was no grossly apparent loss of integrity, and virus inactivation was reproducible (Fig. 4D–F).
Alternative 3D printing materials
In addition to the PA12 material, we tested the inactivation of select viruses on 3 materials commonly used in 3D printing applications. These included a fused deposition modeling (FDM acrylonitrile butadiene styrene (ABS) material (ABS M-30 from Stratasys, Rehovot, Israel), an FDM polylactic acid (PLA) material (PLA from Stratasys, Rehovot, Israel), and a stereolithography acrylic (SLA) material (Surgical Guide from Formlabs, Somerville, MA). These additional materials were selected because the FDM and SLA 3D-printing technology platforms are very frequently utilized, and ABS and PLA are the 2 most common materials for FDM. Further, the surgical guide (SG) material is a very popular SLA material for additional medical applications. Although these 3 materials and PA12 represent the most commonly used materials, other 3D printing materials have been utilized in the healthcare setting. Application of select viruses to these materials found that SLA was more impermeable, requiring longer time intervals for virus drying (>4 hours), while PLA was permeable to the applied virus preparations. In contrast, ABS was similar to the PA12 material studied above. Virus inactivation by chemical disinfection was identical to that observed using the PA12 material (Fig. 5A–C). However, the permeability of PLA to the virus preparation would preclude use in PPE manufacture.
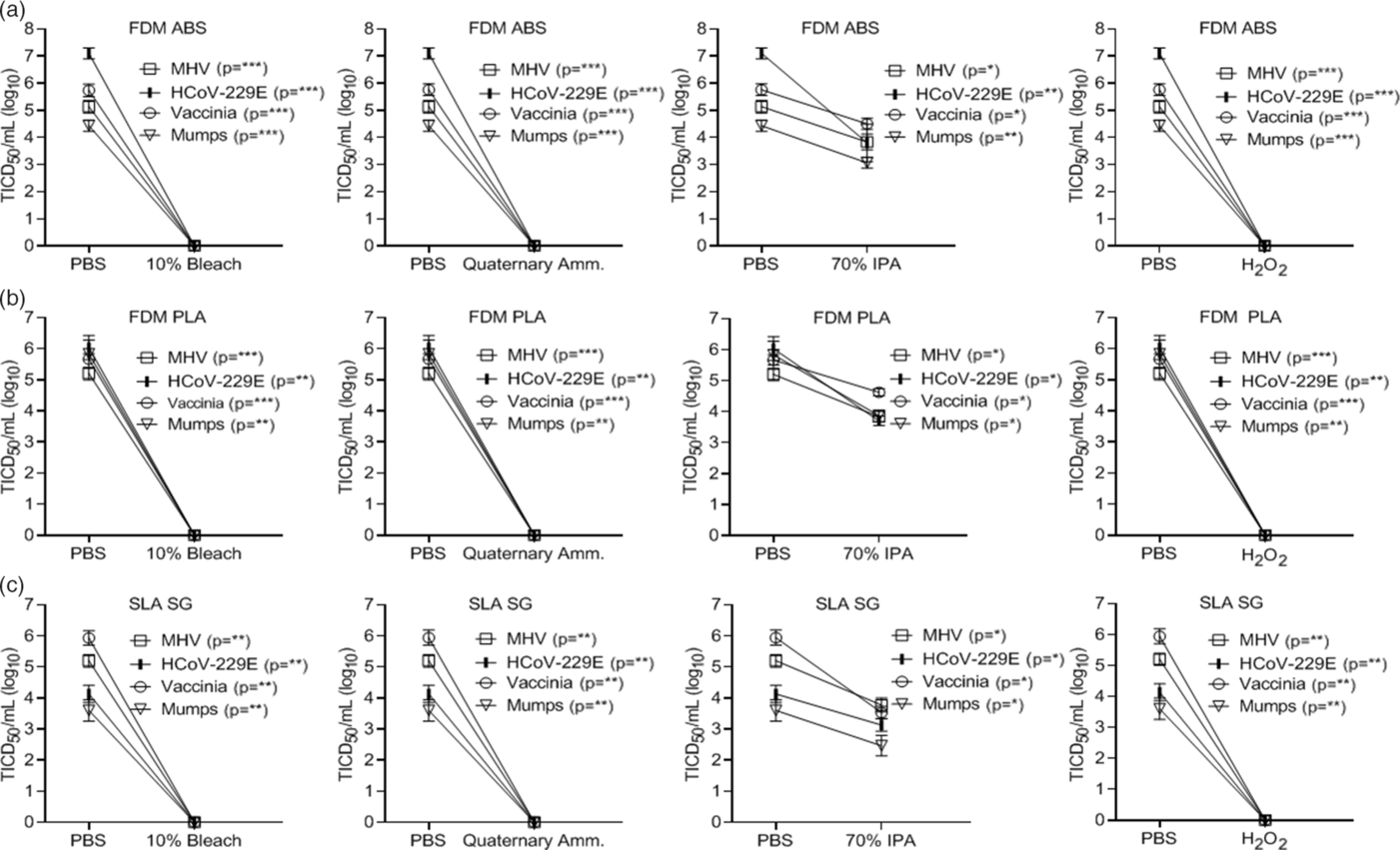
Fig. 5. Chemical inactivation of viruses (log10) on alternative 3D-printed mask materials by type of disinfectant versus controls. Virus recovery from alternative 3D-printed mask material (A) FDM acrylonitrile butadiene styrene (FDM ABS), (B) FDM polylactic acid (PLA), and (C) stereolithography acrylic-surgical guide (SLA SG) after exposure to a single-wipe application of 10% bleach, ammonium quaternary, 70% IPA, and 3% H2O2. Wipe application of PBS acted as the control. Virus titer was completed as described in the Methods section in the cell type indicated in Table 1. Significance was determined using the Student t test. *P < .05; **P< .01; ***P < .001; ****P < .0001; ns, not significant. Error bars represent standard error of the mean (SEM) of triplicate experiments.
Discussion
The shortage of PPE during the COVID-19 pandemic created a critical need for the development of alternative, reusable equipment. However, decontamination guidelines for reusable equipment have not reached consensus and are often limited by incomplete testing against surrogate pathogens. To address the shortage of respiratory PPE, the VHA developed a 3D-printed surgical mask that may be disinfected and reused. 8 In this study, we evaluated practical chemical and thermal decontamination methods for the ability to inactivate SARS-CoV-2, the causative agent of the COVID-19 pandemic, 2 surrogate coronaviruses (MHV and 229E), and a diverse set of clinically relevant human viral pathogens with different viral properties that may influence inactivation.
SARS-CoV-2 and all other viruses tested were completely inactivated by a single application of 10% bleach, ammonium quaternary, and 3% H2O2 formulations. Also, 70°C dry heat completely inactivated viruses including the SARS-CoV-2 surrogate coronaviruses used (MHV and HCoV-229E); however, this decontamination method was not tested against SARS-CoV-2. Furthermore, 70% IPA and 50°C dry heat did not completely inactivate the viruses included in this study, although viral titers were reduced by >90% (>1 log) with these methods. Notably, in these studies, a single-wipe application of 70% IPA did not decontaminate surfaces completely, and a more stringent application of 70% IPA may be required for complete virus inactivation. To address the concern that blood may alter the efficacy of inactivation, we showed that virus present in 50% whole blood was reduced by >93% when treated with a single application of 10% bleach and ammonium quaternary. These studies illustrate the potential for slight operator differences in inactivation, which highlights this potential importance when suboptimal virus inactivation chemicals (IPA) are utilized.
Ionized disinfecting mist behaves like a gas, and in our studies it was applied to environmental surfaces using 2 types of delivery devices (TOMI Environmental Solutions, trade name SteraMist). This technology is EPA-registered as a hospital disinfectant under the name “Binary Ionization Technology (BIT) Solution,” and appears on EPA lists K, L, G and M. This approach was used because the antimicrobial effect is rapid (15 minutes), there is little damage to materials and surfaces, the H2O2 dissipates into water vapor and oxygen after application leaving no toxic residue or odor, and the proprietary delivery system is portable, making the adoption of this technology useful. Reference Cramer, Plana and Yang37 This approach to disinfection was also completely effective (Fig. 2A–C).
In summary, several decontamination approaches (10% bleach, ammonium quaternary, 3% H2O2, and 70°C dry heat) were effective on 3D-printed surgical mask materials. Some approaches were effective for inactivation of the SARS-CoV-2 virus, while others were also effective against its surrogates and other clinically relevant viral pathogens. These results are consistent with previous viral inactivation studies, although those studies included variations in decontamination procedures and did not incorporate virus application onto a 3D-printed material. Reference Kampf, Todt, Pfaender and Steinmann27,Reference Dembinski, Hungnes, Hauge, Kristoffersen, Haneberg and Mjaaland39,Reference Zou, Guo and Gao40 The decontamination of 3D-printed surgical masks may be useful during crisis situations in which the supply of surgical masks is limited. Our results may be used to further study decontamination strategies for 3D-printed materials used in clinical settings.
Acknowledgments
We thank Alisha Loy (University of Iowa Hospitals and Clinics Central Sterilizing Services) and Michael J. Hartley (University of Iowa Hospitals and Clinics Hospital Administration) for assistance with the H2O2 inactivation experiments. We also thank Dr James McLinden and Ms Qing Chang, and Dr Catherine Loc-Carillo (Salt Lake City VA and the University of Utah) for helpful discussions and critical review of the manuscript.
Financial support
This work was supported by service-directed grants from the Veterans’ Affairs (Biomedical Laboratory Research and Development Service grants to JX and JTS), a VA Merit Review Grant (no. BX 000207JTS), and the National Institute of Allergy and Infectious Diseases (grant nos. NIH 5T32AI343 JTS, NIH PO1 AI060699, SP). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication. We thank the NIH AIDS Reagent Program for reagents.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.










