Consumers are generally aware of the health effects of consuming oranges as a rich source of vitamin C. A wide variety of orange derivatives are currently available in several countries as well as on the European market, representing a convenient alternative to the fresh fruit. Among them, freshly squeezed and pasteurised orange juice (containing 100 % orange juice) is the most consumed in Italy (Molfino, Reference Molfino2004). The nutritional profile of this product is similar to that of the fresh fruit (Di Mauro et al. Reference Di Mauro, Maccarone and Marchese2001). These products contain high amounts of vitamin C and lower amounts of other phytochemicals such as carotenoids (namely β-cryptoxanthin, lutein, zeaxanthin and β-carotene) and polyphenols (hesperidin, naringenin, cyanidin-3-glucoside, mainly in blood oranges).
The beneficial properties of oranges and their derivatives have been widely investigated. In particular, the inhibition of breast cancer cell proliferation (So et al. Reference So, Guthrie, Chambers, Moussa and Carroll1996), decrease of colon tumorigenesis (Miyagi et al. Reference Miyagi, Om, Chee and Bennink2000) and antimutagenic properties (Franke et al. Reference Franke, Prá, Giulian, Dias, Yoneama, da Silva, Erdtmann and Henriques2006) have been evidenced in cell-culture and animal models. Moreover, in vivo studies performed on human subjects support a role for orange juice in the protection against oxidative damage to DNA (Franke et al. Reference Franke, Cooney, Henning and Custer2005; Riso et al. Reference Riso, Visioli, Gardana, Grande, Brusamolino, Galvano and Porrini2005).
Among the compounds present in orange juice, vitamin C seems to be the most effective antioxidant, as demonstrated by several in vitro studies (Szeto et al. Reference Szeto, Tomlinson and Benzie2002; Proteggente et al. Reference Proteggente, Pannala, Paganga, Buren, Wagner, Wiseman, Van De Pui, Dacombe and Rice-Evans2002).
Many in vivo studies have investigated the role of vitamin C in the protection against oxidative-induced DNA damage: single-dose trials showed significant antioxidant protection (Green et al. Reference Green, Lowe, Waugh, Alridge, Cole and Arlett1994; Panayiotidis & Collins, Reference Panayiotidis and Collins1997), whereas long-term studies revealed mixed outcomes (Møller & Loft, Reference Møller and Loft2002; Duarte & Lunec, Reference Duarte and Lunec2005).
These discrepancies could be explained by differences in vitamin C absorption (many factors could be involved; for example, source, amount, characteristics of subjects, etc). In particular, high fluctuations in plasma ascorbic acid may be less beneficial in the protection against DNA damage, as recently reported (Møller et al. Reference Møller, Viscovich, Lykkesfeldt, Loft, Jensen and Poulsen2004).
As regards studies evaluating the effect of orange juice intake on DNA damage, results seem to support a protective effect (Franke et al. Reference Franke, Cooney, Henning and Custer2005, Riso et al. Reference Riso, Visioli, Gardana, Grande, Brusamolino, Galvano and Porrini2005). We recently found an inverse correlation between plasma vitamin C concentration and H2O2-induced DNA damage following regular consumption of blood orange juice (BOJ) for 21 d, suggesting a possible protective role for vitamin C (Riso et al. Reference Riso, Visioli, Gardana, Grande, Brusamolino, Galvano and Porrini2005). This increased protection may be due to vitamin C, even if other compounds could be involved.
The aim of the present study was to further extend our understanding of the effect of the intake of one small portion of blood orange juice or vitamin C dissolved in water on mononuclear blood cell (MNBC) resistance to DNA damage.
Subjects and methods
Subjects and study design
Seven healthy, non-smoking female volunteers (age 26·0 (sd 2·1) years, BMI 20·1 (sd 1·4) kg/m2) were enrolled on the basis of a questionnaire concerning their food habits. Vegetarians and anyone taking medication or supplements were excluded from the study.
Subjects were divided into a randomised repeated-measures design in which they had to consume different drinks consisting of BOJ, vitamin C added to water (C-drink) or sugars added to water (S-drink). Single portions (300 ml) of the drinks under study were supplied in the morning to fasting volunteers on three different occasions, 2 weeks apart.
Finger-prick blood samples were drawn from fasting volunteers into heparinised microtubes. After the intake of each drink, blood samples were collected every hour for 8 h and at 24 h. Vitamin C was analysed in every sample taken, while H2O2-induced MNBC DNA damage was determined at 0 h, 3 h (peak of vitamin C in plasma) and 24 h. A standardised meal consisting of antioxidant-controlled foods (bread with ham, cheese, milk ice-cream and decaffeinated coffee with sugar) was provided 4 h after the intake of the drink.
The present study was approved by the local ethics committee. All subjects gave their written informed consent before participation.
Characteristics and analysis of the drinks
The commercial BOJ used (freshly squeezed and pasteurised, containing 100 % orange juice; Fattoria Scaldasole, Italy) is produced by squeezing, immediate freezing and light pasteurising before aseptic packaging and storage at 4°C. A 100 ml portion of juice contained 50·5 ± 0·3 mg vitamin C, 68·6 (sd 1·9) mg hesperidin, 8·2 (sd 0·6) mg narirutin (C Gardana, S Guarnieri, P Riso, P Simonetti, M Porrini, unpublished results) and 12·7 (sd 0·2) g sugars (fructose, glucose and sucrose in the ratio 1:1:2).
Vitamin C was determined by HPLC: the chromatographic system consisted of a model 510 system pump (Waters Corp., Milford, MA, USA), a 5 μm Atlantis C18 column (250 × 4·6 mm internal diameter; Waters, Dublin, Republic of Ireland) and detection was achieved at 245 nm (UV-Vis detector Varian 9050; Varian Inc., Palo Alto, CA, USA). Samples were eluted (1·4 ml/min) with a mobile phase of 0·1 % formic acid. The sample injection volume was 50 μl. Chromatographic data were acquired by a Millennium 4·0 Workstation (Waters Corp). Vitamin C standards (Sigma Chemicals, St Louis, MO, USA) were prepared in 10 % metaphosphoric acid buffer in the range of 5–20 μg/ml.
Flavanone concentrations in BOJ were determined by LC-MS/MS by means of a chromatographic system consisting of an Alliance model 2695 equipped with a model 2996 (Waters Corp) photodiode array detector and a Quattro Micro triple quadrupole mass spectrometer (Micromass, Beverly, MA, USA).
Carbohydrate concentrations were determined by LC-MS. The HPLC system was an Alliance (Waters) coupled to a Quattro Micro triple quadrupole mass spectrometer (Micromass) equipped with an atmospheric pressure chemical ionisation source operating in negative mode.
Vitamin C powder was supplied by Phoenix Srl (Peschiera Borromeo, Italy). Its purity was checked by HPLC (about 99 %) and used to calculate the amount of powder to add to water in order to have a drink with the same amount of vitamin C as in the juice (the C-drink). The final vitamin C concentration in the solution was also measured. The C-drink was prepared just after the volunteers arrived at the laboratory. It was immediately covered and served.
Both the amount of BOJ and C-drink supplied to volunteers (300 ml) provided 150 mg vitamin C; BOJ and the S-drink provided 38 g sugars.
Determination of vitamin C in plasma
The extraction was performed in duplicate on 100 μl plasma (fresh sample) to which 100 μl 10 % metaphosphoric acid solution was added. After vortexing and centrifuging for 1 min, 100 μl of the supernatant fraction were immediately injected for HPLC analysis (conditions have been previously described). Vitamin C standards were prepared daily for plasma analysis.
Evaluation of mononuclear blood cell DNA resistance to oxidative damage
In order to verify the hypothesis that a transient increase in plasma antioxidant compounds following the test drinks might improve cell protection, we evaluated cell ability to counteract an exogenous oxidative insult able to cause consistent DNA damage, as already applied in previous studies (Riso et al. Reference Riso, Visioli, Erba, Testolin and Porrini2004; Porrini et al. Reference Porrini, Riso, Brusamolino, Berti, Guarnieri and Visioli2005).
The resistance of MNBC DNA against oxidative stress (strand breaks after treatment with H2O2 (500 μmol/l) for 5 min) was evaluated by means of the Comet assay as previously reported in detail (Riso et al. Reference Riso, Pinder, Santangelo and Porrini1999). One hundred cells for each slide were electronically captured (400 × magnification) using an epifluorescence microscope (BX 60 OLYMPUS) equipped with an excitation filter BP520-550, dichroic beam-splitter DM565 and BA580-IF barrier filter (OLYMPUS, Olympus Italia S.R.L., Milan, Italy). The light source was a 100 W Hg lamp (Olympus Italia S.R.L.). The microscope was attached to a high-sensitivity charged coupled device (CCD) video camera and to a computer provided with an image analysis system (Comet Program exploited on Image Pro-Plus; Immagini e Computer, Bareggio, Milan, Italy) and set to calculate the levels of strand breaks, as percentage DNA in tail.
For each subject, the average percentage DNA in tail of control cells (cells that were not treated with H2O2) was subtracted from the average percentage DNA in tail of treated cells.
Statistical analysis
Statistical analysis was performed with STATISTICA software (Statsoft Inc., Tulsa, OK, USA). A two-way ANOVA for repeated measures design was employed to compare plasma vitamin C concentration after BOJ, C-drink or S-drink intake and to evaluate the effect of each drink on DNA damage registered at 3 and 24 h. Differences between means were determined by Tukey's test (α = 0·05).
Results
Plasma vitamin C concentrations
Plasma vitamin C concentrations measured from baseline to 24 h after the consumption of each drink are represented in Table 1. Vitamin C concentrations at baseline were not significantly different in the three treatments. They increased significantly 1 h after both BOJ and C-drink consumption (P < 0·001) and reached a peak at 2–3 h (average increase of 26·5 μmol/l for BOJ and 33·3 μmol/l for the C-drink, corresponding to 34 and 45 % respectively). The increases in vitamin C concentration registered at 2 and 3 h were not significantly different after the two drinks containing vitamin C. Then, the vitamin concentration significantly decreased after 4 h (BOJ; P < 0·05) and 6 h (C-drink; P < 0·005). On the whole, ANOVA did not show any significant difference between plasma vitamin C responses following BOJ or C-drink consumption. In addition, area under the curve was used to evaluate the response rate of plasma ascorbic acid after BOJ or C-drink intake for each subject. No significant difference was registered between mean values (724 (sd 121) μmol/l × h for BOJ and 772 (sd 102) μmol/l × h for the C-drink).
Table 1 Plasma vitamin C concentration from baseline to 24 h after the intake of blood orange juice (BOJ), vitamin C dissolved in water (C-drink) and sugars in water (S-drink)
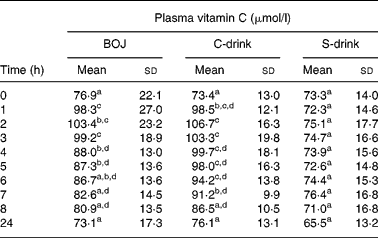
a,b,c,d Mean values within a column with unlike superscript letters were significantly different (P < 0·05).
At 24 h vitamin C was comparable and not different from the baseline values in each trial. Similar trends were observed in a previous study carried out in our laboratory (amount of vitamin C administered was 564 mg).
S-drink intake did not affect plasma vitamin C concentration.
Mononuclear blood cell DNA resistance to oxidative damage
Table 2 outlines the results of DNA strand breaks registered at each time point (0, 3 and 24 h) within each treatment (BOJ, C-drink and S-drink). Values refer to untreated cells (controls), H2O2-treated cells and subtracted values (treated minus untreated cells).
Table 2 Strand breaks in mononuclear blood cells, evaluated by the comet assay, at baseline, and at 3 and 24 h after the intake of blood orange juice (BOJ), vitamin C dissolved in water (C-drink) and sugars in water (S-drink)* (Mean values and standard deviations)
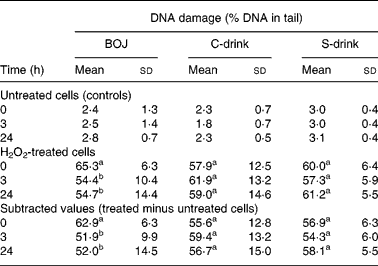
a,b Mean values within a column with unlike superscript letters were significantly different (P < 0·05).
* Oxidative treatment was performed by means of H2O2 (500 μmol/l) for 5 min. Average values of untreated cells (controls), H2O2-treated cells and subtracted values (treated minus untreated cells) are outlined.
Strand breaks in controls before each treatment were not significantly different and were comparable after drink intake.
Two-way ANOVA performed on subtracted values showed a significant effect of the interaction between treatment (BOJ, C-drink or S-drink) and time (0, 3 and 24 h) (P < 0·005). A significant increase in resistance to DNA damage was registered 3 h after BOJ intake (about 18 %; P < 0·01). This trend was found in all the subjects and the protection observed remained nearly constant at 24 h (with a decrease of about 16 %; P < 0·01). In contrast, C-drink or S-drink consumption did not affect DNA damage.
ANOVA was also performed on single-strand breaks after H2O2 treatment (without subtracting controls), confirming the results previously reported.
Correlations
Baseline vitamin C concentrations were not correlated to the maximum increase registered for each subject, suggesting that plasma vitamin C increases were independent from baseline values. Also the reduction of H2O2-induced DNA damage (at 3 h) was independent of initial levels of oxidative-induced DNA damage.
Moreover, despite an inverse correlation observed between plasma vitamin C and DNA damage 3 h after orange juice intake, it was not confirmed at 24 h.
Discussion
The results of the present study provide further evidence that whole foods can increase cell resistance to oxidative stress better than single compounds, and that BOJ is a source of bioavailable vitamin C.
In fact, we have shown a decrease in H2O2-induced oxidative DNA damage in MNBC 3 h after the consumption of a moderate amount of BOJ and this effect was maintained until 24 h. The protection observed at 3 h corresponded to an increase in plasma vitamin C concentration, but at 24 h vitamin C returned to its baseline value, suggesting that different compounds were also involved. This observation is supported by the fact that the same amount of vitamin C, provided as a supplement, did not affect cell DNA damage, despite the similar increase in plasma vitamin C.
Other authors have reported positive effects of single doses of vitamin C on DNA damage in healthy volunteers, with the highest DNA protection within 4 h after ingestion. However, the amount of vitamin C used in these studies was higher than in the present study: 35 mg (Green et al. Reference Green, Lowe, Waugh, Alridge, Cole and Arlett1994) and 1 g (Panayiotidis & Collins, Reference Panayiotidis and Collins1997). Moreover, the intervention with vitamin C for weeks or months provided conflicting results regarding the modulation of DNA damage in human subjects, as recently reviewed by Møller & Loft (Reference Møller and Loft2002). In agreement with these authors, we think that, beyond metabolic and genetic individual differences, one possible explanation for the discrepancies among different studies could be related to the initial plasma vitamin C levels in different groups of volunteers.
In fact, it has been suggested that subjects with basal levels higher than 60 μmol/l could not benefit from vitamin C supplementation (Prieme et al. Reference Prieme, Loft, Nyyssönen, Salonen and Poulsen1997; Porkkala-Sarataho et al. Reference Porkkala-Sarataho, Salonen, Nyyssönen, Kaikkonen, Salonen, Ristonmaa, Diczfalusy, Brigelius-Flohe, Loft and Poulsen2000). In contrast, vitamin C supplementation seems to be effective in patients with conditions associated with oxidative stress and low plasma vitamin C levels (Duarte & Lunec, Reference Duarte and Lunec2005). In the present study, we used healthy non-smoker subjects with high baseline levels of plasma vitamin C (average of about 77 μmol/l), and this could explain the lack of effect observed when one single dose of supplement was provided. Another possible explanation could be related to the intracellular vitamin C saturation that has been reported to occur before plasma saturation (Duarte & Lunec, Reference Duarte and Lunec2005). To clarify this fact, it might have been useful to determine vitamin C concentration in MNBC.
Besides the previous observations, the interesting finding coming from the present study is that our healthy subjects benefited from the consumption of a small single portion of BOJ. In fact, despite the small number of subjects enrolled, all of them showed an increase in resistance to DNA damage.
This suggests that several compounds (for example, cyanidin-3-glucoside, flavanones, carotenoids, etc) other than vitamin C are bioavailable from BOJ (Riso et al. Reference Riso, Visioli, Gardana, Grande, Brusamolino, Galvano and Porrini2005; Franke et al. Reference Franke, Prá, Giulian, Dias, Yoneama, da Silva, Erdtmann and Henriques2006) and could be involved in the protective effect observed. These compounds could act in synergy or sequentially, depending on their absorption. This could help in explaining the correlation between DNA damage and vitamin C concentration 3 h after BOJ intake but the lack of correlation at 24 h.
We also evaluated whether sugars present in the juice could affect DNA damage; some authors found that fructose could be responsible or take part in the antioxidant effect of some fruit, causing an increase in plasma urate (Lotito & Frei, Reference Lotito and Frei2004). The present study does not support a role for fructose, glucose and sucrose on cell antioxidant protection, at least against DNA damage.
The present results are in agreement with those previously reported by Collins et al. (Reference Collins, Horská, Hotten, Riddoch and Collins2001) who found a reduction of single strand breaks at 3 h, which persisted after 8 and 24 h from the intake of a single portion of kiwi-fruit juice (providing about 415 mg vitamin C). In addition to this, the authors, by using an in vitro test, showed that an extract of kiwi fruit was more effective than a solution of vitamin C (with the same concentration), in protecting DNA from oxidative damage.
In conclusion, we reported, in a small number of subjects, that the protective effect of the intake of a small portion of BOJ is not accounted for by vitamin C alone as no effect of the vitamin supplementation was observed. In addition, the maintenance of DNA protection for 24 h observed after BOJ intake supports the importance of the regular consumption of fruit and vegetables, rich in protective compounds, in order to limit cell susceptibility to oxidative damage.
Acknowledgements
We gratefully acknowledge Phoenix Srl (Peschiera Borromeo, Milan, Italy) for supplying the vitamin C supplement and Dr Claudio Gardana for the HPLC analysis of sugars and flavanones.




