Introduction
Cranioplasty is a common need for neurosurgical patients. Those who undergo craniectomy for a variety of indications may require reconstruction for a cranial defect as a result. In up to 20%–50% of cases, the patient’s autologous bone cannot be replaced due to infection, fragmentation, bone resorption, or other causes. Reference Broughton, Pobereskin and Whitfield1,Reference Andrabi, Sarmast, Kirmani and Bhat2 In these cases, a synthetic bone flap is constructed to repair the defect. Unfortunately, large defects or those involving bony areas with complex contours are difficult to reconstruct with a “free-hand” technique during surgery. This necessitates the use of advanced manufacturing methods to create patient-specific implants.
While patient-specific implants are available through some medical device companies, cost and production time are major limitations that impede their wider adoption. Individual implants often cost more than CA$10,000, so neurosurgeons must be judicious in choosing which patients will benefit most from these implants. Reference Binhammer, Jakubowski, Antonyshyn and Binhammer3 Production time often takes weeks, making utilization of commercial solutions limited in cases of trauma, or when urgent reconstruction is required. In light of these limitations, surgeons commonly opt for ‘free-hand’ reconstruction of cranial defects, either utilizing polymethyl methacrylate (PMMA), titanium mesh, or a combination of the two. Results with this method are less predictable, commonly leading to suboptimal restoration of cranial vault contour and overall cosmesis. Reference Oliver, Banuelos, Abu-Ghname, Vyas and Sharaf4
As 3D printing technology improves, there has been a dramatic increase in the availability of inexpensive desktop 3D printers. As a result, this technology is becoming increasingly used in hospital settings for the purpose of teaching and surgical planning. Reference Pucci, Christophe, Sisti and Connolly5,Reference Dong, Chen and Li6 There exists a small number of previous reports in the literature of successful cases utilizing desktop 3D printers for cranial reconstruction; the majority of these cases originated from countries with limited healthcare resources. Reference De La Peña, De La Peña-Brambila, Pérez-De La Torre, Ochoa and Gallardo7,Reference Abdel Hay, Smayra and Moussa8
One of the major barriers to the adoption of these techniques is the stringent regulatory oversight at most Canadian neurosurgical centers. Lal et al. described a technique used in an Indian hospital of 3D printing an open-air mold and then using wax pour-up as intermediate step before converting it to PMMA using lost-wax and compression molding. Reference Lal, Ghosh, Agarwal, Gupta and Roychoudhury9 De La Peña et al. described a technique used in a Mexican hospital of directly 3D printing the cranioplasty in polylactic acid (non-implantable PLA), then creating a plaster mold around it and pouring PMMA into the plaster mold for final implant. Reference De La Peña, De La Peña-Brambila, Pérez-De La Torre, Ochoa and Gallardo7 Both techniques require custom sterilization of the final implant prior to surgery. In Canada, this would require an externally validated sterilization protocol. Given the degree of customization, this would increase cost and complexity, making these techniques challenging to adopt.
To overcome this, a few authors have reported their experience with 3D printing a mold instead and shaping FDA-approved implantable PMMA over the mold to create the cranioplasty. Reference Dong, Chen and Li6,Reference De La Peña, De La Peña-Brambila, Pérez-De La Torre, Ochoa and Gallardo7 For example, Abdel Hay et al. presented 2 cases out of Lebanon of using patients’ CT scans to generate a convex surface which was used to fill the defect. Reference Abdel Hay, Smayra and Moussa8 An ‘open-air’ mold was then generated and 3D printed in PLA. The mold was placed into a sterile plastic bag, and the final implant was contoured over the mold. Building on some of these techniques, we have made further refinements to make the process easier and more efficient. To our knowledge, this is the first Canadian study with the aim to report experience with a novel, cost-effective method for cranioplasty utilizing 3D printed molds that provides similar outcomes to commercially available patient-specific implants.
Methods
A retrospective review was performed at a single Canadian center between 2018 and 2020. Selected patients had undergone craniectomy for a variety of indications (trauma, infection, intraosseous neoplastic invasion), whereby autologous bone flap replacement was not possible (initial surgery performed out of country, infected bone flap, neoplastic invasion necessitating resection of intraosseous component). Patients underwent cranioplasty using 3D printed molds, either a two-piece self-align or open-air mold. Post-operatively, clinical and radiologic data were reviewed for complications. Cosmetic outcome was measured on a five-point ordinance scale (‘poor’, ‘fair’, ‘good’, ‘very good’, ‘excellent’) to grade satisfaction with cosmesis. Costs associated with each case were estimated from the hospital case–cost database per purchasing agreement with medical device vendors at our institution.
Image Processing
Image processing was performed on an iMac running the latest iOS operating system (Apple, CA, USA). Patient’s CT head was imported in DICOM format into OsiriX MD (OsiriXTM, Switzerland). Alternatively, Horos is a free version of the software utilizing the original open-source OsiriX code (Horos Project). CT head with 2 mm or thinner cuts is preferred for smoother surface model creation. All image processing was performed on a hospital-approved workstation in compliance with network security and health information regulations.
For patients with paired pre- and post-operative scans from their previous surgery, a two surface model was generated of the bony anatomy (typically using 300HU cut-off) and exported as de-identified STL files. Models were imported to MeshMixer (free to use, AutoDesk, California, USA) for further processing. In patients with only a post-operative scan, the contralateral (normal) side was mirrored and used as a template. Figure 1 shows a detailed illustration of the DICOM/MeshMixer workflow and intraoperative photos.

Figure 1: DICOM/MeshMixer Workflow and Intraoperative Photos. (A) STL model of the cranial defect. (B) The baseline model is lined up with the defect model. (C) A Boolean-subtract function used to obtain the difference between the 2 models and create a virtual cranioplasty. (D) Mold generation by extruding the outer and inner surfaces, this illustrates extrusion of the inner surface followed by a ‘plane cut’ function to obtain the bottom mold. (E) To avoid an overly thick cranioplasty, the outer surface is extracted and a surface-normal extrusion performed to obtain a uniform thickness (e.g. 5 mm). (F) and (G): Final mold creation with insertion of alignment posts. (H) and (I): Intraoperative photos of the casting process. (J): The cranioplasty continues to polymerize on the sterile table to prevent overheating the plastic.
Mold Creation
For single piece ‘open-air’ mold, the defect and the baseline models were lined up visually in a MeshMixer. The baseline model was then recessed by ˜5mm to create the desired thickness of cranioplasty. Our preferred method, however, is the two-piece mold with alignment posts to facility self-centering.
In MeshMixer, once the models were aligned, the defect model was thickened by 2 mm. This allows clearance for soft tissue around the cranioplasty and prevents the model from sitting proud. A Boolean-subtraction function was used, and this resulted in a precise model of the ‘virtual’ bone flap (Figure 1A-C). The top and bottom surfaces can be extruded separately at this point to generate the two-piece mold (Figure 1D). However, our experience has shown that this often results in an overly thick cranioplasty that will require extra drilling and/or soft tissue resection for proper fit. Instead, we recommend extracting the outer surface only and use the surface-normal extrusion function to create a 5 mm uniform-thickness cranioplasty (Figure 1E). The molds are then generated based on this model with the addition of alignment posts (Figure 1F,G).
Once the STL files of the molds are created, they are sent to a slicer software to generate the final G-code. Several open-source slicer programs are available; we used Ultimaker Cura with standard print parameters (freeware under the GNU license). This is then sent to a desktop 3D printer. We initially used a Makerbot Replicator 2X (US$3000) but have found the delta printer (Kossel series and their open-source derivatives ˜CA$500) to have a better build envelope and print speed. Molds are printed in PLA filament with a build plate temperature of 60°C and a filament temperature of 210°C. Print times varied between 4 and 12 h depending size of cranioplasty.
Cranioplasty Insertion
On the day of OR, the molds are pre-treated with alcohol and chlorhexidine solution and then placed in sterile plastic bags. C-arm covers were used for optimal thickness and ease of handling. The PMMA is poured into the concave mold, and the top piece is pressed into place (Figure 1H,I). Once the mold edges line up and alignment posts are in good contact, the PMMA is allowed to cure. The exothermic reaction can generate a significant amount of heat. PMMA pre-treatment with chlorhexidine solution acts as both added prevention against contamination and partial cooling. Fogging of the inside of the plastic bags serves as a visual indicator of polymerization. The PMMA is removed shortly after in a partially cured state, and it is transferred out of the mold and allowed to fully cure on the sterile OR table (Figure 1J).
Although no formal sterilization process is undertaken prior implantation, the PMMA does not come in contact with any unsterile surface throughout. The fully cured cranioplasty is then implanted into the patient. Mini-plates and screws (Stryker cranial fixation system, Stryker, MI USA) were used to secure implants to the skull in all cases. The exact number of screws and type of plates varied depending on the case, at the surgeon’s discretion. In cases with temporalis involvement, suspension of the muscle was attempted where possible via suturing to the mini-plates or pre-drilled holes through the PMMA implant. In cases where intraosseous neoplastic invasion necessitated craniectomy, cranioplasty was performed at time of resection.
All patients received 24-hour course of post-operative antibiotics (typically cefazolin, otherwise Vancomycin if the patient has penicillin allergy). A subgaleal drain was placed prior to scalp closure, and all drains were removed on post-operative day one. Use of plastic bag can create some wrinkles on the surface of the cranioplasty but did not appear to impact its structural integrity (Figure 1J).
Results
Between 2018 and 2020, seven patients at the Hamilton General Hospital underwent synthetic cranioplasty for a variety of indications (mean age 42.3, SD 16.2). Please see Table 1 for detailed summary of patient demographics, indications, outcomes, and complications. All patients had large cranial defects (6 out of 7 patients had defect >10cm) that would make traditional free-hand reconstruction challenging. The most common type of prior procedure was a decompressive craniectomy (57.1%), followed by boney invasion from intraosseous neoplasm (28.6%). The most common reason for synthetic cranioplasty was infection. Two patients required reconstruction at time of resection of their intraosseous meningioma. One patient presented with bone flap resorption.
Table 1: Summary of results of the seven patients who underwent cranioplasty with the aid of cost-effective 3D printing technique at the Hamilton general hospital. Baseline demographics are presented here as well as side and size of defect, method of reconstruction and complications
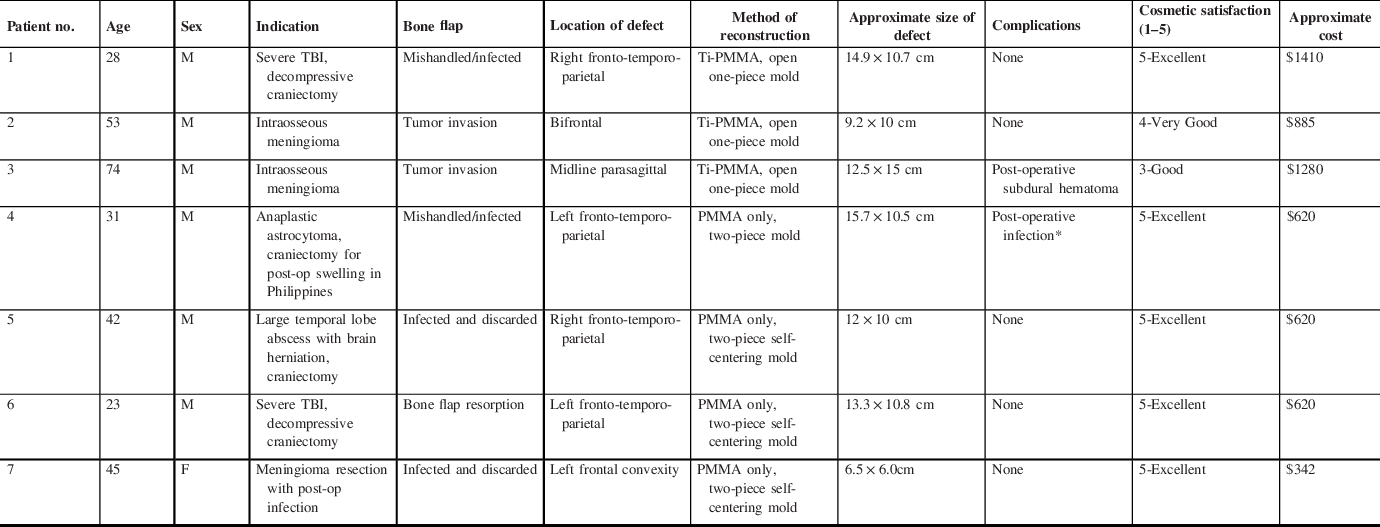
* Patient 4 had significant infection risks, as described in Results
Three initial implants were constructed using an open-air Ti-PMMA construct, while the final four were produced using a PMMA-only construct from a two-piece mold. Figure 2 illustrates the pre- and post-operative CT scans of the three cases done with an open-air mold using a Ti/PMMA construct. Figure 3 illustrates the pre- and post-operative CT scans of the four cases done with a two-piece mold. All patients had ‘Good’ to ‘Excellent’ cosmetic with a mean score of 4.6 out of 5 (SD 0.8).

Figure 2: Pre- and post-operative CT scans of the three cases done with an open-air mold using a Ti/PMMA construct. The mold is cleaned and placed into a sterile plastic bag. The titanium mesh is then manually contoured (using scissors and/or mesh bender) over the mold. PMMA is then poured onto the mesh and allowed to harden over the mold. Panels A-B, C-D, E-F correspond to pre- and post-operative CT scans of patients 1, 2, and 3, respectively. G shows the model of the open-air mold for patient 2. H shows the 3D printed open-air mold for patient 1.

Figure 3: Pre- and post-operative CT scans of the four cases done with a two-piece mold. The utilization of the two-piece mold allowed us to remove the titanium mesh altogether. A-B, C-D, E-F, G-H correspond to pre- and post-operative CT scans of patients 4, 5, 6, and 7, respectively. All patients had self-rated ‘good’ to ‘excellent’ cosmetic results.
In terms of complications, one patient (No. 3) suffered a symptomatic post-operative acute subdural hematoma and required to take back to the OR and revision surgery. Another patient (No. 4) persistently picked at his incision post-op and presented with an area of wound dehiscence 1 month later. He was taken to the OR for washout and cranioplasty removal. Due to previous scalp radiation (anaplastic astrocytoma treated 2 years prior) and behavioral issues, further cranioplasty was not performed. None of the other patients developed delayed infection or any new onset focal neurological deficits with a mean follow-up period of 11.8 months (range 3 to 22 months).
Utilization of the two-piece mold allowed us to remove the titanium mesh altogether. A 90 × 90 mm Stryker Dynamic Ti-mesh costs CA$395 at our institution (Stryker, MI, USA), while a bag of antibiotic-infused PMMA costs CA$120. Average cost for Ti-PMMA implants was approximately $1192, while PMMA-only implants reduced cost to $550. Elimination of the titanium mesh reduced both the cost and complexity of the custom cranioplasty. In addition, patient satisfaction improved with this modification from an average satisfaction score of 4/5 to 5/5.
Discussion
The seven presented cases represent our early utilization of cost-effective 3D printing strategy to create custom cranioplasty implants for cranial reconstruction for various defects. The turnover for cases can be done in under 24 h, compared with several weeks to order a custom implant.
In developing this method, we identified opportunities for improvement and iteratively refined our technique. For the first three cases, a single open-air mold was printed and a Ti-PMMA cranioplasty implant was molded over the ABS construct. While the titanium mesh provides anchoring points for screw fixation to the bone, it does increase the overall cost of the cranioplasty. The addition of titanium mesh may also increase the complication rate, although there is scant evidence in literature to elucidate this issue. Reference Kwiecien, Rueda and Couto10 Most small case series have been limited to in-situ molding of PMMA over titanium mesh. Reference Binhammer, Jakubowski, Antonyshyn and Binhammer3,Reference Kwiecien, Rueda and Couto10
For the latter four cases, the two-piece mold was used with the addition of alignment posts to allow for self-centering. This allowed for a PMMA-only construct that provided a more natural cosmetic appearance when placed on the patient’s skull. The upfront cost of adopting this strategy is relatively inexpensive. The software used are all open-source and a typical iMac or equivalent costs around CA$2000. The cost of a good-quality desktop 3D printer can be obtained for CA$1000–$3000.
In terms of OR cost-effectiveness, the total cost for PMMA-only implant ranged from CA$342 to 620, including cost of 3D printing and antibiotic-loaded PMMA (larger cranioplasties usually require 2 bags of PMMA). The cost of straight plates and screws contributed CA$192–320 of this cost. It should be noted that plates and screws would also be required for commercially available patient-specific implants. Therefore, they provide no additional cost to the case. The addition of titanium mesh would add another CA$395–790 to the cost of the procedure. There is opportunity for further cost reduction by using regular PMMA which only costs $25 per bag. The evidence for antibiotic-loaded PMMA used in arthroplasty appears to be inconclusive. Reference van Vugt, Arts and Geurts11 Comparison of antibiotic-loaded PMMA versus regular PMMA in cranioplasty would be a topic for future study.
Morales-Gomez et al recently reported the largest series of cranioplasties using desktop 3D printing. Twenty-two patients underwent PMMA cranioplasty using 3D printed molds at a single center in Mexico. The authors’ methodology was similar to ours, utilizing a two-piece mold to help with the casting process. Reference Morales-Gómez, Garcia-Estrada and Leos-Bortoni12 We found that with the addition of alignment posts in our method further expedited this process. The authors’ process for mold creation also seems more complicated by manually sketching the defect area and drawing contour lines for reconstruction. This increases the number of hours needed during postprocessing, but it can be advantageous for defects with no baseline scan and no ‘normal’ side to mirror (e.g. midline defects). In this case, a virtual reconstruction must be done based on a ‘standard skull’ or equivalent custom contour lines.
A 2017 study of cranioplasty complications in the US population from 2007 to 2014 using the MarketScan database reported an average of approximately 1020 cases per year. Reference Li, Azad and Veeravagu13 Extrapolating to the Canadian population, an estimated 160 ‘large’ cranioplasty cases take place in Canada each year. Many of these patients will likely not receive commercially available patient-specific implants due to the high cost (average $15,000 CAD). At our center, only 1 or 2 cases were done each year with this type of implant, while the remainder received PMMA ± Ti Mesh constructs. The wider adoption of cost-effective 3D printing strategies, such as the technique that we presented here, provides patients with custom cranioplasties and better cosmesis while also potentially reducing the cost to the Canadian healthcare system by over $2M per year.
Complications observed in this series are not unique to this method of cranioplasty. One patient in this small series developed a post-operative hematoma, while one patient with multiple predisposing factors developed a post-operative infection. Although the risk for hematoma and infection was higher in this small series as a result of the small number of patients included (14.1% for both vs. 3.2% overall for hematoma, and 8.3% for infection in large cranioplasties), overall the complication rate (28.6%) is lower than the literature suggests, considering the largest retrospective series reported a complication rate of 41.7%. Reference Li, Azad and Veeravagu13
This study illustrates the feasibility of cost-effective 3D printing in the Canadian healthcare system. The limitations of our study include the small number of patients included in the series. Given the limited numbers, our complication rate did not appear to be higher than what is reported in literature. Reference Li, Azad and Veeravagu13 Further improvements of the methodology include identifying temperature resistant sterile plastic bag, or 3D printing in materials that would be able to tolerate sterilization. This has the benefit of removing the plastic bag and preventing wrinkling on the PMMA cranioplasty. PEEK is a material that can be used for this purpose; however, there is significantly increased cost associated with both filament and 3D printer. Reference Honigmann, Sharma, Okolo, Popp, Msallem and Thieringer14
Conclusion
The use of cost-effective 3D printing for cranioplasty appears to be feasible. It provides similar cosmetic outcomes compared to commercially available patient-specific implants at a fraction of the cost. The adverse events also did not appear to be higher than historical data. In the current economic environment, it is important to consider benefits to both individual patients and our healthcare system to improve access to healthcare across Canada.
Disclosures
The authors have no conflicts of interest to declare.
Statement of Authorship
ML – Participated in the design of the study, data gathering and analysis, and helped draft the manuscript as well as addressing revisions. AA – Participated in the design of the study, performed the ORs and clinical follow-up, and helped edit the manuscript. WA – Participated in the design of the study, performed the ORs and clinical follow-up, and helped edit the manuscript. BHW – Conceived the study, participated in its design and coordination, performed the ORs and clinical follow-up, and helped draft and edit the manuscript and revisions.






