The association between maternal nutritional status and fetal growth is well known. For example, the weight, BMI and fat content of the maternal body correlate positively with infant birth weight(Reference Butte, Ellis and Wong1, Reference Baker, Michaelsen and Rasmussen2). The maternal insulin-like growth factor (IGF) system has been suggested to be a mediator between maternal nutritional status and fetal growth(Reference Owens3), because it is affected by nutritional status(Reference Thissen, Ketelslegers and Underwood4) and influences placental development, function and nutrient transfer(Reference Roberts, Owens and Sferruzzi-Perri5, Reference Forbes and Westwood6), as well as maternal retention of nutrients(Reference Gargosky, Owens and Walton7). This system includes the ligands IGF-I and IGF-II (IGF) and six binding proteins (IGF binding protein (IGFBP)-1–6). Free IGF have either immediate anabolic insulin-like effects on protein and carbohydrate metabolism or long-term effects on cell replication and differentiation(Reference Jones and Clemmons8). In the circulation, most IGF are bound to binding proteins. This provides a mechanism to regulate the biological activity of the free IGF. Binding protein IGFBP-3 binds 80–90 % of all IGF in serum. However, during pregnancy, proteases degrade IGFBP-3(Reference Sakai, Iwashita and Takeda9–Reference Olausson, Lof and Brismar12), which decreases its affinity for IGF. This may increase the metabolic action of IGF-I on target cells such as those in the placenta, possibly stimulating fetal growth. IGFBP-1 binds only about 2 % of serum IGF(Reference Frystyk, Hojlund and Rasmussen13), but it is important for the short-term regulation of IGF activity, and has a role in blood glucose regulation(Reference Baxter14).
Reports of relationships between serum concentrations of IGF-I in pregnant women and birth weight of their babies are contradictory as some studies(Reference Hall, Hansson and Lundin15–Reference Clapp, Schmidt and Paranjape18), but not all(Reference Hills, English and Chard19), have shown such relationships to be positively significant. In a previous study on healthy, pregnant, Swedish women, we found serum concentrations of IGF-I to be significantly correlated with fetal weight in the third trimester(Reference Olausson, Lof and Brismar12). In these women, we also observed a decrease in the serum concentration of IGF-I in the very beginning of pregnancy. Women with a high concentration before pregnancy had a more pronounced decrease in this concentration than women with a low concentration before pregnancy(Reference Olausson, Lof and Brismar12). Furthermore, we identified significant negative correlations between the maternal serum concentration of IGFBP-1 in gestational weeks 20, 32 and 35 and birth weight of infants(Reference Olausson, Lof and Brismar12). A few studies have investigated relationships in pregnant and non-pregnant women between serum concentrations of IGF-I and IGFBP-1 and body weight, BMI or body composition. In non-pregnant women of reproductive age, the results are contradictory(Reference Lukanova, Lundin and Zeleniuch-Jacquotte20–Reference Ahmed, Thomas and Schmitz22). Thus, Jernström & Olsson(Reference Jernström and Olsson21) reported a positive correlation between IGF-I serum concentrations and body weight, whereas others have reported that such concentrations are unrelated to body fat(Reference Ahmed, Thomas and Schmitz22) and BMI(Reference Lukanova, Lundin and Zeleniuch-Jacquotte20). In pregnant women, Hills et al. (Reference Hills, English and Chard19) reported a significant positive relationship between serum IGF-I and body weight. Comparable studies for IGFBP-1 demonstrate that the serum concentration of this protein is negatively related to body weight, BMI and/or the body fat content of women before(Reference Ahmed, Thomas and Schmitz22) as well as during pregnancy(Reference Hills, English and Chard19, Reference Baldwin, Chung and Rogers23, Reference Holmes, Holly and Soothill24). However, to understand whether the maternal IGF system represents a mediator between maternal nutritional status and fetal growth, further studies of the relationships between serum concentrations of IGF-I and IGFBP-1 and nutritional status in terms of body weight and composition are needed in women.
Our previous study in healthy Swedish women included longitudinal assessments, before and during pregnancy, of an extensive set of variables including components of the IGF system as well as accurate measures of body composition. This dataset has been used in a number of studies on pregnancy in relation to, for example, energy metabolism(Reference Lof, Olausson and Bostrom25, Reference Lof and Forsum26), body composition(Reference Lof and Forsum26–Reference Forsum, Lof and Olausson28) and serum concentrations of hormones(Reference Lof, Olausson and Bostrom25, Reference Eriksson, Lof and Olausson29), including the IGF system(Reference Olausson, Lof and Brismar12). In the present study, we took advantage of the longitudinal design when testing the hypothesis that the maternal IGF system may act as a mediator between maternal nutritional status and fetal growth. This was carried out, first, by investigating relationships, before and during pregnancy, between maternal serum concentrations of IGF-I and IGFBP-1 and maternal body weight and composition. In addition, we explored how concentrations of IGF-I, before and in early pregnancy, may impact infant birth weight when combined with maternal body weight and composition.
Subjects and methods
Subjects
Thirty-nine women planning pregnancy were recruited via the health care system or via advertisements in a local newspaper in the city of Linköping. Twenty-three of these became pregnant and completed the study. The women were all healthy non-smokers with no eating disorders and with a BMI before conception ranging from 18 to 39. Before pregnancy, the women had normal concentrations of blood glucose and serum insulin, and none was diagnosed with gestational diabetes(Reference Olausson, Lof and Brismar12). Data regarding their serum insulin concentrations and insulin resistance before and during pregnancy have been published previously(Reference Eriksson, Lof and Olausson29). Relevant data regarding the women and their infants are given in Table 1. Body weight and composition of the women before and during pregnancy have been described previously(Reference Lof and Forsum26). Infant birth weight and serum IGF-I and IGFBP-1 data have been reported by Olausson et al. (Reference Olausson, Lof and Brismar12). Each woman delivered one healthy infant after 280 (sd 10; range 256–296) d of pregnancy. At birth, the infants weighed 3740 (sd 510; range 2600–4540) g and were 51 (sd 2; range 46–57) cm long.
Table 1 Characteristics of women before pregnancy and in gestational weeks 14 and 32
(Mean values, standard deviations and ranges, n 23)

IGF-I, insulin-like growth factor-I; IGFBP-1, IGF binding protein-1.
Study outline
The measurements were done before conception and at weeks 14 and 32 of pregnancy. Women arrived early in the morning after an overnight fast for measurements of body weight, blood collection and administration of a dose of 2H. Stage of gestation was assessed using ultrasound, generally at weeks 12–14(Reference Jorgensen30). Infant body weight and length were measured just after delivery. The study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Research Ethics Committee of the University of Linköping, Sweden. Verbal informed consent, witnessed and formally recorded, was obtained from all subjects.
Body composition and anthropometry
Women
Body weight was recorded using a digital balance (KCC150; Mettler-Toledo, Albstadt, Germany). Fat-free body weight was calculated from total body water estimated using 2H dilution as described previously(Reference Lof and Forsum27). Total body water was divided by the appropriate hydration fraction (before pregnancy, 0·718; week 14, 0·722; and week 32, 0·747) to assess fat-free body weight(Reference Lof and Forsum27). Body fat was calculated as body weight minus fat-free body weight.
Infants
Body weight was recorded using an electronic column scale, and length using a length board. These measurements were obtained from hospital records. Corrected infant birth weight, to account for differences in birth weight due to gestational age and sex, was calculated as recorded birth weight times the ratio of recorded birth weight and mean birth weight of healthy Swedish newborns with the appropriate gestational age and sex(Reference Niklasson and Karlberg31).
Analyses of blood samples
Blood was collected by cubital vein puncture. Samples were kept at +4°C for a maximum of 4 h before being centrifuged at 1500 g for 10 min. Serum was harvested and stored at − 70°C. Total IGF-I in serum was measured using RIA after acid–ethanol extraction(Reference Bang, Eriksson and Sara32). The intra-assay CV were 4·3 % at 149 μg/l and 8·6 % at 526 μg/l, while the inter-assay CV was 11 %. IGFBP-1 was analysed using RIA as described by Póvoa et al. (Reference Póvoa, Roovete and Hall33). The intra-assay CV were 8·4 % at 18 μg/l and 6·0 % at 90 μg/l. The inter-assay CV was 10 %.
Statistical analyses
Results presented are means and standard deviations. Simple linear regression and correlation analyses were used. To identify significant differences between slopes of regression lines, we evaluated whether there was an interaction between an independent variable and stage of gestation in the effect on the dependent variable. This evaluation was carried out using multiple regression analysis including an interaction term between the independent variable and stage of gestation. Multiple regression analysis was also used to explore how corrected infant birth weight varied in response to two independent variables (serum IGF-I concentration in combination with body weight and body fat). P ≤ 0·05 was considered statistically significant. Statistical analyses were carried out using Statistica 6.1 (StatSoft, Inc., Tulsa, OK, USA) and SPSS (version 6.0; Chicago, IL, USA).
Results
Serum concentrations of insulin-like growth factor-I and insulin-like growth factor binding protein-1 in relation to maternal body weight and composition
Correlation coefficients for relationships between serum concentrations of IGF-I and variables describing body weight and composition are given in Table 2. Before pregnancy, no correlations were found between serum IGF-I and body weight, body fat (kg and %) or fat-free body weight (kg). However, in gestational weeks 14 and 32, significant positive correlations with serum IGF-I were found for body weight and fat-free body weight, while body fat (kg and %) was not correlated with serum IGF-I on these measurements. Table 2 also shows the corresponding correlations for serum concentrations of IGFBP-1. Before pregnancy and in gestational weeks 14 and 32, serum concentrations of IGFBP-1 were significantly correlated with body weight and body fat (kg and %), but not with fat-free body weight. Fig. 1 shows the regression lines obtained when IGFBP-1 in serum is regressed on body fat (%) in gestational week 14 and before pregnancy (Fig. 1(a)) and on body fat (%) in gestational week 32 and before pregnancy (Fig. 1(b)). The slope of the regression line obtained before pregnancy was significantly different from the corresponding value obtained in gestational week 14 (P = 0·0001) as well as from the corresponding value obtained in gestational week 32 (P = 0·0001). In addition, Fig. 2 illustrates the relationships obtained when the serum concentrations of IGFBP-1 during pregnancy minus the corresponding values obtained before pregnancy were regressed on body fat (%) before pregnancy. These regression lines were significant with negative slopes when values in gestational week 14 (a) as well as in gestational week 32 (b) were used.
Table 2 Correlation coefficients (r) and their probabilities (P) for linear relationships obtained when serum concentrations of insulin-like growth factor-I (IGF-I) or IGF binding protein-1 (IGFBP-1) are regressed on body weight, body fat and fat-free body weight, respectively*
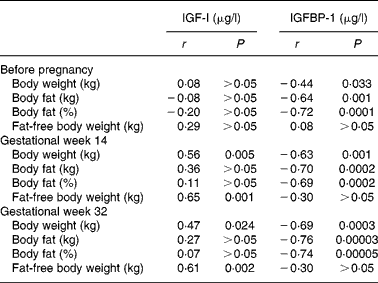
* Results were obtained from twenty-three healthy women before pregnancy and in gestational weeks 14 and 32.

Fig. 1 Serum concentration of insulin-like growth factor binding protein-1 (IGFBP-1; μg/l) (y) regressed on body fat (%) (x) (a) before pregnancy (●) and in gestational week 14 (○; n 23), and (b) before pregnancy (●) and in gestational week 32 (△; n 23). Regression equations are as follows: y = 56·4 − 1·1x, r − 0·720, P = 0·0001 (before pregnancy); y = 346·0 − 6·0†x, r − 0·693, P = 0·0002 (gestational week 14); y = 333·8 − 6·0†x, r − 0·743, P = 0·00 005 (gestational week 32). † The slope of the regression line was significantly different from the corresponding value before pregnancy assessed after identification of an interaction between body fat (%) and stage of gestation in the effect on the serum concentration of IGFBP-1 (P = 0·0001). This assessment was made by means of multiple regression analysis, where an interaction term between body fat (%) and stage of gestation was included.

Fig. 2 Difference in insulin-like growth factor binding protein-1 (ΔIGFBP-1) serum concentrations (μg/l) (y) (a) between values obtained in gestational week 14 and before pregnancy (n 23), and (b) between values obtained in gestational week 32 and before pregnancy (n 23) regressed on body fat (%) before pregnancy (x). Regression equations are as follows: (a) y = 267 − 4·4x, r − 0·613, P = 0·002; (b) y = 260 − 4·3x, r − 0·664, P = 0·001. * ΔIGFBP-1: the serum concentration in gestational week 14 minus the corresponding concentration before pregnancy. † ΔIGFBP-1: the serum concentration in gestational week 32 minus the corresponding concentration before pregnancy.
Serum concentrations of insulin-like growth factor-I before pregnancy and in gestational week 14 and maternal body weight and composition in relation to corrected infant birth weight
Both maternal body weight (kg) and body fat content (kg) before pregnancy were significantly correlated with corrected infant birth weight (r 0·436, P = 0·038 and r 0·443, P = 0·034, respectively). Similarly, in gestational week 14, significant correlations were observed between maternal body weight (kg) and corrected infant birth weight (r 0·449, P = 0·032), as well as between the body fat content (kg) and corrected infant birth weight (r 0·438, P = 0·037). No significant correlations were observed between serum IGF-I (μg/ml) and corrected infant birth weight either before pregnancy (r 0·015, P = 0·947) or in gestational week 14 (r − 0·174, P = 0·428).
Table 3 shows adjusted r 2 for multiple linear relationships relating corrected infant birth weight (y) to two independent variables, including the serum concentration of IGF-I (either before pregnancy or in gestational week 14) and either maternal body weight or body fat content (before pregnancy or in gestational week 14). Combinations including IGF-I in gestational week 14 explained significant (P = 0·001–0·021) fractions of the variation in corrected infant birth weight (adjusted r 2 0·251–0·435), while combinations including IGF-I before pregnancy did not explain any significant (P = 0·105–0·121) fraction of this variation (adjusted r 2 0·109–0·122). Fig. 3 shows a graphical representation of two of the relationships given in Table 3. These relationships show the effect on corrected infant birth weight of using the serum concentration of IGF-I before pregnancy (a) v. in gestational week 14 (b) as one of the two independent variables when body weight before pregnancy was the second independent variable.
Table 3 Multiple linear regression analyses relating corrected infant birth weight (dependent variable, y)* to two independent variables, including insulin-like growth factor-I (IGF-I) in maternal serum (before pregnancy or in gestational week 14) and a variable describing maternal body weight or body fat (n 23)

* Corrected infant birth weight was calculated as recorded birth weight times a ratio between recorded birth weight and mean birth weight of healthy Swedish newborns with the appropriate gestational age and sex(Reference Niklasson and Karlberg31).

Fig. 3 A graphical representation of the relationships between corrected infant birth weight (g) (dependent variable) and the maternal serum concentration of insulin-like growth factor-1 (IGF-I; μg/l) (first independent variable) before pregnancy (a) or in gestational week 14 (b), and maternal body weight (kg) before pregnancy (second independent variable). Regression equations are given in Table 3.
Discussion
In a previous paper(Reference Forsum, Lof and Olausson28) based on the dataset that was the same as that used in the present study, we reported relationships between maternal body weight and composition and infant birth weight. In the present paper, we investigated whether the maternal IGF system has a role in mediating the kind of relationships observed in our previous study(Reference Forsum, Lof and Olausson28). Our dataset provided a unique opportunity to investigate this area for the following reasons: it has a longitudinal design and includes measurements before conception and in early pregnancy, as well as information on infant birth weight. Furthermore, our body composition results are based on assessments of total body water using 2H dilution and application of appropriate hydration factors, a procedure known to result in accurate estimates of body fat. A combination of these accurate and longitudinal data, thus, provided a unique opportunity to study interactions between maternal body weight and composition, the maternal IGF system and infant birth weight.
An intriguing observation is that the serum IGF-I concentration becomes correlated with maternal body weight and fat-free body weight after the serum concentration of IGF-I decreases during the first part of pregnancy. In addition, the multiple regression models presented in Table 3 and Fig. 3 suggest that lower concentrations of IGF-I in early pregnancy are favourable for fetal growth when combined with maternal body weight or fat content. A possible interpretation is that a regulatory activity is involved, originating in the product of conception with the purpose of controlling growth of the maternal body. Early pregnancy represents a period of balance between anabolism and catabolism, and it is important for the fetus that growth of the maternal body is maintained within appropriate limits. A certain proliferation of the maternal body is advantageous for the fetus, but if this growth becomes excessive, the fetus may be unable to compete successfully for available nutrients. It should be pointed out, however, that this interpretation certainly is speculative and requires confirmation. In this context, it is also relevant to note that an experimental study in animals has shown that maternal circulating concentrations of IGF-I from early gestation to mid-gestation may impact fetal growth and placental transport near term(Reference Roberts, Owens and Sferruzzi-Perri5).
Figs. 1 and 2 show that women with a low body fat content before pregnancy have higher increases during pregnancy in their serum concentrations of IGFBP-1 than women with a high body fat content before pregnancy. This is noteworthy since high serum concentrations of IGFBP-1 during the second half of pregnancy are associated with low birth weight(Reference Olausson, Lof and Brismar12), and therefore also with slow fetal growth. This suggests the presence of a regulatory process responsible for maintaining a fetal growth rate appropriate to the nutritional status of the mother. It is possible that low serum concentrations of IGFBP-1 result in higher concentrations of free IGF-I, with the possible consequence being a stimulating effect on fetal growth via the stimulating actions of free IGF-I on the placenta. However, it is unlikely that this mechanism explains more than a part of the relationship between maternal serum IGFBP-1 concentrations and fetal growth rate since IGFBP-1 binds only about 2 % of all IGF-I. Insulin and blood glucose also influence serum concentrations of IGFBP-1(Reference Baxter14), and therefore it is possible that the observed relationship between serum IGFBP-1 and fetal growth is due to the glucoregulatory effects of IGFBP-1 and its relationship to insulin. However, in our women, neither serum concentrations of insulin nor insulin resistance(Reference Eriksson, Lof and Olausson29) was significantly correlated with corrected birth weight of their infants. In this context, it may be relevant to note that in our women, serum concentrations of insulin and insulin resistance were significantly correlated with body fat (%)(Reference Eriksson, Lof and Olausson29). Insulin resistance during pregnancy is thought to promote fetal growth, since women with gestational diabetes tend to deliver infants with high birth weight(Reference Ostlund, Hanson and Bjorklund34). The mechanism behind the enhanced fetal growth is thought to be increased exposure to all major fuel sources i.e. glucose, NEFA and amino acids(Reference Jansson, Cetin and Powell35). Franks et al. (Reference Franks, Looker and Kobes36) recently reported that blood glucose concentrations are related to infant birth weight also in non-diabetic pregnant women. Hence, it is possible that the mechanisms explaining the relationships found in the present study regarding serum concentrations of IGFBP-1, the maternal body fat content and corrected infant birth weight are related to interactions between insulin, blood glucose and IGFBP-1.
One weakness of the present study is the small number of women studied. It is noteworthy, however, that their BMI before pregnancy and weight gain during pregnancy are quite typical for Swedish women of childbearing age, and our women are similar to many other such women in the Western countries. A potential problem with our small sample size is the risk for type II errors, e.g. a failure to identify existing relationships as significant. Using a simple linear regression model with twenty-three observations gives a power of 80 % to identify a correlation coefficient of 0·55 as significant. All relationships identified as non-significant in Table 2 have correlation coefficients well below 0·55. This is also true for correlation coefficients obtained for the relationships between corrected infant birth weight and serum concentrations of IGF-I before pregnancy and in gestational week 14.
In conclusion, we have reported findings confirming and extending the statement that the IGF system has a role in mediating the effect of the maternal nutritional status on fetal growth. The present results provide new aspects on this role of the IGF system, and suggest that the mechanisms involved are complicated and interact in an intricate way. Studies in populations of pregnant women with different nutritional status may be valuable for increasing our understanding regarding the role of the IGF system in fetal growth regulation.
Acknowledgements
The study was funded by the Family Erling-Persson Foundation, the General Maternity Hospital Foundation, Lions Forskningsfond mot folksjukdomar, the Magnus Bergvalls Foundation, the Swedish Research Council project nos 04224, 12172 and 15402, the Swedish Society of Medicine and the Swedish Nutrition Foundation. The authors thank all the women and their infants for their participation, and acknowledge Elisabeth Norén-Krog for skilful laboratory work. The authors' responsibilities are as follows: E. F. designed the study; M. L. and H. O. conducted the study; K. B. was responsible for the hormone analyses; H. O., E. F. and A. S. analysed the data, interpreted the results and drafted the manuscript, which was reviewed by the other authors. None of the authors has a conflict of interest.








