CVD is the leading cause of death in North America1 and it is well recognized that LDL-cholesterol is a major risk factor associated to CVDReference Ross2. However, not all CVD patients show high LDL-cholesterol concentrations and it has been shown that variations in the physical properties and composition of LDL particles such as LDL oxidation could explain, at least in part, the atherogenicity of LDLReference Robbesyn, Salvayre and Negre-Salvayre3, Reference Klouche, May, Hemmes, Messner, Kanse, Preissner and Bhakdi4 even in the absence of high LDL-cholesterol concentrations. As oxidized LDL (OxLDL) are not recognized by the LDL receptor but rather by scavenger receptors at the surface of sub-endothelial resident macrophages, LDL oxidation favours the unrestricted uptake of cholesterol, thereby leading to the formation of foam cells, which is the first step in the formation of the earliest atherosclerotic lesions known as fatty streaksReference Ross2.
Migration of monocytes from the bloodstream into the sub-endothelial space requires that cell adhesion molecules such as the intercellular cell adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1) and E-selectin be expressed by endothelial cells. This process is referred to as endothelial activationReference Ross2. Interestingly, soluble forms of these different cell adhesion molecules can be measured in plasma probably as a result of the vascular endothelium breakdown, and these concentrations have been shown to correlate with cardiovascular mortalityReference Blankenberg, Rupprecht, Bickel, Peetz, Hafner, Tiret and Meyer5.
Numerous studiesReference Chu and Liu6–Reference Fuhrman and Aviram8 have shown that antioxidants and more specifically polyphenolic compounds can inhibit LDL oxidation, which in turn has been suggested to reduce OxLDL-induced expression of cell adhesion molecules by endothelial cellsReference Couillard, Ruel, Archer, Pomerleau, Bergeron, Couture, Lamarche and Bergeron9, Reference Kume, Cybulsky and Gimbrone10. Cranberry is a potent source of different antioxidant compounds such as flavonols, anthocyanidins, proanthocyanidinsReference Chen, Zuo and Deng11, Reference Wang, Du and Francis12, as well as resveratrolReference Wang, Catana, Yang, Roderick and van Breemen13 and phenolic acidsReference Chen, Zuo and Deng11. Furthermore, cranberries also contain acetylsalicylic acidReference Duthie, Kyle, Jenkinson, Duthie, Baxter and Paterson14 that possesses anti-inflammatory activity and that has been shown to decrease VCAM-1 expression in vitro Reference Eisele, Schwedhelm, Schieffer, Tsikas and Boger15. The present study was therefore undertaken in order to examine the effect of consuming increasing doses of low-calorie cranberry juice cocktail (CJC) for a period of 12 weeks on plasma concentrations of OxLDL and cell adhesion molecules in men.
Material and methods
Subjects
Thirty-one healthy and sedentary men were recruited through the media to participate in a 12-week intervention. Inclusion and exclusion criteria are described in Table 1. Briefly, subjects had to have a waist circumference ≥ 90 cm, a fasting plasma LDL-cholesterol concentration between 3·0 and 5·0 mmol/l and absence of diabetes, CVD and renal, hepatic and endocrine disorders. A written consent was provided by each volunteer to participate in the study which was approved by the Medical Ethics Committee of Laval University (project no. 2003-131). During the course of the study, one subject dropped out of the project due to personal reasons not related to the intervention. Therefore, the data presented refer to the thirty men who completed the study.
Table 1 Exclusion and inclusion criteria of the study

Intervention
Upon entry into the study, subjects met with a dietitian and were instructed to maintain their usual nutritional habits throughout the entire intervention. Participants were subjected to a 4-week Run-In period which consisted of consuming 500 ml/d of a cranberry-flavoured low-calorie placebo juice (PJ). During the Run-In period, participants were instructed to reduce their alcohol consumption to a maximum of one drink/d (or < 15 g alcohol/d) and to refrain from consuming any vitamin, antioxidant or mineral supplements. This Run-In period was followed by three 4-week periods during which subjects successively consumed 125 ml (phase 1), 250 ml (phase 2) and 500 ml (phase 3) of CJC daily. These volumes were adjusted to 500 ml liquid/d by adding 375, 250 or 0 ml PJ/d for phases 1, 2 and 3 respectively. This was achieved in order to minimize the impact of incorporating increasing quantities of liquid in the daily diet of subjects and blind subjects from the level of treatment they were assigned to.
PJ and CJC were provided to the subjects in 125 ml ready-to-drink TetraBrik boxes from Tetra-Pak© (Richmond Hill, Ontario, Canada) that were packaged at Laval University. Both the PJ and CJC were kindly provided to us by Ocean Spray Cranberries Inc. who also monitored the packaging sessions to ensure adequate reconstitution and quality of both the PJ and CJC. A detailed description of the PJ and CJC that were used in the present study has been previously publishedReference Ruel, Pomerleau, Couture, Lemieux, Lamarche and Couillard16. In an effort to keep the subjects' sugar consumption to a minimum and limit possible detrimental changes in plasma TAG concentrations, we provided subjects with the no added sugar version of Ocean Spray's Low-calorie CJC. Both the PJ and CJC were artificially sweetened with sucralose (Splenda®; McNeil Nutritionals LLC, Fort Washington, PA, USA). One box of 125 ml CJC or PJ contained 5·46 g of carbohydrates, and provided 91·3 kJ energy. A detailed description of both CJC and PJ is in Table 2.
Table 2 Detailed description of the content of a portion (125 ml) of placebo juice (PJ) and low-calorie cranberry juice cocktail (CJC)†
(Mean values and standard deviations for five determinations)

ND, none determined.
† Other ingredients included in PJ and CJC are filtered water, cranberry juice concentrate (CJC only), fructose, pectin, sodium citrate, ascorbic acid, sucralose and acesulfame-K.
Blood pressure and lipid measures
At each visit to the investigation unit, blood pressure was measured by a nurse with a manual sphygmomanometer with the subject in a supine position after a 5 min rest. Blood samples were obtained from the subjects' antecubital vein in the morning after a 12 h fast. Upon collection, cholesterol and TAG concentrations were determined in plasma by enzymatic methods using a Technicon RA-1000 analyser (Bayer Corporation Inc., Tarrytown, NY, USA) as previously describedReference Moorjani, Dupont, Labrie, Lupien, Brun, Gagne, Giguere and Belanger17. Plasma VLDL (density < 1·006 g/ml) were isolated by ultracentrifugation and the HDL fraction was obtained after precipitation of LDL from the infranatant (density>1·006 g/ml) with heparin and MnCl2Reference Burstein, Scholnick and Morfin18. The cholesterol and TAG contents of the infranatant fraction were measured before and after the precipitation step. LDL-cholesterol was obtained by substracting the HDL-cholesterol concentration from the cholesterol content of the density>1·006 g/ml fraction. Apolipoprotein (apo) B concentration was measured by nephelometry (Dade Behring, Mississauga, Ontario, Canada) in total plasma as well as in the density < 1·006 g/ml fraction (LDL-apo B). The lyophilized serum standards for apo B measurements were prepared at the Lipid Research Center of Laval University Medical Center and calibrated with reference standards obtained from the Centers for Disease Control (Atlanta, GA, USA). Commercial ELISA kits were used to determine plasma soluble forms of E-selectin, ICAM-1, VCAM-1 (R&D Systems, Minneapolis, MN, USA) as well as OxLDL (ALPCO Diagnostics, Windham, NH, USA) concentrations.
Statistical analyses
Data are presented as means and standard deviations unless stated otherwise. Treatment effect and changes between CJC doses on the different variables were determined using the MIXED model procedure for repeated measures. When needed, covariance matrix structures were treated for compound symmetry, order 1 autoregressive or Huynh-Feldt. Associations between changes in variables were quantified with Spearman correlation coefficients. Changes in men with (n 9) or without (n 21) the metabolic syndrome were tested for significance using Student's paired t-tests. All analyses were performed with the SAS statistical package version 8.2 (SAS Institute, Cary, NC, USA) and P ≤ 0·05 was considered significant.
Results
Subjects' physical and metabolic characteristics upon their entry into the study are presented in Table 3. As shown in Fig. 1, the intervention yielded a decrease in plasma OxLDL (P < 0·001), which reached significance from baseline after the 250 ml/d ( − 5·5 (sd 27·1) %, P < 0·05) and 500 ml/d ( − 20·7 (sd 21·1) %, P < 0·001) phases. As reported and discussed in a previous publication by our groupReference Ruel, Pomerleau, Couture, Lemieux, Lamarche and Couillard16, the intervention yielded a significant increase in plasma HDL-cholesterol (P = 0·0010) concentration but failed to significantly affect circulating TAG (P = 0·0553) as well as levels of total cholesterol (P = 0·85) and LDL-cholesterol (P = 0·52) (Table 4).
Table 3 Baseline physical and metabolic characteristics of the thirty men

sICAM-1, soluble intercellular adhesion molecule-1; sVCAM-1, soluble vascular cell adhesion molecule-1.

Fig. 1 Changes in plasma oxidized LDL (P < 0·0001 across doses) concentrations during the intervention. Values are means with their standard errors depicted by vertical bars. Mean values were significantly different from baseline (0 ml CJC/d): *P < 0·05.
Table 4 Changes in lipids, blood pressure and heart beat during the intervention

Mean values were significantly different from those of the 0 ml group: *P < 0·05.
The effects of consuming increasing daily doses of CJC on plasma cell adhesion molecule concentrations are illustrated in Fig. 2. We found that both soluble VCAM-1 (sVCAM-1; P < 0·05) and soluble ICAM-1 (sICAM-1; P < 0·0001) were decreased following the intervention, reductions reaching significance compared to baseline values after consumption of the 500 ml/d CJC dose for both sVCAM-1 ( − 6·4 (sd 11·5) %, P < 0·01) and sICAM-1 ( − 7·5 (sd 15·4) %, P = 0·001). There was no effect of the intervention on plasma E-selectin concentrations (P = 0·66).

Fig. 2 Changes in plasma soluble vascular cell adhesion molecule-1 (A; P < 0·05 across doses), intercellular adhesion molecule-1 (B; P = 0·0001) and soluble E-selectin (C; P = 0·66) concentrations during the intervention. Values are means with their standard errors depicted by vertical bars. Mean values were significantly different from baseline (0 ml CJC/d): *P < 0·05. Mean values were significantly different from week 8 (250 ml CJC/d): †P < 0·05.
In an effort to characterize the relationships between the responses in plasma sVCAM-1, sICAM-1, soluble E-selectin and OxLDL concentration (0–500 ml CJC/d), we computed Spearman correlation coefficients between these variables. As shown in Fig. 3, we found a positive correlation between change in sVCAM-1 and OxLDL concentrations (r 0·62, P < 0·005) but an inverse association between sICAM-1 and OxLDL concentrations (r − 0·40, P < 0·05).

Fig. 3 Associations between changes in plasma oxidized LDL and soluble vascular cell adhesion molecule-1 (sVCAM-1; A), soluble intercellular adhesion molecule-1 (sICAM-1; B) as well as soluble E-selectin (sE-selectin; C) concentrations over the course of the entire intervention.
We also examined the impact of being characterized by the metabolic syndrome, according to the National Cholesterol Education Program Adult Treatment Panel III guidelines19, on the responses of OxLDL and adhesion molecules to the intervention. As shown in Fig. 4, we found that consuming low-calorie CJC for a period of 12 weeks significantly reduced plasma OxLDL and sICAM-1 concentration in both men with and without the metabolic syndrome. However, circulating sVCAM-1 were signficantly lower at the end of the intervention only in men without the metabolic syndrome. We found no effect of the presence of the metabolic syndrome on the response of plasma soluble E-selectin concentrations to the intervention (data not shown).
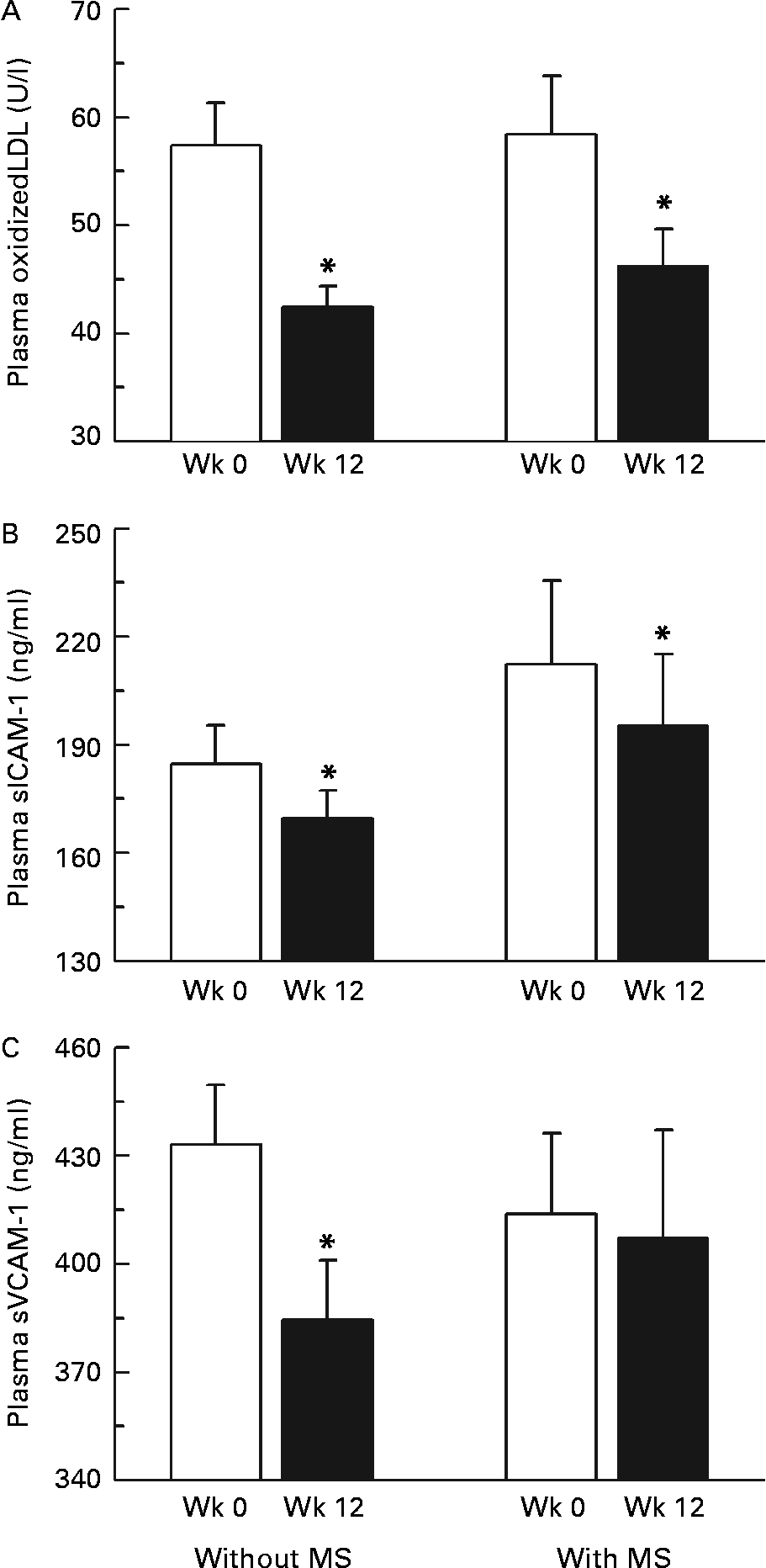
Fig. 4 Changes in plasma oxidized LDL (A), soluble intercellular adhesion molecule-1 (sICAM-1; B) and vascular cell adhesion molecule-1 (sVCAM-1; C) concentrations in response to the intervention in men with or without the metabolic syndrome (MS). Values are means with their standard errors depicted by vertical bars. Mean values were significantly different from week 0 (Wk 0): *P < 0·05.
As shown in Table 4, a significant variation was noted in systolic blood pressure (P across doses < 0·05) during the intervention which was accounted for by a significant decrease between the 125 and 500 ml CJC/d ( − 7 (sd 12) mmHg, P = 0·0036) doses. On the other hand, we noted no significant change in diastolic blood pressure or heart beat in response to the 12-week intervention (Table 3).
Finally, we examined the relationships between changes in systolic blood pressure and plasma cell adhesion molecule concentrations following the intervention. A significant association was noted between change in plasma VCAM-1 concentrations and systolic blood pressure (r 0·42, P < 0·05). However, no correlation was noted between changes in systolic blood pressure and circulating ICAM-1 (P = 0·51) or E-selectin (P = 0·87) concentrations.
Discussion
In the present study, we report that consumption of increasing doses of low-calorie CJC on a daily basis for a period of 12 weeks is associated with reductions in plasma OxLDL as well as sICAM-1 and sVCAM-1 concentrations but has no effect on circulating E-selectin concentrations in men. It is, to our knowledge, the first time that low-calorie CJC supplementation has been associated with reductions in plasma cell adhesion molecule concentrations in man. In this sense, the present observations tend to give further support to the cardioprotective potential of cranberries that has been already suggestedReference Reed20.
The significant reduction in plasma OxLDL concentrations with consumption of CJC observed in the present study is in agreement with results from previous investigations having shown that cranberry antioxidants decrease metal ion-induced LDL oxidation in vitro Reference Chu and Liu6, Reference Wilson, Porcari and Harbin7 and with a previous study from our group in which we observed a decrease in OxLDL concentration following a 2-week CJC supplementation in menReference Ruel, Pomerleau, Couture, Lamarche and Couillard21. Consumption of other fruit juices rich in flavonoids such as grapeReference Castilla, Echarri and Davalos22 and pomegranateReference Aviram, Dornfeld, Rosenblat, Volkova, Kaplan, Coleman, Hayek, Presser and Fuhrman23, which share similarities with cranberries in regards to their polyphenolic content, has also been shown to reduce plasma OxLDL concentrations.
Although, plasma LDL-cholesterol concentrations and LDL oxidation are closely relatedReference Holvoet24, the reduction in circulating OxLDL concentrations we report herein cannot be explained by changes in LDL-cholesterol concentrations as these remained stable throughout the entire intervention, as previously reportedReference Ruel, Pomerleau, Couture, Lemieux, Lamarche and Couillard16. Furthermore, it has been shown that small dense LDL particles are more susceptible to oxidationReference Parthasarathy, Santanam, Ramachandran and Meilhac25. Although LDL size was not measured in the present study, we have previously shown that a 2-week CJC supplementation was not associated with any significant variation in LDL size despite a reduction in plasma OxLDL concentrationsReference Ruel, Pomerleau, Couture, Lamarche and Couillard21. In addition, there was no change in the LDL-apo B/LDL-cholesterol ratio, a crude marker of LDL density, during the current intervention. This suggests that LDL size may not be a major contributor to the decrease in OxLDL observed in the present study. We believe that the reduction of plasma OxLDL concentrations in response to low-calorie CJC supplementation is likely to be attributable to polyphenolic compounds of the cranberries as they exert a potent antioxidant activityReference Sun, Chu, Wu and Liu26, Reference Hakkinen, Karenlampi, Heinonen, Mykkanen and Torronen27 and have been shown to prevent in vitro LDL oxidationReference Chu and Liu6, Reference Wilson, Porcari and Harbin7. On the other hand, it may be argued that consuming low-calorie CJC may have affected the total antioxidant capacity of the subjects through increases and/or preservation of endogenous antioxidant defences. However, evidence against such changes have been recently provided with data indicating that CJC supplementation for 2 weeks had no effect on fasting blood glutathione peroxidase, catalase and superoxide dismutase activities nor on fasting circulating glutathione concentrationsReference Duthie, Jenkinson, Crozier, Mullen, Pirie, Kyle, Yap, Christen and Duthie28. However, the possibility of improvements in these parameters over the course of the 12-week low-calorie CJC supplementation such as the one we report herein cannot be excluded.
Consumption of grape juice for 14 d was associated with decreases in plasma sICAM-1, an effect that was attributed to polyphenolic grapesReference Coimbra, Lage, Brandizzi, Yoshida and da Luz29. The present results are supportive of the effect of polyphenols on ICAM-1 production. Interestingly, similarities exist in the polyphenol content of grapes and cranberries. Both have been characterized as potent sources of quercetin, myricetin and resveratrolReference Hakkinen, Karenlampi, Heinonen, Mykkanen and Torronen27, Reference Flamini30, which have been identified as strong antioxidantsReference Hakkinen, Karenlampi, Heinonen, Mykkanen and Torronen27. In contrast, other polyphenol-rich foods like tea have shown equivocal results on their effects on circulating adhesion molecule concentrationsReference Hodgson, Puddey, Mori, Burke, Baker and Beilin31–Reference Lee, Min, Chun, Lee, Park, Lee do, Lee and Son33. Differences in the design of these studies but mostly in the type of flavonoids found in tea compared to cranberries are likely to explain the discrepancies between these previous observations and the present results.
The most extensively characterized pathway for cell adhesion molecule production involves the activation of NF-κB. Briefly, stimulation of a phosphorylation cascade will ultimately lead to the cleavage of the nuclear factor kappa B (NF-κB) inhibitor (IκB)/NF-κB complex and nuclear translocation of NF-κB where it will be able to regulate the transcription of numerous genes such as ICAM-1 and VCAM-1Reference Robbesyn, Salvayre and Negre-Salvayre3. Many stimuli have been identified to activate the IκB/NF-κB pathway including many inflammatory mediators and OxLDLReference Robbesyn, Salvayre and Negre-Salvayre3. The present observations are somewhat supportive of the OxLDL-induced production of sVCAM-1 as the change in plasma OxLDL concentration following the intervention was positively correlated to the change in circulating sVCAM-1. Unfortunately, NF-κB activation was not assessed in the current study and thus our hypothesis remains to be ascertained.
On the other hand, the contribution of OxLDL to the reduction in plasma sICAM-1 concentrations following the intervention is not so clear as we found an inverse relationship between the decrease in circulating OxLDL and sICAM-1 concentrations. Although statistically significant, the physiological relevance of that relationship remains to be better understood. Results from in vitro studies suggest that molecules like quercetinReference Blanco-Colio, Valderrama and Alvarez-Sala34, Reference Choi, Choi, Park, Kang and Kang35, resveratrolReference Leiro, Arranz, Fraiz, Sanmartin, Quezada and Orallo36, Reference Kaplan, Morgan, Bisleri, Cheema, Akman, Topkara and Oz37, proanthocyanidinReference Bagchi, Bagchi, Stohs, Ray, Sen and Preuss38, anthocyanidinReference Youdim, McDonald, Kalt and Joseph39, hydroxycinnamic acidReference Youdim, McDonald, Kalt and Joseph39 and acetylsalicylic acidReference Kopp and Ghosh40, Reference Weber, Erl, Pietsch and Weber41 have all been shown to prevent TNF-α-induced expression of VCAM-1 and/or ICAM-1 through their inhibition of NF-κB activation. Because cranberries are rich in quercetin, it is likely that the 12-week low-calorie CJC supplementation may have lowered plasma sICAM-1 through a direct effect of the increase in flavonoid intake.
Abdominal obesity is associated with a cluster of metabolic alterations including hypertriglyceridaemia, low HDL-cholesterol and impaired glucose–insulin homeostasis that has been defined as the metabolic syndromeReference Grundy, Brewer, Cleeman, Smith and Lenfant42. Furthermore, the metabolic syndrome per se is now recognized as a risk factor of cardiovascular eventsReference Malik, Wong, Franklin, Kamath, L'Italien, Pio and Williams43. It is therefore relevant to develop strategies aimed at improving the metabolic condition of abdominally obese individuals that could reduce their CVD risk. In the present study, we found that consuming low-calorie CJC for a period of 12 weeks significantly reduced plasma OxLDL and sICAM-1 concentrations in both men with and without the metabolic syndrome. However, our intervention yielded a significant reduction of sVCAM-1 concentrations only in subjects without the metabolic syndrome.
High blood pressure is an important risk factor for CVD19. In the present study, we noted a slight significant decrease in systolic blood pressure over the course of the intervention. We found no effect of drinking low-calorie CJC on a daily basis on diastolic blood pressure. Previous studies with fruit juice have also reported a blood pressure-lowering effect. Indeed, pomegranateReference Aviram and Dornfeld44, Concord grape juiceReference Park, Kim and Kang45 and sweetie juice (mix of grapefruit and pummelo)Reference Reshef, Hayari, Goren, Boaz, Madar and Knobler46 in man as well as grape juice with wine vinegar in ratsReference Honsho, Sugiyama, Takahara, Satoh, Nakamura and Hashimoto47 have all been shown to lower blood pressure. Among possible mechanisms underlying the hypotensive effect of these beverages, inhibition of the activity of the angiotensin-converting enzyme has been suggested as most likely. On the other hand, dark chocolate, a food also rich in flavonoids, has been reported to increase resting brachial artery diameterReference Vlachopoulos, Aznaouridis, Alexopoulos, Economou, Andreadou and Stefanadis48 which is concordant with a blood pressure-lowering effect. Neither angiotensin-converting enzyme activity nor brachial artery diameter were measured in the present study and the decrease in systolic blood pressure emergent herein remains unexplained.
One important drawback of the present study is that we are not able to clearly assess whether the favourable changes in plasma OxLDL, ICAM-1 and VCAM-1 concentrations we noted were the result of increasing doses of CJC or duration of the intervention. Furthermore, although a similar study design has been previously used in numerous studiesReference Ruel, Pomerleau, Couture, Lemieux, Lamarche and Couillard16, Reference Ruel, Pomerleau, Couture, Lamarche and Couillard21, Reference Stein, Keevil, Wiebe, Aeschlimann and Folts49–Reference O'Byrne, Devaraj, Grundy and Jialal53, the absence of a placebo control group is also unfortunate and surely imposes a limit to the present study. These issues are presently under investigation in our laboratory.
In summary, results of the present study show that a 12-week supplementation with low-calorie CJC reduces plasma OxLDL concentrations in men. Furthermore, to our knowledge, the present study is the first to show that consuming low-calorie CJC leads to a decrease in circulating sICAM-1 and sVCAM-1 concentrations in man. Although further research is needed to warrant our observations, the present study reinforces the notion that consuming fruits and vegetables, or their derived products, can have significant benefits on the CVD risk profile.
Acknowledgements
This study was supported by an unrestricted grant from the Canadian Cranberry Growers Coalition. Charles Couillard, Simone Lemieux and Patrick Couture are research scholars from the Fonds de la recherche en santé du Québec (FRSQ). Charles Couillard is also supported by the Chair in Nutrition, Lipidology and Cardiovascular Disease of Laval University. Benoît Lamarche holds a Canada Research Chair in Nutrition and Cardiovascular Health. The authors had no conflicts of interest to disclose. G. R. was responsible for data collection, data analysis and drafting of the manuscript. S. P. recruited the subjects and contributed to data collection and analysis. P. C. was responsible of the medical monitoring of the subjects during the intervention. S. L. was responsible for the conception and design of the study. B. L. was responsible for the conception and design of the study and provided assistance for statistical analyses. C. C. was responsible for the conception and design of the study as well as obtaining funding for the study. All authors were also implicated in the critical review of the manuscript for important intellectual content. The authors would like to thank Marge Leahy PhD and Robin Roderick MSc from Ocean Spray Cranberries Inc. for kindly assessing the composition of CJC and placebo juice as well as supervising the packaging sessions at Laval University. We also acknowledge the contributions of Danielle Aubin (R.N) as well as Mélanie Martineau, Jocelyne Giasson, Raoul Géra, Pascal Cliche and Bernard Béliveau (Food Transformation Laboratory, Laval University). Finally, we would like to thank the subjects without whom no clinical research would be possible.










