Implications
Sperm interaction with the cow reproductive tract after semen deposition has a profound influence on pregnancy rates and provides perplexing fundamental questions that are unresolved despite considerable study. The fertilizing sperm are selected by the tract from the millions or billions of sperm deposited at mating or artificial insemination (AI). Successful sperm interact with luminal fluid and epithelia, while evading destruction by the immune system. They respond to rheotactic, chemical and adhesive stimuli to undergo functional changes and arrive at the site of fertilization. An understanding of how these processes are coordinated can improve in vitro fertilization success, contraception effectiveness, sperm lifespan in the oviduct, improved semen storage and fertility.
Introduction
Normally only one sperm fertilizes an oocyte despite that billions of sperm are deposited by natural mating into the vagina, or millions are deposited by AI into the uterus of a cow. The remarkable journey that successful sperm take to reach the oocyte is long and tortuous, filled with viscous fluid, dead ends and potentially hostile immune cells. Rather than a simple race to get to the oocyte, there is much evidence that complex mechanisms influence sperm transport, immunological tolerance of sperm, sperm selection, sperm storage and release, all before actual fertilization. At steps along the way to the site of fertilization, sperm may interact with the fluid in which they are suspended and the epithelium lining the tract. The very dynamic process of sperm transport helps ensure that there is an appropriate number of fertile sperm at the site of fertilization so that the oocyte can be fertilized by only one sperm. This review considers sperm interaction with fluid in the reproductive tract as well as sperm adhesion to the epithelium. It also reviews how sperm, foreign cells in the female reproductive tract, are tolerated by the immune system. Although it emphasizes literature about cattle, concepts developed in other species are included.
Sperm in the vagina and cervix
Sperm are transported through the vagina, cervix and uterus to the oviduct where they can fertilize oocytes. In cattle and many other mammals, estrus occurs before ovulation so sperm are deposited in the female reproductive tract before ovulation. At normal copulation in cattle, semen is deposited in the cranial vagina. Vaginal fluid is the first luminal medium to which sperm are exposed after semen deposition. The acidic pH of the vagina makes it inhospitable for sperm, although buffers found in semen neutralize the local pH. The cow produces a large volume of vaginal fluid and up to 100 ml can accumulate (reviewed by Rutllant et al., Reference Rutllant, Lopez-Bejar and Lopez-Gatius2005). The rheological properties of vaginal fluid appear to influence sperm motility characteristics, although fertilizing sperm may spend only a short time in the vagina (Rutllant et al., Reference Rutllant, Lopez-Bejar and Lopez-Gatius2005).
It is likely that bovine sperm, like human sperm (Suarez and Pacey, Reference Suarez and Pacey2006), that are candidates to fertilize oocytes enter the cervical canal quickly avoiding damage due to the low vaginal pH. The cervix contains many folds and grooves that are filled with mucus. The mucus within the canal is a major barrier to sperm, particularly those that have abnormal motility (Katz et al., Reference Katz, Slade and Nakajima1997). The composition and structure of cervical mucus changes near estrus, allowing sperm with normal motility to advance, typically through what have been called ‘privileged paths’ that are found in the grooves produced by folds that extend through the cervical canal (Mullins and Saacke, Reference Mullins and Saacke1989). A microfluidic model has confirmed that sperm migration through these privileged paths is controlled by microgrooves and a gentle flow of fluid (Tung et al., Reference Tung, Hu, Fiore, Ardon, Hickman, Gilbert, Suarez and Wu2015b).
Sperm are foreign cells and can induce an immune response in the cervix. In rabbits, neutrophil infiltration was observed within 30 min of mating (Tyler, Reference Tyler1977). Immunoglobulins IgG and IgA (Kutteh et al., Reference Kutteh, Prince, Hammond, Kutteh and Mestecky1996) and complement proteins have been detected in human cervical mucus (Mathur et al., Reference Mathur, Rosenlund, Carlton, Caldwell, Barber, Rust and Williamson1988). Therefore, sperm retained in the cervix might be attacked by the immune system before moving into the uterus.
Sperm in the uterus
After natural mating, sperm move from the cervical canal into the uterus. In cattle, AI is used frequently. When performing AI, the technician deposits semen directly into the uterine body, so sperm do not enter the vagina and cervix. Depositing sperm directly in the uterus reduces the number of sperm needed for routine AI to 10-20 million (Moore and Hasler, Reference Moore and Hasler2017). As few as two million sperm are often inseminated when using sperm separated based on their sex chromosome, a process used to bias the sex of offspring (DeJarnette et al., Reference DeJarnette, Nebel and Marshall2009). Experiments in which the uterotubal junction (UTJ) in heifers was ligated at various times after mating revealed that it took 6-8 h for sperm to move through the cervix and uterus to infiltrate the oviduct in numbers sufficient for oocyte fertilization (Wilmut and Hunter, Reference Wilmut and Hunter1984). Sperm are transported through the uterus with the aid of uterine smooth muscle contractions in the direction of the oviduct (Hawk, Reference Hawk1987). To measure fluid movement and uterine contractions, technetium-labeled albumin-macrospheres were deposited in the uterus of women. These macrospheres (5-40 μm diameter) could be detected by high-resolution ultrasound. They were transported from the uterus to the oviduct more rapidly in the late follicular phase (Kunz et al., Reference Kunz, Beil, Deininger, Wildt and Leyendecker1996) which, along with other experiments, indicates that uterine contractions that transport sperm are under endocrine control. Further, this result demonstrates that materials in addition to sperm can move through the UTJ.
Sperm in the uterus of cattle and other species are retained in uterine glands in low numbers per gland (Hunter, Reference Hunter1995; Rijsselaere et al., Reference Rijsselaere, Van Soom, Van Cruchten, Coryn, Gortz, Maes and de Kruif2004). Retention, at least in swine, is accomplished by sperm binding to uterine epithelial cells (Rath et al., Reference Rath, Knorr and Taylor2016). Sperm attachment to uterine cells stimulates the production of both pro- and anti-inflammatory cytokines (Lovell and Getty, Reference Lovell and Getty1968). There is evidence that porcine sperm bind to sialic acid-containing glycans on the surface of uterine epithelial cells (Rath et al., Reference Rath, Knorr and Taylor2016). For example, a sialic acid lectin that recognizes sialic acid binds to uterine epithelial cells and blocks sperm binding, in vitro. Although it is not clear whether many sperm in uterine glands move into the oviduct, the fate of the majority of sperm in the uterus is elimination.
Rapid removal of sperm may help reduce the acquired immune response against sperm (Hansen, Reference Hansen2011). Little is known about the immune response elicited by semen deposition in cattle but it has been studied more in rodents and horses (Katila, Reference Katila2012; Bromfield, Reference Bromfield2014; Christoffersen and Troedsson, Reference Christoffersen and Troedsson2017). The primary function of the inflammatory response is to clear excess sperm, seminal debris and bacteria from the uterus. Following semen deposition, there is an infiltration of polymorphonuclear leukocytes (PMNs). In addition to activation of innate immunity, adaptive immunity is also involved. Several classes of antibodies have been isolated from uterine fluid. In addition to cytokines released from the uterine endometrium, seminal plasma itself contains immune system modulators that affect uterine and oviduct immune cells (Robertson, Reference Robertson2007; Schjenken and Robertson, Reference Schjenken and Robertson2014 and Reference Schjenken and Robertson2015). There is evidence that a seminal vesicle protein may allow the uterus to tolerate sperm (Kawano et al., Reference Kawano, Araki, Yoshida, Hibino, Ohnami, Makino, Kanai, Hasuwa, Yoshida, Miyado and Umezawa2014). Interestingly, the seminal fluid fraction of semen also improves preimplantation development and has interesting long-term effects on offspring (Bromfield et al., Reference Bromfield, Schjenken, Chin, Care, Jasper and Robertson2014). This non-traditional role of seminal plasma has been studied most in rodents; the amount of seminal plasma in cattle that mate normally is low and even lower when AI is used.
Sperm entry into the oviduct through the utero-tubal junction
In the bovine UTJ, sperm move through a slit-like lumen with a mucosal pad and into the lower portion of the oviduct, the isthmus, which contains four to eight primary grooves in tubal segment (Wrobel et al., Reference Wrobel, Kujat and Fehle1993). Compared with the major part of the upper oviduct, the ampulla, the isthmus has a narrower lumen with fewer folds but a thicker layer of smooth muscle. Although macrospheres seem to have the ability to pass through the UTJ (discussed above), there is evidence that sperm, at least in mice, require a specific protein to be recognized and to pass through the UTJ into the isthmus. Mouse sperm deficient in ADAM3, due to mutation of the ADAM3 gene or genes whose products affect ADAM3 are not detected beyond the UTJ (Nakanishi et al., Reference Nakanishi, Isotani, Yamaguchi, Ikawa, Baba, Suarez and Okabe2004; Yamaguchi et al., Reference Yamaguchi, Yamagata, Ikawa, Moss and Okabe2006; Yamaguchi et al., Reference Yamaguchi, Muro, Isotani, Tokuhiro, Takumi, Adham, Ikawa and Okabe2009; Okabe, Reference Okabe2013). Even if sperm from a chimeric male derived from a normal and a mutant embryo were deposited, only the normal sperm moved into the oviduct (Nakanishi et al., Reference Nakanishi, Isotani, Yamaguchi, Ikawa, Baba, Suarez and Okabe2004). Thus, the presence of normal sperm does not aid in opening the UTJ to allow ADAM3 mutant sperm to pass into the oviduct.
In addition to ADAM3, there also appears to be a rheological barrier in the porcine UTJ, perhaps the viscous mucus present in the grooves of this structure (Hunter, Reference Hunter2002; Tienthai, Reference Tienthai2015). The rabbit and mouse UTJ and oviduct fluid contain proteoglycans with sulfated glycosaminoglycan chains and hyaluronan (Jansen, Reference Jansen1978; Suarez et al., Reference Suarez, Brockman and Lefebvre1997). In addition to changing the viscosity and affecting sperm motility, the abundance of hyaluronan in fluid and its receptor, CD44 on the epithelial cells of the UTJ, suggest that CD44 signal transduction might affect the function of the UTJ and lower oviduct (Bergqvist et al., Reference Bergqvist, Yokoo, Bage, Sato and Rodriguez-Martinez2005a and Reference Bergqvist, Yokoo, Heldin, Frendin, Sato and Rodriguez-Martinez2005b).
In cattle and other species, there appears to be a valve at the UTJ that can constrict the lumen, restricting sperm entry. This valve is formed by a vascular plexus and surrounded by a thick muscle layer that, in total, can contract the lumen (Wrobel et al., Reference Wrobel, Kujat and Fehle1993). The physical constriction, mucus barrier and protein signature requirements emphasize how stringently entrance to the oviduct is regulated.
Sperm in the oviduct
Once sperm enter the lower oviduct, the isthmus, they can bind to the epithelial cell surface or remain in oviduct fluid. Many studies of the intact oviduct have been performed in mice because the uterus and oviduct can be transilluminated so that sperm can be observed (Demott and Suarez, Reference Demott and Suarez1992). Sperm from transgenic mice that have enhanced green fluorescent protein in their acrosomes and red fluorescent protein in their midpiece mitochondria have been followed in the female tract after natural mating (La Spina et al., Reference La Spina, Puga Molina, Romarowski, Vitale, Falzone, Krapf, Hirohashi and Buffone2016). The location of live sperm and their acrosomal status can be followed using fluorescence microscopy.
When sperm in the lumen of the isthmus were observed, groups of sperm were carried by fluid that was moved alternately toward the uterus and then toward the ampulla (back and forth) by contractions of oviduct smooth muscle (Ishikawa et al., Reference Ishikawa, Usui, Yamashita, Kanemori and Baba2016). These contractions were not observed in the ampulla. Most of the sperm in the isthmus were acrosome-intact (La Spina et al., Reference La Spina, Puga Molina, Romarowski, Vitale, Falzone, Krapf, Hirohashi and Buffone2016). Relatively few sperm were found in the ampulla and most were acrosome-reacted (La Spina et al., Reference La Spina, Puga Molina, Romarowski, Vitale, Falzone, Krapf, Hirohashi and Buffone2016; Muro et al., Reference Muro, Hasuwa, Isotani, Miyata, Yamagata, Ikawa, Yanagimachi and Okabe2016), consistent with the recent evidence that the acrosome reaction of fertilizing mouse sperm occurs before contact with the cumulus–oocyte complex (Jin et al., Reference Jin, Fujiwara, Kakiuchi, Okabe, Satouh, Baba, Chiba and Hirohashi2011; La Spina et al., Reference La Spina, Puga Molina, Romarowski, Vitale, Falzone, Krapf, Hirohashi and Buffone2016).
Oviduct fluid affects sperm function
The fluid in the oviduct is highly viscous, unlike the culture medium in which studies of mammalian fertilization are usually performed. Fluid viscosity is often overlooked in studies of sperm function within the oviduct. More viscous fluid has more internal friction so the wake from a sperm swimming in viscous medium is relatively small compared with less viscous medium (Kirkman-Brown and Smith, Reference Kirkman-Brown and Smith2011). Studies of human sperm demonstrate that resistance of the fluid to be moved results in a sperm tail with multiple bends while beating (Kirkman-Brown and Smith, Reference Kirkman-Brown and Smith2011; Hyakutake et al., Reference Hyakutake, Suzuki and Yamamoto2015). In contrast, in less viscous medium, the tail has fewer bends and, instead, remains mostly straight while simply swinging or flapping back and forth (Kirkman-Brown and Smith, Reference Kirkman-Brown and Smith2011; Hyakutake et al., Reference Hyakutake, Suzuki and Yamamoto2015). Consequently, in viscous fluid, a motile sperm will have less side-to-side movement (yaw) than in a standard viscosity medium (Kirkman-Brown and Smith, Reference Kirkman-Brown and Smith2011). Sperm also tend to swim near and against solid surfaces, for example, epithelial walls or the corners of microchannels (Denissenko et al., Reference Denissenko, Kantsler, Smith and Kirkman-Brown2012). Sperm that are close to the channel wall swim faster than those moving in the center of the channel (El-Sherry et al., Reference El-Sherry, Elsayed, Abdelhafez and Abdelgawad2014). Viscoelastic medium induces bovine sperm to swim in coordinated groups that may facilitate sperm migration (Tung et al., Reference Tung, Lin, Harvey, Fiore, Ardon, Wu and Suarez2017). The majority of sperm orient their swimming so that they swim against the flow of medium when the flow rate is intermediate (33-134 μm/s) (Miki and Clapham, Reference Miki and Clapham2013; El-Sherry et al., Reference El-Sherry, Elsayed, Abdelhafez and Abdelgawad2014; Tung et al., Reference Tung, Ardon, Roy, Koch, Suarez and Wu2015a). This appears to guide sperm upstream in oviduct fluid (Miki and Clapham, Reference Miki and Clapham2013). There is controversy about whether a signaling process in sperm aids in orienting sperm in the upstream direction or if sperm rheotaxis is a passive process (Miki and Clapham, Reference Miki and Clapham2013; Hyakutake et al., Reference Hyakutake, Suzuki and Yamamoto2015).
Interestingly, the viscosity of oviduct fluid varies during the estrous cycle; tenacious mucus is found in the rabbit oviduct lumen at estrus and disappears after ovulation (Jansen, Reference Jansen1978). Most studies of sperm–oviduct interaction or fertilization have used standard culture medium and ignored its low viscosity, compared with oviduct fluid. A few have tried to recapitulate the viscosity of oviduct fluid by adding components like methylcellulose or polyvinylpyrrolidone to medium (Suarez and Dai, Reference Suarez and Dai1992; Alasmari et al., Reference Alasmari, Costello, Correia, Oxenham, Morris, Fernandes, Ramalho-Santos, Kirkman-Brown, Michelangeli, Publicover and Barratt2013; Gonzalez-Abreu et al., Reference Gonzalez-Abreu, Garcia-Martinez, Fernandez-Espin, Romar and Gadea2017). In addition to effects on normal motility, discussed above, physiological viscosity converts the wild thrashing motion and high yaw of hyperactivated sperm to motility with less yaw and a more forward movement (Suarez and Dai, Reference Suarez and Dai1992).
In addition to the rheological properties of oviduct fluid, specific components of oviduct fluid such as secreted proteins, proteoglycans and lipids may influence fertilization by affecting sperm function (Coy et al., Reference Coy, Lloyd, Romar, Satake, Matas, Gadea and Holt2010; Killian, Reference Killian2011). This complex fluid can affect sperm before encountering the oocyte and during fertilization (Rodriguez-Martinez, Reference Rodriguez-Martinez2007; Killian, Reference Killian2011). For example, bovine sperm take up phospholipids that are abundant in oviduct fluid (Evans and Setchell, Reference Evans and Setchell1978; Killian et al., Reference Killian, Chapman, Kavanaugh, Deaver and Wiggin1989). Oviduct fluid glutathione peroxidase, superoxide dismutase and catalase can protect bovine sperm from damage by reactive oxygen species that may otherwise reduce sperm viability and motility (Lapointe and Bilodeau, Reference Lapointe and Bilodeau2003). Proteoglycans found in oviduct fluid promote capacitation of bovine sperm through their glycosaminoglycan side chains (Parrish et al., Reference Parrish, Susko-Parrish, Handrow, Sims and First1989; Bergqvist et al., Reference Bergqvist, Ballester, Johannisson, Hernandez, Lundeheim and Rodriguez-Martinez2006).
Oviduct fluid components, for example, glycosaminoglycans, can also cause proteolysis or loss of sperm membrane proteins, including those that are implicated in sperm binding to the oviduct epithelium. The best studied of these proteins originate from accessory gland secretions and bind to sperm at ejaculation. Some bovine Binder of Sperm (BSPs) and porcine sperm adhesins are lost as sperm are capacitated (Topfer-Petersen et al., Reference Topfer-Petersen, Ekhlasi-Hundrieser and Tsolova2008; Hung and Suarez, Reference Hung and Suarez2010). Although the significance of protein loss or proteolysis is uncertain, in sperm bound to the oviduct epithelium, it might contribute to their release before fertilization (Topfer-Petersen et al., Reference Topfer-Petersen, Ekhlasi-Hundrieser and Tsolova2008; Hung and Suarez, Reference Hung and Suarez2010).
In addition to losing proteins, sperm also gain proteins while they reside in the oviduct. The first of two examples is oviduct-specific glycoprotein (OGP) or oviductin, also known as OVGP1, found in oviducts of many mammals. Although it has homology to the chitinase family of proteins, OGP does not have enzymatic activity (Jaffe et al., Reference Jaffe, Arias, O’Day-Bowman, Donnelly, Mavrogianis and Verhage1996; Araki et al., Reference Araki, Nohara, Yoshida-Komiya, Kuramochi, Ito, Hoshi, Shinkai and Sendai2003). Bovine sperm incubated in OGP have improved motility and viability (Abe et al., Reference Abe, Sendai, Satoh and Hoshi1995). Hamster sperm treated with recombinant OGP have increased phosphorylation of tyrosine residues on proteins, an indication that capacitation was enhanced (Yang et al., Reference Yang, Zhao, Yang and Kan2015). There is also evidence in mice and swine that OGP binds to the zona pellucida to increase fertilization success by rendering the zona matrix more permissive to penetration by sperm (Lyng and Shur, Reference Lyng and Shur2009; Algarra et al., Reference Algarra, Han, Soriano-Ubeda, Aviles, Coy, Jovine and Jimenez-Movilla2016).
A second example of an oviduct protein that affects sperm is osteopontin. Although it is already bound to bovine sperm before semen is deposited in females (Erikson et al., Reference Erikson, Way, Chapman and Killian2007), addition of osteopontin during in vitro fertilization reduces polyspermy (Goncalves et al., Reference Goncalves, Chapman, Bertolla, Eder and Killian2008). Neither osteopontin nor OGP is necessary for fertility in mice because animals deficient in each are fertile (Rittling et al., Reference Rittling, Matsumoto, McKee, Nanci, An, Novick, Kowalski, Noda and Denhardt1998; Araki et al., Reference Araki, Nohara, Yoshida-Komiya, Kuramochi, Ito, Hoshi, Shinkai and Sendai2003).
In addition to oviduct fluid proteins being added as peripheral membrane proteins, integral membrane proteins could be added by fusion with sperm of oviductosomes secreted by the oviduct. For example, a portion of the major Ca2+ efflux pump is added to mouse sperm by oviduct exosomes (Al-Dossary et al., Reference Al-Dossary, Bathala, Caplan and Martin-DeLeon2015). The proteins secreted by bovine oviduct cells and found in oviduct fluid have recently been profiled and include growth factors, metabolic regulators, immune modulators, enzymes and extracellular matrix components (Lamy et al., Reference Lamy, Labas, Harichaux, Tsikis, Mermillod and Saint-Dizier2016; Pillai et al., Reference Pillai, Weber, Phinney and Selvaraj2017). They function in immune homeostasis, gamete maturation, fertilization and early development (Pillai et al., Reference Pillai, Weber, Phinney and Selvaraj2017). The abundance of some depend on the stage of the estrous cycle and whether they were found in oviducts ipsilateral or contralateral to the ovary that ovulated (Lamy et al., Reference Lamy, Labas, Harichaux, Tsikis, Mermillod and Saint-Dizier2016).
The oviduct as a functional sperm reservoir
The oviduct, along with the UTJ in some species, appears to be the major location in which sperm are stored before fertilization. In contrast, although sperm are retained in the cervix or uterus, it is not clear that they are eventually released to move to the oviduct. So the UTJ and oviduct appear to be the major sperm storage sites in many mammals. To be a true ‘functional sperm reservoir’, as coined by Hunter et al. (Reference Hunter, Nichol and Crabtree1980), in addition to retaining sperm, the oviduct must affect sperm function and lengthen sperm lifespan beyond the inherent longevity of sperm (Orr and Zuk, Reference Orr and Zuk2014). More than simple adhesion occurs because binding to the oviduct epithelium prolongs the lifespan of sperm and suppresses capacitation and motility (Pollard et al., Reference Pollard, Plante, King, Hansen, Betteridge and Suarez1991; Rodriguez-Martinez et al., Reference Rodriguez-Martinez, Saravia, Wallgren, Tienthai, Johannisson, Vazquez, Martinez, Roca, Sanz and Calvete2005; Rodriguez-Martinez, Reference Rodriguez-Martinez2007; Hung and Suarez, Reference Hung and Suarez2010). Thus, the oviduct isthmus meets these requirements. However, the ability of sperm reservoirs described in a variety of species to prolong the lifespan of a highly differentiated and transcriptionally inactive cell is enigmatic.
The reservoir also releases a finite number of stored sperm, acting as a buffer for sperm number to prevent polyspermy but still provide an appropriate number of fertile sperm to the upper oviduct (Hunter and Leglise, Reference Hunter and Leglise1971). The isthmic epithelium binds and retains preferentially sperm that have intact acrosomes and normal morphology (Teijeiro et al., Reference Teijeiro, Dapino and Marini2011; Teijeiro and Marini, Reference Teijeiro and Marini2012). All together, the isthmus functions to increase the probability that a suitable number of fertile sperm are present at the site of fertilization.
The oviduct epithelium retains sperm and modulates sperm function
In mammals, the oviduct epithelium binds and retains sperm so they accumulate to form the reservoir. Adhesion is very specific. The sperm head binds to oviduct epithelial cells but not all cells (Pacey et al., Reference Pacey, Hill, Scudamore, Warren, Barratt and Cooke1995; Kervancioglu et al., Reference Kervancioglu, Saridogan, Aitken and Djahanbakhch2000). Moreover, the ability of sperm binding to maintain viability is not a common property of all cells (Boilard et al., Reference Boilard, Bailey, Collin, Dufour and Sirard2002). The ability to maintain viability requires direct contact between sperm and oviduct epithelial cells (Dobrinski et al., Reference Dobrinski, Smith, Suarez and Ball1997; Murray and Smith, Reference Murray and Smith1997; Smith and Nothnick, Reference Smith and Nothnick1997). Adhesion to the oviduct regulates sperm capacitation (Dobrinski et al., Reference Dobrinski, Smith, Suarez and Ball1997; Boilard et al., Reference Boilard, Bailey, Collin, Dufour and Sirard2002; Fazeli et al., Reference Fazeli, Elliott, Duncan, Moore, Watson and Holt2003) and suppresses the normal increase in sperm intracellular free calcium that occurs during capacitation (Dobrinski et al., Reference Dobrinski, Ignotz, Thomas and Ball1996; Dobrinski et al., Reference Dobrinski, Smith, Suarez and Ball1997).
Studies performed in several mammals have concluded that glycans are the components in oviduct epithelial cells that bind sperm (Lefebvre et al., Reference Lefebvre, Lo and Suarez1997; Green et al., Reference Green, Bredl, Holt, Watson and Fazeli2001; Suarez, Reference Suarez2001; Cortes et al., Reference Cortes, Orihuela, Zuniga, Velasquez and Croxatto2004; Topfer-Petersen et al., Reference Topfer-Petersen, Ekhlasi-Hundrieser and Tsolova2008). The evidence in most studies underpinning a role for oviduct glycans is a competition assay in which different glycans are added to sperm before challenging these sperm by allowing them to bind oviduct epithelial cells in vitro. If few sperm bind to oviduct cells, this result is interpreted as an indication that the specific glycan is related to the authentic oviduct glycan that binds sperm. A frequent problem with these studies is that most test high concentrations of a small number of monosaccharides or small oligosaccharides.
Identification of glycans that bind porcine sperm using a glycan array
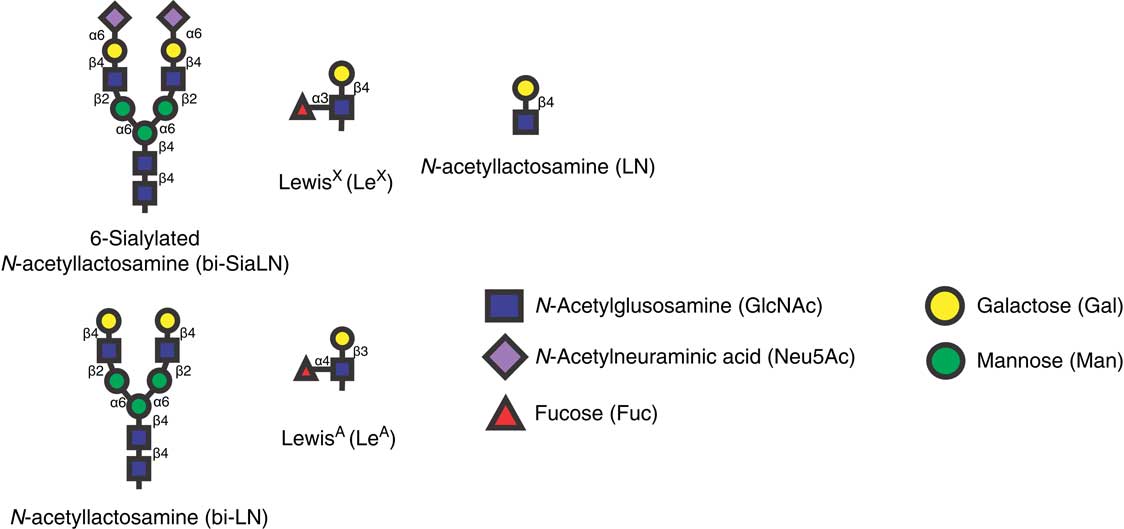
The development of glycans immobilized on an array provided an opportunity to test hundreds of glycans for their ability to bind sperm. Using such an array, nearly 400 glycans were tested for their ability to bind porcine sperm (Kadirvel et al., Reference Kadirvel, Machado, Korneli, Collins, Miller, Bess, Aoki, Tiemeyer, Bovin and Miller2012). All the glycans that bound sperm contained one of two glycan motifs, either a Lewis X trisaccharide (LeX) or a structure with core mannose and two antennae terminating in the sialylated lactosamine trisaccharide bi-SiaLN or in simply lactosamine (Figure 1). There were several examples demonstrating that sperm bound these two motifs with high specificity. In all sialic acid-containing structures that bound sperm, sialic acid was linked to the 6 position of galactose; structures that were identical except that sialic acid was attached to galactose at the 3 position did not bind sperm. Furthermore, the branched structure on a mannose core was required because single sialylated lactosamine trisaccharides (Neu5Acα2-6Galβ1-4GlcNAc) did not bind sperm (Kadirvel et al., Reference Kadirvel, Machado, Korneli, Collins, Miller, Bess, Aoki, Tiemeyer, Bovin and Miller2012).

Figure 1 Structures of glycans that bind bovine (LeA) and porcine sperm (bi-SiaLN, bi-LN, and LeX), and the related glycan that does not (LN). LeA is found on the bovine oviduct epithelium. bi-SiaLN is abundant on the epithelium of the porcine ampulla and isthmus including ciliated and non-ciliated cells. LeX is found in the porcine isthmus but not the ampulla.
The LeX trisaccharide was found as a monomer, dimer or trimer in the remaining glycans that bound sperm (Kadirvel et al., Reference Kadirvel, Machado, Korneli, Collins, Miller, Bess, Aoki, Tiemeyer, Bovin and Miller2012). This trisaccharide is composed of Gal and Fuc linked to GlcNAc (Figure 1). The LeX trisaccharide also bound sperm with high specificity; the closely related Lewis A trisaccharide (LeA, a positional isomer; the carbons in GlcNAc to which Gal and Fuc are linked are exchanged) did not bind porcine sperm. Contrarily, bovine sperm bind LeA but not LeX (Suarez et al., Reference Suarez, Revah, Lo and Kolle1998). Binding specificity was further supported because porcine sperm did not bind to Galβ1-4GlcNAc; fucose substitution on LeX was necessary (Kadirvel et al., Reference Kadirvel, Machado, Korneli, Collins, Miller, Bess, Aoki, Tiemeyer, Bovin and Miller2012).
To confirm that the glycans on the array that bound sperm were present in the oviduct isthmus and to determine the complete structures of the oviduct glycans that bound sperm, oviduct glycans and glycolipid structures were identified by tandem MS (Kadirvel et al., Reference Kadirvel, Machado, Korneli, Collins, Miller, Bess, Aoki, Tiemeyer, Bovin and Miller2012). The LeX and branched sialylated motifs (bi-SiaLN) that bound sperm were found on larger structures that were the most abundant of the complex-type glycans on epithelial cells (Kadirvel et al., Reference Kadirvel, Machado, Korneli, Collins, Miller, Bess, Aoki, Tiemeyer, Bovin and Miller2012). Nearly all of the complex-type oligosaccharides linked to proteins through asparagine residues were branched with two antennae and several had a sialyl residue on at least one terminus. Some biantennary glycans had both motifs, a sialyl residue on one terminus and a Lewis structure on the second. This kind of hybrid glycan was not present on the array but it is possible that, because it includes both motifs, it might bind sperm with even higher affinity than glycans with a single motif.
As tandem MS did not distinguish between LeA and LeX and between glycans with sialyl residues attached to the 6-carbon and the 3-carbon of Gal, an additional strategy was used. An antibody and specific lectin, Sambucus nigra agglutinin were used that recognize sialic acid attached to galactose in an α-2,6 linkage preferentially and not sialic acid attached to galactose in an α-2,3 linkage (Naito et al., Reference Naito, Takematsu, Koyama, Miyake, Yamamoto, Fujinawa, Sugai, Okuno, Tsujimoto, Yamaji, Hashimoto, Itohara, Kawasaki, Suzuki and Kozutsumi2007; Song et al., Reference Song, Yu, Chen, Lasanajak, Tappert, Air, Tiwari, Cao, Chokhawala, Zheng, Cummings and Smith2011). Both reagents detected 6-sialylated structures that were abundant on the epithelium throughout the oviduct including on ciliated and non-ciliated cells (Kadirvel et al., Reference Kadirvel, Machado, Korneli, Collins, Miller, Bess, Aoki, Tiemeyer, Bovin and Miller2012).
Similarly, an antibody to LeX was also used to confirm the identity of the oviduct Lewis trisaccharide structures identified by MS (Kadirvel et al., Reference Kadirvel, Machado, Korneli, Collins, Miller, Bess, Aoki, Tiemeyer, Bovin and Miller2012). Interestingly, LeX was found in a punctate pattern at the luminal surface of porcine isthmic epithelial cells (Machado et al., Reference Machado, Kadirvel, Daigneault, Korneli, Miller, Bovin and Miller2014) but was not found in the ampulla.
bi-SiaLN and LeX glycan motifs bind to the porcine sperm head
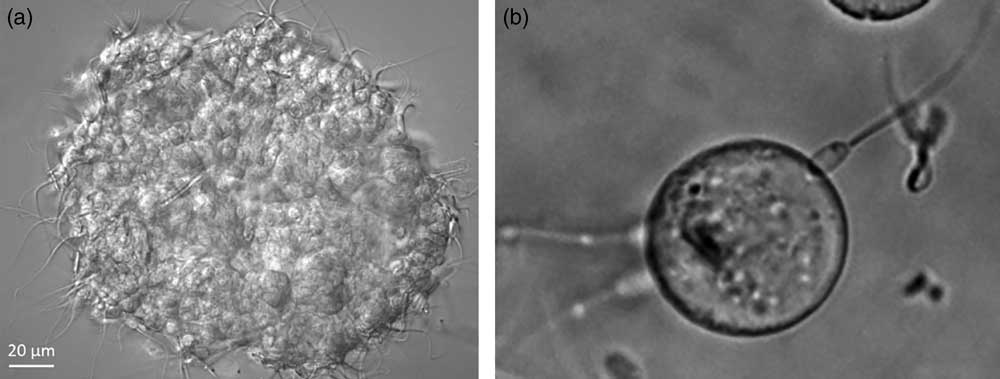
The head is the portion of sperm that binds to the oviduct epithelium and is where (Suarez et al., Reference Suarez, Redfern, Raynor, Martin and Phillips1991) authentic receptors for glycans with bi-SiaLN and/or LeX motifs should be localized. Fluorescein-labeled LeX and bi-SiaLN bound preferentially to the apical edge of the head in 60%-70% sperm before capacitation (Kadirvel et al., Reference Kadirvel, Machado, Korneli, Collins, Miller, Bess, Aoki, Tiemeyer, Bovin and Miller2012; Machado et al., Reference Machado, Kadirvel, Daigneault, Korneli, Miller, Bovin and Miller2014). Binding of fluoresceinated glycans could be displaced by an excess of the same glycan that did not have a fluorescent tag. The binding specificity was confirmed by testing sperm binding to oviduct glycans attached to Sepharose beads (Figure 2). Tethering a motile cell to a solid phase glycan rather than a soluble glycan more closely mimics sperm binding to the oviduct and requires a higher affinity.
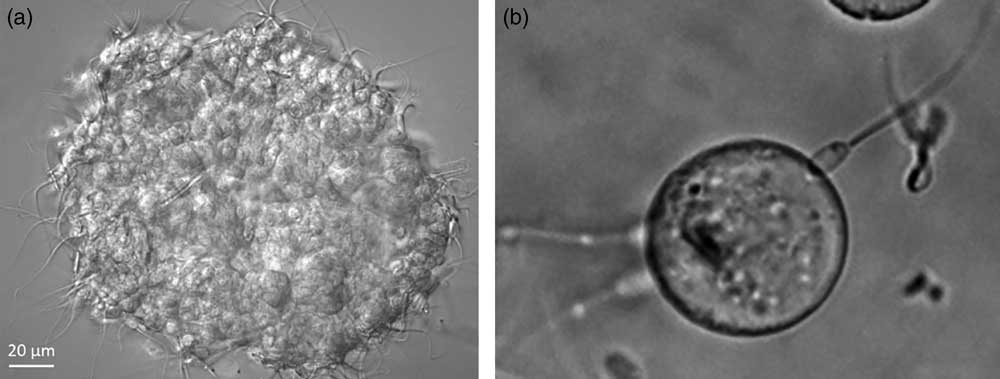
Figure 2 Sperm bind to oviduct cell aggregates isolated from the isthmus (a; porcine sperm) and beads to which Lewis A (LeA) trisaccharide has been attached (b; bovine sperm).
Porcine sperm binding to oviduct cells requires glycans with bi-SiaLN and LeX
Experiments using immobilized glycans (i.e. the glycan array and glycans linked to Sepharose) showed that bi-SiaLN and LeX were each sufficient to tether a motile sperm. Necessity experiments were performed in which either the glycans or putative receptors were blocked. The result of blocking was assessed by sperm binding to aggregates of epithelial cells stripped from the isthmus (Figure 2). Results of these experiments indicated that each glycan or glycan receptor was necessary for sperm to bind oviduct cells.
Receptors on sperm for oviduct glycans
The identity of the receptors that bind oviduct glycans are controversial. Sperm from different species bind different glycans and the receptors they use may also be unique. Using bovine cells, one group provided evidence that two oviduct proteins, the chaperones GRP78 and HSP60, bound to sperm, although the glycans each bound were not determined (Boilard et al., Reference Boilard, Reyes-Moreno, Lachance, Massicotte, Bailey, Sirard and Leclerc2004). A second group completed more detailed studies by and proposed that oviduct plasma membrane annexins containing fucose bind sperm proteins originating from accessory gland secretions added to sperm at ejaculation (Ignotz et al., Reference Ignotz, Cho and Suarez2007). This result was a bit surprising because annexins are usually considered cytosolic proteins and they lack signal peptides that would direct them through the secretory pathway to become fucosylated. A proteomic study found that annexin A1 is the most abundant protein in oviduct fluid (Lamy et al., Reference Lamy, Labas, Harichaux, Tsikis, Mermillod and Saint-Dizier2016). Perhaps it is released into fluid without passing through the secretory pathway. However, in the fluid, it would be expected to compete with annexin A1 located on oviduct epithelial cells and decrease sperm binding to the oviduct.
Studies of porcine sperm also implicated accessory gland secretions added to sperm (Ekhlasi-Hundrieser et al., Reference Ekhlasi-Hundrieser, Gohr, Wagner, Tsolova, Petrunkina and Topfer-Petersen2005; Topfer-Petersen et al., Reference Topfer-Petersen, Ekhlasi-Hundrieser and Tsolova2008). The spermadhesin AQN1 originating from accessory gland secretions is a glycan-binding protein (Ekhlasi-Hundrieser et al., Reference Ekhlasi-Hundrieser, Gohr, Wagner, Tsolova, Petrunkina and Topfer-Petersen2005; Topfer-Petersen et al., Reference Topfer-Petersen, Ekhlasi-Hundrieser and Tsolova2008). Spermadhesins represent 90% of the total boar seminal plasma protein and they become peripherally associated with the sperm plasma membrane after ejaculation (Sanz et al., Reference Sanz, Calvete, Mann, Gabius and Topfer-Petersen1993). Sperm AQN1 is reported to bind mannose and galactose residues on oviduct cells, but not LeX or bi-SiaLN structures (Ekhlasi-Hundrieser et al., Reference Ekhlasi-Hundrieser, Gohr, Wagner, Tsolova, Petrunkina and Topfer-Petersen2005).
The observation that the accessory gland proteins do not bind LeX and bi-SiaLN motifs (Ekhlasi-Hundrieser et al., Reference Ekhlasi-Hundrieser, Gohr, Wagner, Tsolova, Petrunkina and Topfer-Petersen2005) and sperm obtained from the cauda epididymis are still able to bind oviduct cells, although in reduced number (Petrunkina et al., Reference Petrunkina, Gehlhaar, Drommer, Waberski and Topfer-Petersen2001), suggested that other glycan receptors were important. Indeed, in cattle there is no evidence that the fertility of epididymal sperm, not exposed to accessory gland proteins, is lower that normal ejaculated semen that includes accessory gland secretions (Amann and Griel, Reference Amann and Griel1974). The fertility of cauda epididymal sperm motivated the investigation of glycan receptors on porcine sperm from the epididymis, which also avoided interference from the very abundant accessory gland proteins (Silva et al., Reference Silva, Kadirvel, Jiang, Bovin and Miller2014).
Membrane lysates from porcine cauda epididymal sperm were separated chromatographically and each fraction was subjected to SDS-PAGE, transferred to nitrocellulose and incubated with biotinylated LeX and bi-SiaLN. This ‘glycan blot’ was used to identify proteins with appropriate glycan affinity. Several proteins were identified including the peripheral membrane protein MFG-E8, also known as lactadherin, P47 or SED1 (Silva et al., Reference Silva, Frost, Li, Bovin and Miller2017). Competition experiments showed that lactadherin bound to oviduct cells and that inhibition reduced sperm binding (Silva et al., Reference Silva, Frost, Li, Bovin and Miller2017).
Although there is compelling evidence that oviduct glycans are at least partially responsible for sperm binding, there is also evidence that sperm binding to oviduct epithelial cells is mediated to some degree by other interactions. Perturbation of glycans or their candidate receptors decreases sperm binding to oviduct cell aggregates by a maximum of 60% (Kadirvel et al., Reference Kadirvel, Machado, Korneli, Collins, Miller, Bess, Aoki, Tiemeyer, Bovin and Miller2012; Machado et al., Reference Machado, Kadirvel, Daigneault, Korneli, Miller, Bovin and Miller2014). Protein-based interactions may account for the residual binding. For example, fibronectin from oviduct cells can bind α5β1 integrin on bovine sperm (Osycka-Salut et al., Reference Osycka-Salut, Castellano, Fornes, Beltrame, Alonso, Jawerbaum, Franchi, Diaz and Perez Martinez2017) and the adhesion protein E-cadherin is found in both sperm and oviduct epithelial cells (Pollard et al., Reference Pollard, Plante, King, Hansen, Betteridge and Suarez1991; Lefebvre et al., Reference Lefebvre, Chenoweth, Drost, LeClear, MacCubbin, Dutton and Suarez1995; Caballero et al., Reference Caballero, Gervasi, Veiga, Dalvit, Perez-Martinez, Cetica and Vazquez-Levin2014).
Oviduct epithelial cells respond to sperm binding
In addition to the effect of adhesion on sperm, sperm adhesion to the oviduct modifies the transcriptional profile of oviduct epithelial cells (Fazeli et al., Reference Fazeli, Affara, Hubank and Holt2004; Georgiou et al., Reference Georgiou, Sostaric, Wong, Snijders, Wright, Moore and Fazeli2005; Georgiou et al., Reference Georgiou, Snijders, Sostaric, Aflatoonian, Vazquez, Vazquez, Roca, Martinez, Wright and Fazeli2007; Lopez-Ubeda et al., Reference Lopez-Ubeda, Garcia-Vazquez, Romar, Gadea, Munoz, Hunter and Coy2015). Genes related to the inflammatory response, molecular transport, protein trafficking and cell-to-cell signaling are among those most affected by sperm (Lopez-Ubeda et al., Reference Lopez-Ubeda, Garcia-Vazquez, Romar, Gadea, Munoz, Hunter and Coy2015). In the sow, there is evidence that the ovary has a local effect on the transcriptome of the oviduct. Unilateral ovariectomy reduces expression of genes believed to be involved in sperm survival and early embryonic development (Lopez-Ubeda et al., Reference Lopez-Ubeda, Munoz, Vieira, Hunter, Coy and Canovas2016). The effect of sperm on the sperm reservoir appears conserved between birds and mammals. Infiltration of porcine sperm into the UTJ and rooster sperm into the chicken utero-vaginal junction alters the expression of genes involved in pH regulation and immune-modulation (Atikuzzaman et al., Reference Atikuzzaman, Alvarez-Rodriguez, Vicente-Carrillo, Johnsson, Wright and Rodriguez-Martinez2017). Even more surprisingly, the transcriptional response of oviduct cells is different in response to insemination of either X chromosome- or Y chromosome-bearing sperm (Alminana et al., Reference Alminana, Caballero, Heath, Maleki-Dizaji, Parrilla, Cuello, Gil, Vazquez, Vazquez, Roca, Martinez, Holt and Fazeli2014). Thus, the presence of sperm changes the behavior of oviduct cells in addition to its consequences for sperm. The result of altered production of specific proteins by oviduct cells is not clear.
Sperm release from oviduct epithelial cells
For successful fertilization, sperm must be released from the reservoir in the isthmus to meet the oocyte in the ampulla. There are several hypotheses to explain how sperm are released. The first is that a signal, perhaps from follicular fluid or the ovulated cumulus–oocyte complex stimulates the release of sperm. This would assure that some sperm are released at the appropriate time. An alternate hypothesis is that a small fraction of sperm is released almost continuously so that there is always a small number of sperm prepared to fertilize an oocyte. It is possible that both mechanisms exist; that is, release in response to a signal is superimposed on top of the more spontaneous release of fractions of sperm. In any case, sperm release is due to a change in sperm behavior, in oviduct cell function or in the oviduct fluid surrounding the cells.
An important maturation that sperm complete in the oviduct is capacitation. After capacitation, sperm have a reduced ability to bind oviduct glycans (Kadirvel et al., Reference Kadirvel, Machado, Korneli, Collins, Miller, Bess, Aoki, Tiemeyer, Bovin and Miller2012; Machado et al., Reference Machado, Kadirvel, Daigneault, Korneli, Miller, Bovin and Miller2014), supporting the hypothesis that during capacitation, glycan receptors are modified. How capacitation might affect glycan receptors is unclear, but there is some preliminary evidence in cattle and swine that, during capacitation, they may be targeted for proteolysis. The molecular mass of one of the BSPs is altered during capacitation (Hung and Suarez, Reference Hung and Suarez2012). Furthermore, a candidate glycan receptor on porcine sperm, MFG-E8, co-precipitates in sperm lysates with a proteasomal subunit suggesting it may also be degraded (Miles et al., Reference Miles, O’Gorman, Zhao, Samuel, Walters, Yi, Sutovsky, Prather, Wells and Sutovsky2013).
Another alternative is that the development of hyperactivated motility may be sufficient to detach a sperm from the oviduct epithelium (Curtis et al., Reference Curtis, Kirkman-Brown, Connolly and Gaffney2012). In support of this, mouse sperm deficient in CatSper calcium channels that cannot hyperactivate do not detach from the oviduct (Ho et al., Reference Ho, Wolff and Suarez2009).
There is evidence that the cumulus cells of the ovulated cumulus–oocyte complex can release chemical signals, such as progesterone (Schoenfelder et al., Reference Schoenfelder, Schams and Einspanier2003; Tosca et al., Reference Tosca, Uzbekova, Chabrolle and Dupont2007), which might activate localized sperm release by promoting Ca2+ influx through CatSper channels (Lishko et al., Reference Lishko, Kirichok, Ren, Navarro, Chung and Clapham2012). Release may also be controlled by components from the oviduct itself, such as disulfide reducants (Talevi et al., Reference Talevi, Zagami, Castaldo and Gualtieri2007; Brussow et al., Reference Brussow, Ratky and Rodriguez-Martinez2008), glycosidases that cleave oviduct glycans from the epithelium (Carrasco et al., Reference Carrasco, Coy, Aviles, Gadea and Romar2008a and Reference Carrasco, Romar, Aviles, Gadea and Coy2008b), and oviduct smooth muscle contractions (Chang and Suarez, Reference Chang and Suarez2012). There is evidence that locally produced anandamide activates cannabinoid receptors and TRPV1 to induce a Ca2+ influx and sperm release (Gervasi et al., Reference Gervasi, Osycka-Salut, Sanchez, Alonso, Llados, Castellano, Franchi, Villalon and Perez-Martinez2016). Anandamide may also activate nitric oxide production by sperm to promote their release (Osycka-Salut et al., Reference Osycka-Salut, Gervasi, Pereyra, Cella, Ribeiro, Franchi and Perez-Martinez2012). Finally, the production of unknown sulfated glyconjugates may release sperm by competing for binding sites on the oviduct epithelium (Talevi and Gualtieri, Reference Talevi and Gualtieri2010). The dynamic nature of sperm interaction with the oviduct suggests that a variety of factors may regulate sperm release that may aid in providing a constant supply of competent fertilizing sperm.
Immunological tolerance of sperm in the oviduct
The oviduct lumen must maintain an aseptic state for successful fertilization and early embryonic development while regulating maternal responses to allogenic sperm and semi-allogenic embryos (Marey et al., Reference Marey, Yousef, Kowsar, Hambruch, Shimizu, Pfarrer and Miyamoto2016). Under pathologic conditions, the mucosal immune system produces a proinflammatory response. However, bovine sperm binding to oviduct epithelial cells induces an upregulation of IL-10, TGFβ and increased production of prostaglandin E2, inducing an anti-inflammatory response (Marey et al., Reference Marey, Yousef, Kowsar, Hambruch, Shimizu, Pfarrer and Miyamoto2016; Yousef et al., Reference Yousef, Marey, Hambruch, Hayakawa, Shimizu, Hussien, Abdel-Razek, Pfarrer and Miyamoto2016). This produces an environment that suppresses sperm phagocytosis by PMNs and allows sperm greater opportunity to survive in the oviduct and fertilize oocytes. In essence, sperm induce their own protection from an immune response in the oviduct.
Practical applications
Sperm reservoirs have a remarkable ability to prolong the viability of sperm and, if we can understand how that is accomplished, we may be able to modify sperm diluents to lengthen sperm lifespan outside of the oviduct (McGetrick et al., Reference McGetrick, Reid and Carrington2014). Being able to store bovine sperm for several days would be advantageous in regions of the world where liquid nitrogen is not available or where fresh semen is used routinely due to short transportation times before use (Vishwanath and Shannon, Reference Vishwanath and Shannon2000). There is already some evidence that addition of an oviduct protein can improve viability or bovine, porcine and caprine sperm after a 24-48 h incubation (Elliott et al., Reference Elliott, Lloyd, Fazeli, Sostaric, Georgiou, Satake, Watson and Holt2009; Lloyd et al., Reference Lloyd, Elliott, Fazeli, Watson and Holt2009 and Reference Lloyd, Fazeli, Watson and Holt2012; Holt et al., Reference Holt, Del Valle and Fazeli2015).
There is also another important application of this research, development of a method to increase sperm lifespan in the oviduct. If sperm survived longer after insemination or natural mating, cows that ovulated well after semen deposition would have a higher likelihood of pregnancy. This might reduce the requirement for frequent estrus detection in females because a precise estimate of ovulation time might not be as crucial. Fertility despite the uncoupling of mating with ovulation has been accomplished by some mammals, notably some species of bats that store sperm for months, as well as snakes, reptiles and insects (Holt and Fazeli, Reference Holt and Fazeli2016). Although the opportunity to reduce estrus detection by lengthening sperm lifespan may be overly optimistic, the examples in nature of species that store sperm for a long duration suggest that it may be possible.
Acknowledgments
Work in the author’s laboratory was supported by Agriculture and Food Research Initiative Competitive Grant No. 2011-67015-20099 and 2015-67015-23228 from the USDA National Institute of Food and Agriculture and the National Science Foundation. The author acknowledges Rebecca Winters and Lantana Grub for comments that improved the manuscript and apologizes for being unable to discuss other important work due to length restrictions.
Declaration of interest
The author has no conflict of interest.
Ethics Statement
None of the original studies described herein by the author used live animals.
Software and Data Repository Resources
Glycan array data are deposited with the Center for Functional Glycomics, which is publicly available.