INTRODUCTION
On 1 May 2009, the first two cases of 2009 pandemic influenza A(H1N1) were identified in France [Reference Vaux1]. Up to the end of June, all probable and confirmed cases of influenza A(H1N1) 2009 were hospitalized, whatever their severity. This policy was discontinued with the start of sustained community transmission. In order to assess the impact of the pandemic wave in terms of severe morbidity and mortality, a national surveillance of confirmed or probable hospitalized A(H1N1) 2009 influenza cases was set up on 1 July 2009 by the National Institute for Public Health Surveillance (Institut de Veille Sanitaire, InVS). On 2 November due to a sharp rise in the number of hospitalizations, this surveillance was restricted to patients with severe disease, essentially those admitted to intensive-care units (ICUs). At this time, consistent with reports from other countries, ICU admissions represented 20% of hospitalizations for influenza A(H1N1) [Reference Denholm2–4].
In this paper, we describe the incidence and characteristics of adult ICU admissions due to pandemic influenza A(H1N1) 2009 virus.
METHODS
In France, a nationwide hospital-based surveillance system was implemented on 1 July 2009. Clinicians were requested to report to the InVS all hospitalized cases of pandemic influenza through a standardized notification form available on the InVS website. Notification included patients with: (i) a positive RT–PCR performed on a nasal swab or bronchoalveolar lavage, (ii) severe clinical influenza, likely to be caused by 2009 pandemic influenza virus according to the clinician, even in the absence of laboratory confirmation, and (iii) clinical influenza and an epidemiological link with a confirmed case of pandemic influenza. In order to ensure a high completeness of notification, several procedures were implemented. First, the French Ministry of Health informed all hospital clinicians about the notification procedure. Second, until November 2010, the forms received were cross-checked with individual positive results from the network of laboratories performing the A(H1N1) 2009 RT–PCR. If a case with positive laboratory result was not notified, the clinician was contacted by phone, informed about the notification system and asked to return the notification form. This procedure was stopped in mid-November when the number of cases increased sharply. Instead, from mid-November, each of the 17 regional units of the InVS (CIRES) contacted the ICUs in their own region at least once a week to ensure that the notification procedure was used. Third, this hospital surveillance was strongly supported by all ICU medical practitioners' societies. Follow-up data for cases were collected weekly by telephone contact between InVS staff (epidemiologists or physicians) and physicians in charge of the patient until discharge or death. At this time, clinicians were asked to return a second notification form also available on the InVS website. On 2 November due to a sharp rise in the total number of hospitalizations, the surveillance of hospitalized pandemic influenza cases was restricted to patients requiring admission to an ICU or dying while hospitalized with suspected influenza.
This analysis concerns laboratory-confirmed cases in adults aged ⩾15 years, admitted to an ICU from 1 July 2009 to 15 February 2010. Patients transferred between ICUs were counted as a single admission. Data on follow-up were available up to 8 April 2010. All the data were collected through the standardized notification and follow-up forms completed by the clinician in charge of the patient. Risk factors were notified through a list of conditions including pregnancy, chronic respiratory diseases, asthma, diabetes, chronic cardiac disease, immunosuppression, obesity [defined as a body mass index (BMI) ⩾30 kg/m2], and morbid obesity (BMI ⩾40 kg/m2). In the present analysis, asthma was considered as a chronic respiratory disease. The clinician was asked whether the case presented or not with acute respiratory distress syndrome (ARDS). A severe outcome was defined as the need for mechanical ventilation or death.
The weekly ICU admission numbers were compared with the weekly estimated incidence of influenza-like illness (ILI) consultations, obtained through a network of sentinel general practitioners (GPs) (http://www.sentiweb.org/). Population figures provided by the French National Institute for Statistic and Economic Studies (INSEE) were used to calculate age-specific incidence rates.
Descriptive statistics included frequency analysis (percentages) for categorical variables and median and interquartile ranges (IQR) for continuous variables. The differences in medical and demographic characteristics according to outcomes were tested using χ2 test for categorical variables and Mann–Whitney rank sum test for continuous variables. A P value <0·05 was considered statistically significant. Odds ratios (ORs), including 95% confidence intervals (CIs), were calculated through multivariate logistic regression analysis. In this analysis, age was included as a categorical variable and presence or absence of each of the following underlying risk factors: chronic respiratory disease (including asthma), pregnancy, diabetes, obesity and morbid obesity, immunosuppression and chronic cardiac disease as dichotomous variables. The variable assessing the time between onset of symptoms and antiviral therapy was dichotomized (⩽2 days vs. >2 days). Goodness of fit of the models was assessed by Pearson goodness-of-fit χ2 tests. The analysis was performed with Stata version 9 (StataCorp., USA).
This surveillance system was approved by the Commission Nationale de l'Informatique et des Libertés (CNIL), the French Data Protection Authority.
RESULTS
From 1 July 2009 to 15 February 2010, 1297 patients admitted to an ICU for 2009 pandemic influenza were reported to InVS. There were 1117 (86%) adults aged ⩾15 years. Out of those 1117 cases, 1065 (95%) were laboratory confirmed. Almost all cases (n=1032, 97%) were RT–PCR confirmed as 2009 pandemic influenza A(H1N1), the other 33 cases being influenza A patients with undetermined subtype A.
The number of ICU admissions increased sharply from mid-October until the end of November (Fig. 1). The weekly distribution of ICU admissions and ILI consultations were very similar, apart from a first small increase seen by GPs in late September, reflecting a large circulation of rhinovirus in the community [Reference Casalegno5].
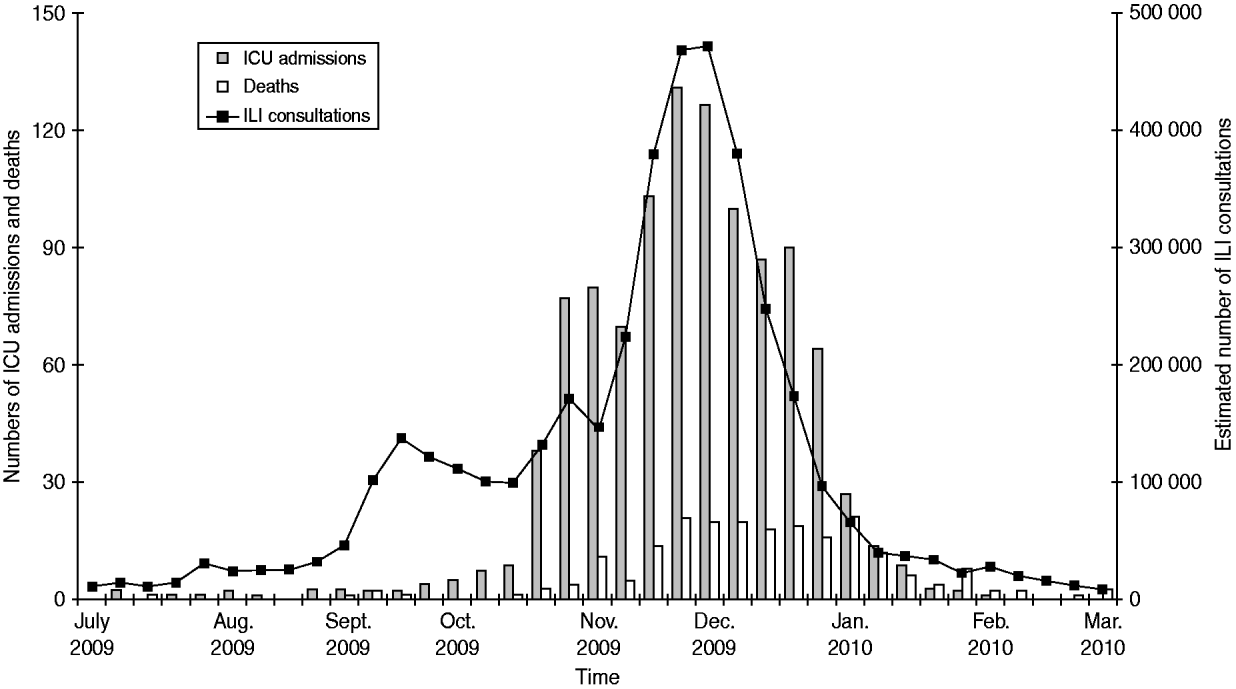
Fig. 1. Weekly number of estimated influenza-like illness (ILI) consultations (http://www.sentiweb.fr), intensive-care unit (ICU) admissions and in-hospital deaths associated with pandemic H1N1 influenza in adults, France, 1 July 2009 to 15 February 2010.
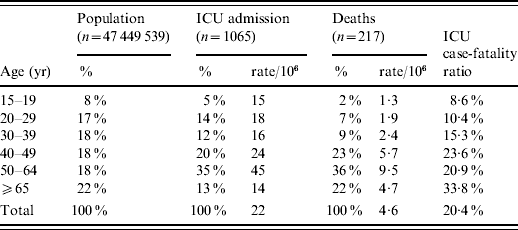
The incidence of ICU admissions and in-hospital deaths increased with age up to 64 years (Table 1). Only 13% of the cases were aged ⩾65 years. Compared to the French population, the 50–64 years age group was over-represented among patients admitted to ICUs and those who died. The ICU case-fatality ratio increased with age, from 9% in adolescents (15–19 years) to 34% in the elderly (⩾65 years).
Table 1. Incidence rates, age distribution of intensive-care unit (ICU) admissions and deaths due to A(H1N1) influenza compared to the age distribution of the French population and ICU case-fatality ratio for adults, France, 1 July 2009 to 15 February 2010
Baseline characteristics of the cases are presented in Table 2. Of the ICU patients, 82% had at least one underlying condition: 33% had chronic respiratory disease (including asthma, 14%), 6% were pregnant women, 20% and 6%, respectively, were obese or morbidly obese. Only 16 cases were vaccinated against pandemic influenza but the vaccine was given <7 days prior to onset of the disease for 15 cases and given 16 days before for one case.
Table 2. Characteristics at admission to intensive-care units (ICUs) of adults with A(H1N1) influenza, in France, 1 July 2009 to 15 February 2010
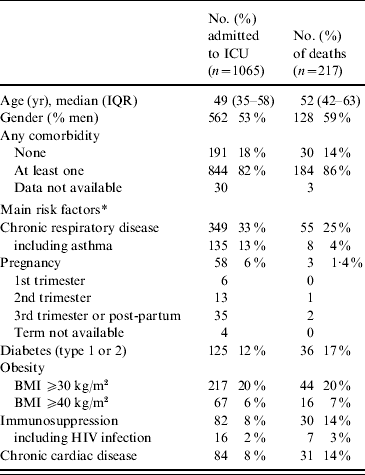
IQR, Interquartile range; BMI, body mass index.
* Several co-existing conditions can be described for the same patient.
Of the 1065 adults, 217 died (20%); 843 (79%) had been discharged from the ICU, and five (0·5%) were still hospitalized in the ICU on 8 April 2010 and lost to follow-up since that date (Table 3). The median length of stay in the ICU was 7 days for patients discharged and 9 days for those who died. Mechanical ventilation (invasive or non-invasive) was required for 67% of patients with a median duration of 10 days, and extra-corporeal membrane oxygenation (ECMO) was required for 7%. The clinician notified ARDS for 49% of cases. The case-fatality ratio was 28% in patients undergoing mechanical ventilation and 49% in those undergoing ECMO. One third of the deaths occurred within the first 4 days of hospitalization. Compared to survivors, non-survivors were older (median 52 and 47 years, respectively, P<0·001), more frequently males (59% and 51%, P=0·04), and more frequently had an underlying condition (86% and 80%, P<0·05).
Table 3. Characteristics of intensive-care unit (ICU) stays related to A(H1N1) influenza in France, 1 July 2009 to 15 February 2010
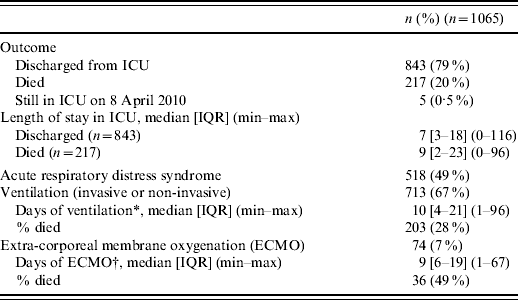
IQR, Interquartile range.
* Available for 548 (77%).
† Available for 62 (84%).
The time from first symptoms to hospital admission was available for 920 (86%) cases. The median time-interval was 3 days (IQR 1–5) and did not differ according to outcome (died or discharged). Data on administration of antiviral therapy (oseltamivir) and time since onset of symptoms were available for 647 (61%) patients. Of these, 609 (94%) received antiviral therapy and 231 (36%) received this treatment within 48 h of the onset of symptoms.
Factors associated with death or mechanical ventilation
On multivariate analysis, the factors independently associated with the use of ventilatory support or death were increasing age [40–64 years: adjusted OR (aOR) 2·4, 95% CI 1·7–3·3; ⩾65 years: aOR 3·5, 95% CI 2·2–5·8] and obesity (aOR 2·4, 95% CI 1·6–3·6). Conversely, chronic respiratory disease was associated with less severe outcome (aOR 0·4, 95% CI 0·3–0·5) (Table 4). Pregnancy was not significantly associated with severe outcome (aOR 0·7, 95% CI 0·4–1·3). Late antiviral therapy (>48 h) was associated with a greater risk of severe outcome in ICU patients with predisposing risk factors (age: aOR 2·0, 95% CI 1·4–3·0) but not in those without risk factors (aOR 1·9, 95% CI 0·8–4·8).
Table 4. Factors associated with severe outcome (ventilation or death), for adults admitted to intensive-care units (ICUs) with A(H1N1) influenza, France, 1 July 2009 to 15 February 2010
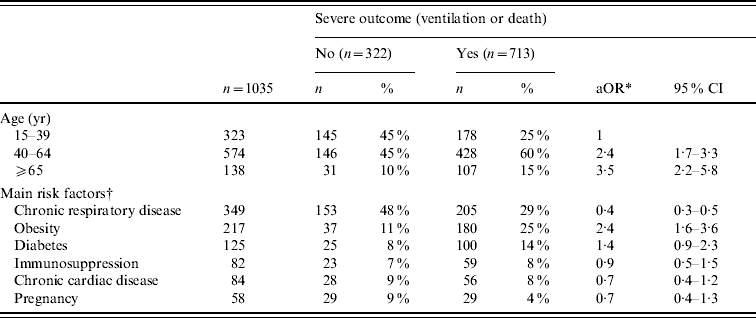
* Adjusted odds ratio for age and underlying condition; CI, confidence interval.
† Risk factors were available for 1035 ICU patients. Several co-existing conditions can be described for the same patient.
DISCUSSION
This first, large series of severe cases in Europe highlights the specificities of this new influenza virus compared to seasonal influenza.
From July 2009 to February 2010, 1065 confirmed A(H1N1) 2009 adult severe cases were notified to InVS, 95% of which occurred in the 13 weeks between mid-October and mid-January. As this surveillance was implemented specifically for the 2009 pandemic, it is difficult to compare these data with those of previous years. Nevertheless, according to the monthly data obtained from the French national hospital discharge database (PMSI) between October and December 2009, 1748 patients were admitted to ICUs with an influenza ICD-10 code (J9–J11), either laboratory confirmed or not, compared to 148, 172 and 344, respectively, during the seasonal influenza epidemics of 2006–2007, 2007–2008 and 2008–2009 (PMSI, unpublished data). This increase reflects the H1N1 severity compared to seasonal influenza, even if increased PCR testing, in the pandemic context, may have led to more frequent laboratory diagnosis than during seasonal flu, and could have partially contributed to this increase.
The ICU admission rate was 22 per million adult inhabitants. From the incidence available per age group [4], we estimated the incidence of ICU admission around 30 per million for the ⩾25 years group in Australia and New Zealand, close to those estimated in our study. The ICU admission rates and in-hospital death rates increased with age up to age 65 years and decreased in the elderly. Less than a quarter of deaths occurred in the elderly while >90% of deaths from seasonal influenza occurred in this age group [6, Reference Carrat and Valleron7]. However, the highest ICU case-fatality ratio (33%) was in the elderly, meaning that they were largely protected from infection but not from complications and deaths once infected. Furthermore, increasing age was a risk factor for severe disease after adjustment for the presence of comorbidities. Our results are consistent with other reports on 2009 pandemic influenza. In England, the incidence of pandemic A(H1N1) was higher in children and young adults but case-fatality ratios increased with age [Reference Donaldson8]. In Australia and New Zealand, the rate of ICU admission was low in the elderly but the risk of death increased with age [4]. In Mexico, confirmed cases aged ⩾70 years had the lowest mortality rate but the highest death/hospital admission ratio [Reference Echevarria-Zuno9].
For ICU patients, early antiviral treatment was associated with a reduced risk of severe outcome (ventilatory support requirement or death) for high-risk patients, even when the delay between the onset of symptoms and the hospitalization was taken into account. This association was not significant in patients without predisposing risk factors, but the point estimates of the OR and the low band of the CI close to 1 could reflect a lack of power of the analysis. As already suggested, those data are in favour of the effectiveness of early initiation of antiviral therapy for the reduction of severity of illness [Reference Jain3, Reference Trémolières10].
About 18% of adults admitted to ICUs and 14% of those who died did not have any underlying disease or risk factors. Although data are lacking for a rigorous comparison, those figures appear higher than for seasonal influenza. The underlying conditions leading to more severe illness were in accord with those reported in the literature. Chronic respiratory illness was the most prevalent in our case series (33%), as described previously [Reference Jain3, 4, Reference Kumar11]. The prevalence of asthma was 13% in ICU patients compared to 6% in French general population [Reference Delmas and Fuhrman12]. Chronic respiratory illness, including asthma, was not associated with a higher risk of severe disease or death in ICU cases. Severity associated with chronic respiratory disease during influenza illness is related to the exacerbation of the disease, and, particularly for asthma, that is usually responsive to β2 agonist treatment. In contrast, obesity was clearly a risk factor for severity of disease. The prevalence of obesity was 20% in ICU cases whereas it was 12% in the general adult population in France in 2006 (6% and 0·8%, respectively, for morbid obesity) [Reference Charles, Eschwege and Basdevant13]. Furthermore, obesity significantly increased the risk of severe outcome in ICU patients, even after adjustment for comorbidities. Previous studies have suggested that obesity was a risk factor for severe viral pneumonia and for ARDS [Reference Gong14, Reference Rello15], but not for mortality [Reference Kumar11, Reference Gong14, Reference Morris16]. Pregnancy accounted for 6% of admissions to ICUs whereas pregnancy (estimated from the number of live birth in 2008 and the number of abortions in France) represents around 1% of the general population. Our finding confirms that pregnancy should be considered as a risk factor for complications for 2009 pandemic influenza [Reference Jamieson17, Reference Louie18]. However, the total number of severe cases and deaths in pregnancy remained less than feared based on reports from the first affected countries. Pregnant women and health professionals were widely informed about the risk of severe influenza disease related to pregnancy. The widespread use of antiviral drugs for curative or prophylactic treatment in pregnant women could have reduced the impact of 2009 pandemic influenza in this group.
In our series, 67% of patients required ventilatory support, 49% had ARDS and 7% required ECMO. These results are consistent with previously published reports and highlight the predominance and severity of the respiratory illness due to primary viral pneumonia [4, Reference Kumar11].
Our study has several limitations. Although our surveillance was implemented in the context of public health emergency some cases may not have been reported to InVS. However, the clinical reports were cross-matched with laboratory results and local InVS staff regularly contacted ICU physicians in order to complete missing information on received forms and to check the completeness and accuracy of notification. Furthermore, this hospital surveillance was strongly supported by the various ICU medical practitioners' societies. Therefore, the completeness of case detection by the surveillance system was probably high. One of the main French regions assessed the exhaustiveness of the surveillance through direct contact with all ICUs once the wave was over. The exhaustiveness was estimated to be close to 90%. We included non-subtyped influenza A-positive cases. However, these cases represented only 3% of the whole study population and, in the absence of co-circulation of other influenza viruses identified through the active virological surveillance implemented during the epidemic, were highly likely to be A(H1N1) 2009 cases.
This first large series of severe cases in Europe confirms the high contribution of young adults, aged 30–64 years to A(H1N1) 2009 mortality. Not all deaths associated with A(H1N1) 2009 virus are included in this analysis. During the same period, from July 2009 to February 2010, 76 deaths related to probable or confirmed H1N1 that occurred in hospital wards other than ICUs (n=30) or outside the hospital (n=46) have been identified. Furthermore, only deaths that have been attributed to the virus on virological grounds were taken into account. Some indirect mortality in patients with severe comorbidity may have been missed. It will be important to try to assess the extent of this indirect mortality later, through analysis of excess deaths in the death database when completed for the whole duration of the epidemic. However, the total number of deaths identified throughout this first pandemic wave appears less than predicted for a pandemic virus. Apart from the intrinsic characteristics of the virus, its moderate virulence, its sensitivity to the antiviral drugs maintained for the duration of the pandemic wave, other factors may have contributed to this situation. First, the low attack rate in the elderly, probably as a consequence of previous exposure to H1N1 virus having circulated in the first half of the last century inducing cross-protection to A(H1N1) 2009 virus [Reference Miller19]. Persons aged ⩾65 years represented only 3% of ILI diagnosed by GPs during the epidemic but 23% of H1N1 hospital deaths. Second, in the early stage of the epidemic, rapid antiviral prophylaxis or treatment has been recommended for high-risk individuals if symptomatic or in close contact with cases. During the more intense phase of the epidemic, prophylaxis was discontinued but antiviral treatment was recommended for all cases with flu-like symptoms. As our data support the effectiveness of early antiviral treatment for the prevention of severe outcomes, this may have contributed to a decrease in the burden of severe cases. Third, vaccination of high-risk populations may also have resulted in a decrease in the number of severe cases. However, preliminary data from a telephone survey conducted in a random sample of the population support a low coverage in the high-risk population, below 20%. Fourth, rapid access to hospital care, when needed, is universal in France with in theory no financial barrier, even for those in the lowest socioeconomic category or marginalized populations. The median time from onset of symptoms to hospital admission of 3 days illustrates this very high accessibility to hospital for severe A(H1N1) 2009 cases. Furthermore, the moderate virulence of the virus did not lead to overloading of the capacity of critical-care services making it possible to offer to each case the level of care required by their condition.
Notwithstanding its limitations, this study confirms the main results published in the literature and highlights the role of obesity as an independent risk factor for severe disease and the protective role of rapid antiviral treatment. It also highlights the value of a very successful cooperation between clinicians and public health authorities to set up and to maintain a real-time and high-quality hospital-based surveillance throughout a public health crisis.
ACKNOWLEDGEMENTS
We thank all the clinicians who were involved in the clinical surveillance and all the laboratories that contributed with RT–PCR results, particularly contributors to the REVA-Grippe-SRLF registry. We also thank all colleagues at InVS who were directly or indirectly involved in the influenza surveillance for their support, especially Elise Chiron and Etienne Lucas for the data management, Sophie Vaux, the regional units of InVS (CIRES), and Angie Bone for her attentive review of the manuscript. We also thank Jean-Claude Desenclos for scientific advice.
DECLARATION OF INTEREST
None.







