Introduction
Trypetheliaceae is one of the dominant elements of lichen communities in tropical rainforests, dry forests, and savannas (Komposch & Hafellner Reference Komposch and Hafellner2000, Reference Komposch and Hafellner2003; Komposch et al. Reference Komposch, Aptroot and Hafellner2002; Aptroot et al. Reference Aptroot, Lücking, Sipman, Umaña and Chaves2008, Reference Aptroot, Menezes, Lima, Xavier-Leite and Cáceres2013; Aptroot 2009 Reference Aptroota , Reference Aptrootb ). The family was traditionally considered to belong in a suite of crustose, lichen-forming fungi with pyrenocarpous ascomata, and bitunicate (fissitunicate) asci, and was alternatively placed in the orders Pyrenulales or Melanommatales (Barr Reference Barr1979, Reference Barr1987; Harris Reference Harris1984, Reference Harris1991; Kirk et al. Reference Kirk, Cannon, David and Stalpers2001; Eriksson et al. Reference Eriksson, Barah, Currah, Hansen, Kurtzman, Rambold and Laessøe2004; Cannon & Kirk Reference Cannon and Kirk2007). Eriksson (Reference Eriksson1981) elaborated a detailed scheme about the putative evolution of this group, postulating that a trypethelioid precursor, the so-called α-Trypetheliaceae, gave rise to both the Pyrenulaceae and Trypetheliaceae. Pyrenulales are now classified near Verrucariales within the Eurotiomycetes, whereas Melanommatales have been synonymized with Pleosporales in the Dothideomycetes (Berbee Reference Berbee1996; Liew et al. Reference Liew, Aptroot and Hyde2000; Lumbsch & Lindemuth Reference Lumbsch and Lindemuth2001; Weerakoon et al. Reference Weerakoon, Aptroot, Lumbsch, Wolseley, Wijeyaratne and Gueidan2012), which generated ambiguity as to the correct placement of Trypetheliaceae. Inclusion of a single taxon in the first AFTOL study (Lutzoni et al. Reference Lutzoni, Kauff, Cox, McLaughlin, Celio, Dentinger, Padamsee, Hibbett, James and Baloch2004) suggested placement of the family within Dothideomycetes, which was subsequently confirmed by Del Prado et al. (Reference Del Prado, Schmitt, Kautz, Palice, Lücking and Lumbsch2006) with a more target-oriented taxon sampling, and by Nelsen et al. (Reference Nelsen, Lücking, Grube, Mbatchou, Muggia, Rivas Plata and Lumbsch2009) and Schoch et al. (Reference Schoch, Crous, Groenewald, Boehm, Burgess, de Gruyter, de Hoog, Dixon, Grube and Gueidan2009) with phylogenetic studies focusing on Dothideomycetes. However, the family consistently clustered outside the Pleosporales and a separate order, Trypetheliales, was established for it (Aptroot et al. Reference Aptroot, Lücking, Sipman, Umaña and Chaves2008). While Nelsen et al. (Reference Nelsen, Lücking, Grube, Mbatchou, Muggia, Rivas Plata and Lumbsch2009) demonstrated the close relationship between tropical Mycomicrothelia species and Trypetheliaceae, a subsequent phylogenetic study suggested inclusion of these species within the family, together with further species in the collective genera Arthopyrenia and Julella (Nelsen et al. Reference Nelsen, Lücking, Mbatchou, Andrew, Spielmann and Lumbsch2011). A second family, Polycoccaceae, which chiefly includes lichenicolous fungi, has been recently established and included in Trypetheliales (Ertz et al. Reference Ertz, Diederich, Lawrey, Berger, Freebury, Coppins, Gardiennet and Hafellner2015).
Genera in Trypetheliaceae were traditionally separated by thallus structure, ascoma disposition, and ascospore type (Letrouit-Galinou Reference Letrouit-Galinou1957, Reference Letrouit-Galinou1958; Makhija & Patwardhan Reference Makhija and Patwardhan1988, Reference Makhija and Patwardhan1993; Harris 1989 Reference Harrisa , Reference Harrisb , Reference Harris1990, Reference Harris1991, Reference Harris1995; Aptroot 1991 Reference Aptroota , Reference Aptrootb , 2009 Reference Aptroota , Reference Aptrootb ; Aptroot et al. Reference Aptroot, Lücking, Sipman, Umaña and Chaves2008; Lumbsch & Huhndorf Reference Lumbsch and Huhndorf2010). Thus, the core group with corticate thalli and typically astrothelioid ascospores (with diamond-shaped lumina) was divided into five genera according to ascoma disposition and ascospore septation: Trypethelium (solitary to aggregate ascomata with apical, separate ostioles, ascospores transversely septate), Laurera (solitary to aggregate ascomata with apical, separate ostioles, ascospores muriform), Astrothelium (solitary to aggregate ascomata with lateral, separate or fused ostioles, ascospores transversely septate), Cryptothelium (aggregate ascomata with lateral, fused ostioles, ascospores muriform), and Campylothelium (solitary ascomata with lateral, separate ostioles, ascospores muriform). In addition, the genus Pseudopyrenula was distinguished by its white, ecorticate thallus, combined with astrothelioid ascospores, whereas Polymeridium was defined as having an ecorticate thallus and thin-walled ascospores. This classification was not only considered artificial (Harris 1989 Reference Harrisa , Reference Harris1995; Del Prado et al. Reference Del Prado, Schmitt, Kautz, Palice, Lücking and Lumbsch2006; Aptroot et al. Reference Aptroot, Lücking, Sipman, Umaña and Chaves2008), but was also flawed logically, since species with separate or fused ostioles were united under a single genus (Astrothelium) if ascospores were transversely septate but separated into two genera (Campylothelium, Cryptothelium) if ascospores were muriform. Also, species in the Trypethelium eluteriae group have ascospores more similar to those of Polymeridium, lacking diamond-shaped lumina.
Harris (1989 Reference Harrisb , Reference Harris1991, Reference Harris1995) was the first to try and establish more natural genera, such as Bathelium, characterized by a suite of morpho-anatomical and chemical characters. The genera Aptrootia and Architrypethelium were introduced more recently for species with unique ascospore types (Aptroot 1991 Reference Aptrootb ; Lücking et al. Reference Lücking, Sipman, Umaña, Chaves and Lumbsch2007). Molecular phylogenetic studies then revealed the inclusion of some species of Arthopyrenia, Julella, and Mycomicrothelia in a basal position in the family (Nelsen et al. Reference Nelsen, Lücking, Grube, Mbatchou, Muggia, Rivas Plata and Lumbsch2009, Reference Nelsen, Lücking, Mbatchou, Andrew, Spielmann and Lumbsch2011), all with morphologies similar to Polymeridium and Pseudopyrenula (with ecorticate thalli and mostly exposed ascomata).
Generic delimitation within Trypetheliaceae was evaluated in an expanded study (Nelsen et al. Reference Nelsen, Lücking, Aptroot, Andrew, Cáceres, Rivas Plata, Gueidan, da Silva Canêz, Knight and Ludwig2014), illustrating that a number of genera recognized within Trypetheliaceae were well defined, while the boundaries of several others required substantial adjustments. Thus, the bulk of the species with a corticate thallus and astrothelioid ascospores was suggested to be included in a single clade, Astrothelium, regardless of ascomatal disposition or ascospore septation; with such a modified concept, Cryptothelium and Laurera would become synonyms of Astrothelium, although no formal changes were proposed. In contrast, Trypethelium was to be retained for species in the T. eluteriae group, with aggregate, sessile pseudostromata and a unique ascospore type. The genera Aptrootia and Architrypethelium, and presumably also Campylothelium, were confirmed as monophyletic, whereas Bathelium s. str. with muriform ascospores formed a separate clade outside Astrothelium, with some species with transversely-septate ascospores previously assigned to this genus now included in Astrothelium. In addition, the genus Marcelaria was established for the enigmatic species Laurera purpurina and its relatives (Aptroot et al. Reference Aptroot, Menezes, Lima, Xavier-Leite and Cáceres2013). Differences in ascospore type and ontogeny were found to reflect this modern understanding of phylogenetic relationships within the family (Sweetwood et al. Reference Sweetwood, Lücking, Nelsen and Aptroot2012).
Species circumscriptions within Trypetheliaceae were also rather schematically based on ascospore size and septation, and the formation of anthraquinone and perylenequinone pigments, recognizing c. 200 taxa (Harris Reference Harris1984; Aptroot 1991 Reference Aptrootb ; Del Prado et al. Reference Del Prado, Schmitt, Kautz, Palice, Lücking and Lumbsch2006; Aptroot et al. Reference Aptroot, Lücking, Sipman, Umaña and Chaves2008). Characters such as hymenial inspersion, lichexanthone synthesis (Harris Reference Harris1991, Reference Harris1995, Reference Harris1998), and particularly thallus morphology were rarely, if at all, considered to be taxonomically important. The pigments, on the other hand, were subject to numerous rather detailed studies on their chemical structures and possible functions (Stensiö & Wachtmeister Reference Stensiö and Wachtmeister1969; Culberson & Culberson Reference Culberson and Culberson1970; Mathey & Hoder Reference Mathey and Hoder1978; Harris Reference Harris1984; Mathey et al. Reference Mathey, Van Vaeck and Steglich1987, Reference Mathey, Van Roy, Van Vaeck, Eckhardt and Steglich1994; Aptroot 1991 Reference Aptrootb ; Mathey & Lukins Reference Mathey and Lukins2001; Manojlovic et al. Reference Manojlovic, Vasiljevic, Gritsanapan, Supabphol and Manojlovic2010).
Here we present a further, much expanded phylogenetic study based on two loci, the mitochondrial small subunit (mtSSU) and the nuclear large subunit (nuLSU) of the rDNA cistron, including a total of 196 operational taxonomic units (OTU’s) spanning the entire diversity of the family. While providing a much-refined framework for revised generic delimitations, this study also focuses on the problem of species delimitation and the potential importance of phenotypic characters that have hitherto been neglected for taxonomic purposes in this family.
Material and Methods
We included all available mtSSU and nuLSU data on Trypetheliaceae published in previous studies (Lutzoni et al. Reference Lutzoni, Kauff, Cox, McLaughlin, Celio, Dentinger, Padamsee, Hibbett, James and Baloch2004; Del Prado et al. Reference Del Prado, Schmitt, Kautz, Palice, Lücking and Lumbsch2006; Nelsen et al. Reference Nelsen, Lücking, Grube, Mbatchou, Muggia, Rivas Plata and Lumbsch2009, Reference Nelsen, Lücking, Mbatchou, Andrew, Spielmann and Lumbsch2011, Reference Nelsen, Lücking, Aptroot, Andrew, Cáceres, Rivas Plata, Gueidan, da Silva Canêz, Knight and Ludwig2014) and added a total of 155 new sequences (100 mtSSU, 55 nuLSU) for 117 OTUs (Table 1). The only genera putatively placed within the family for which sequence data could not be obtained were Exiliseptum (Harris Reference Harris1984) and Melanophloea (Aptroot & Schumm Reference Aptroot and Schumm2012). Cladosporium cladosporioides was used as outgroup following Nelsen et al. (Reference Nelsen, Lücking, Aptroot, Andrew, Cáceres, Rivas Plata, Gueidan, da Silva Canêz, Knight and Ludwig2014).
Table 1 GenBank Accession numbers and voucher information for taxa and samples used in this study. INB→CR indicates that the collections previously held at INB (National Biodiversity Institute, Costa Rica) are currently in the process of being transferred to CR (National Herbarium, Costa Rica)

For newly generated sequences, the Sigma-Aldrich REDExtract-N-Amp Plant PCR Kit (St. Louis, Missouri, USA) was used to isolate DNA, following the manufacturer’s instructions, except only 10–30 µl of extraction buffer and 10–30 µl dilution buffer were used, and a 20× DNA dilution was then used in subsequent PCR reactions. A portion of the fungal mitochondrial small subunit (mtSSU) was amplified and sequenced using combinations of the following primers: mrSSU1, mrSSU2, mrSSU2R, mrSSU3R (Zoller et al. Reference Zoller, Scheidegger and Sperisen1999), MSU7 (Zhou & Stanosz Reference Zhou and Stanosz2001), mrSSU-1/2-5'-mpn and mrSSU-2/3-3'-mpn (Nelsen et al. Reference Nelsen, Lücking, Mbatchou, Andrew, Spielmann and Lumbsch2011). Additionally, a portion of the fungal nuclear large subunit (nuLSU) was amplified and sequenced using combinations of the primers f-nu-LSU-0116-5'/ITS4A-5' (Nelsen et al. Reference Nelsen, Lücking, Mbatchou, Andrew, Spielmann and Lumbsch2011, Reference Nelsen, Lücking, Andrew, Rivas Plata, Chaves, Cáceres and Ventura2012), AL2R (Mangold et al. Reference Mangold, Martín, Lücking and Lumbsch2008), f-nu-LSU-0287-5'-mpn (Nelsen et al. Reference Nelsen, Lücking, Mbatchou, Andrew, Spielmann and Lumbsch2011), LR3 (Vilgalys & Hester Reference Vilgalys and Hester1990), LR3R (reverse complement of LR3), LR4 (http://www.biology.duke.edu/fungi/mycolab/primers.htm), LR5 and LR6 (Vilgalys & Hester Reference Vilgalys and Hester1990).
The 10 µl PCR reactions consisted of 5 µM of each PCR primer, 3 mM of each dNTP, 2 µl of 10 mg/ml 100X BSA (New England BioLabs, Ipswich, Massachusetts, USA), 1·5 µl 10× PCR buffer (Roche Applied Science, Indianapolis, Indiana, USA), 0·5 µl Taq, approximately 2 µl diluted DNA, and 2 µl water or 2·5–5 µl REDExtract-n-Amp PCR Ready Mix (Sigma-Aldrich, St. Louis, Missouri, USA), 5 µM of each PCR primer, 2 µl diluted DNA and 2–4·5 µl water. The PCR cycling conditions were as follows: 95 °C for 5 min, followed by 35 cycles of 95 °C for 1 min, 53 °C (mtSSU), 55 °C (nuLSU: AL2R/LR3) or 60 °C (nuLSU: f-nu-LSU-0116-5'/ITS4A-5' with LR3 or LR6) for 1 min, and 72 °C for 1 min, followed by a single 72 °C final extension for 7 min. Samples were visualized on an ethidium bromide-stained 1% agarose gel under UV light and bands were gel extracted, heated at 70 °C for 5 min, cooled to 45 °C for 10 min, treated with 1 µl GELase (Epicentre Biotechnologies, Madison, Wisconsin, USA) and incubated at 45 °C for at least 24 h. The 10 µl cycle sequencing reactions consisted of 1–1·5 µl of Big Dye version 3.1 (Applied Biosystems, Foster City, California, USA), 2·5–3 µl of Big Dye buffer, 1–6 µM primer (primers listed above), 0·75–2 µl GELase-treated PCR product and water. Cycle sequencing was performed using one of the following conditions: 96 °C for 1 min, followed by 25 cycles of 96 °C for 10 s, 50 °C for 5 s and 60 °C for 4 min or instead 96 °C for 1 min, followed by 40 cycles of 96 °C for 10 s, 45 °C for 5 s and 60 °C for 4 min. Samples were precipitated and sequenced in an Applied Biosystems 3730 DNA Analyzer (Foster City, California, USA), and sequences assembled in Sequencher 4.9 (Gene Codes Corporation, Ann Arbor, Michigan, USA). DNA analyses were performed at the Pritzker Laboratory for Molecular Systematics and Evolution at the Field Museum.
Sequences were automatically aligned using MAFFT 6.935 with sorting function (Katoh et al. Reference Katoh, Asimenos and Toh2009). The unaligned sequences were also subjected to assessment of alignment ambiguity through the Guidance web server (Penn et al. 2010 Reference Penn, Privman, Ashkenazy, Landan, Graur and Pupkoa , Reference Penn, Privman, Landan, Graur and Pupkob ). No substantial ambiguity was detected for the nuLSU locus, whereas three large, ambiguously aligned regions were found in the mtSSU locus. These were removed from the alignment and separately recoded using PICS-Ord 1.0 (Lücking et al. Reference Lücking, Hodkinson, Stamatakis and Cartwright2011). After testing for potential conflict between the trimmed mtSSU and the nuLSU loci using Compat.py 3.0 (Kauff & Lutzoni Reference Kauff and Lutzoni2002, Reference Kauff and Lutzoni2003), with no conflicts detected, the two loci were merged together with the PICS-Ord codes (see Supplementary Material A, available online) and a combined, partitioned maximum likelihood analysis (by locus and codes) was performed in RAxML 7.2.8 (Stamatakis Reference Stamatakis2006), employing a GTRGAMMA model for the nucleotide partitions and GTR for the code partition. Support was estimated by performing 1000 fast bootstrap pseudoreplicates (Stamatakis et al. Reference Stamatakis, Hoover and Rougemont2008).
For selected portions of the resulting topology, we employed the Shimodeira-Hasegawa (SH) test as implemented in RAxML 8.0.2 to test whether alternative topologies could be rejected.
Results and Discussion
The final dataset consisted of 196 ingroup OTUs and 1272 characters (mtSSU: 690; nuLSU: 491; mtSSU PICS-Ord codes: 91). The basal portion of the best-scoring maximum likelihood tree (Figs 1 & 2) included several clades representing the artificial genera Arthopyrenia, Julella, and Mycomicrothelia, all with ecorticate thalli and more or less euseptate ascospores. Species formerly placed in Arthopyrenia s. lat. and Mycomicrothelia s. lat. did not form monophyletic clades and the backbone in this part of the tree was poorly supported. Therefore, while the name Bogoriella was separately reinstated for the bulk of tropical, lichenized Mycomicrothelia species (Aptroot & Lücking Reference Aptroot and Lücking2016), here we established new genera (see below) for the well-supported core clade of tropical, lichenized Arthopyrenia species (Constrictolumina) and for Mycomicrothelia oleosa (Novomicrothelia), for which monophyly with the other three Mycomicrothelia species (Bogoriella) was rejected (SH test, P < 0·01). No formal change was proposed for Julella fallaciosa since the available data do not allow us to conclude whether this taxon is conspecific with the type of the genus. Two further lineages representing tropical, lichenized species previously assigned to Arthopyrenia were provisionally placed in Constrictolumina, since monophyly of these lineages with the latter could not be rejected (SH test, P > 0·05).
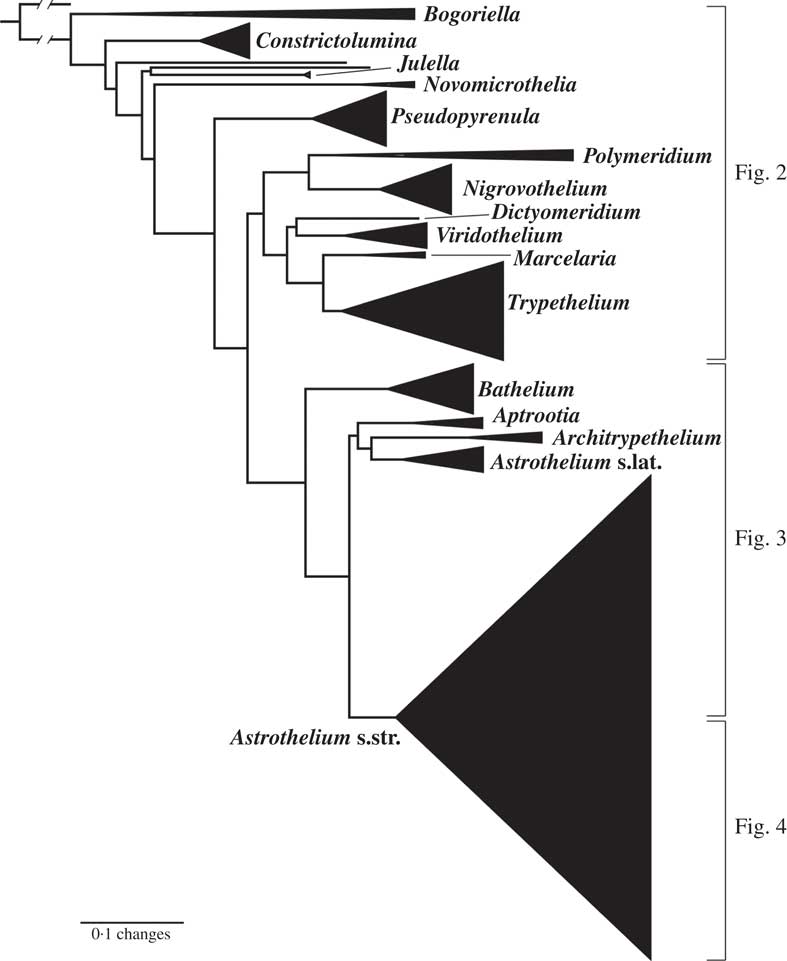
Fig. 1 Combined mtSSU-nuLSU-PICS-Ord cartoon tree of Trypetheliaceae based on maximum likelihood analysis. Accepted genera are labelled. Separate detailed figures (which also include bootstrap support values) are indicated.
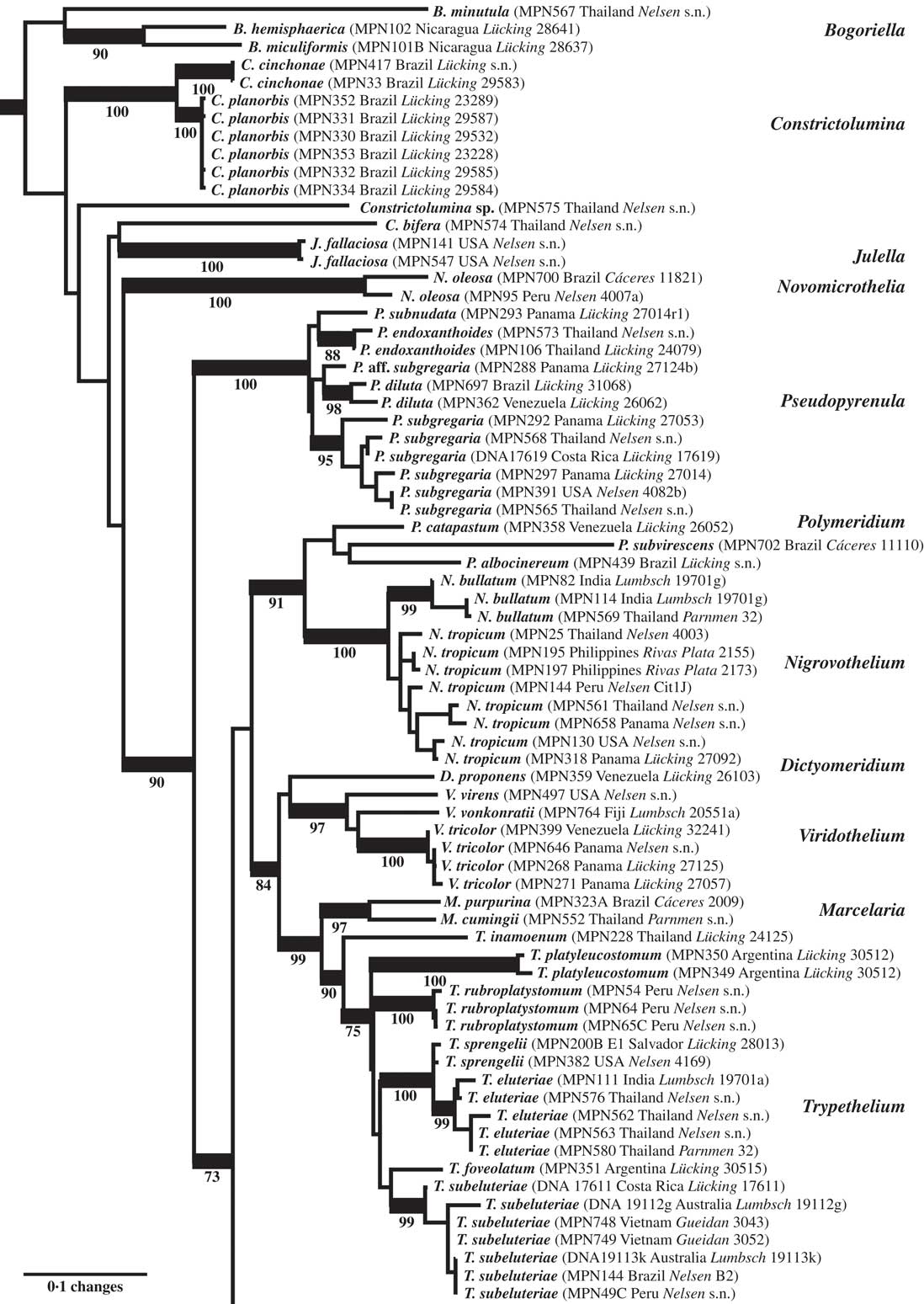
Fig. 2 Combined mtSSU-nuLSU-PICS-Ord tree of Trypetheliaceae (basal and central portion of the tree: Bogoriella to Trypethelium) based on maximum likelihood analysis. Thick lines indicate bootstrap support ≥70% and exact bootstrap support values are given below branches. Accepted genera are in larger font and in bold.
The strongly supported remainder of the tree contained lineages with astrothelioid ascospores and/or corticate thalli. This large clade was rather well supported in most parts, suggesting up to 15 lineages which we mostly interpret as distinct genera (Figs 1–4). Pseudopyrenula formed a supported, monophyletic sister group to all remaining genera which were split into two larger clades (Figs 1 & 2). The first of these two clades included Polymeridium s. str., the Trypethelium tropicum clade (Nigrovothelium), the Polymeridium proponens clade (Dictyomeridium), for which monophyly with Polymeridium s. str. was rejected (SH test, P < 0·01), the Trypethelium virens clade (Viridothelium), Marcelaria, and Trypethelium s. str. (Fig. 2). The Trypethelium virens clade had previously been identified with the name Campylothelium (Nelsen et al. Reference Nelsen, Lücking, Aptroot, Andrew, Cáceres, Rivas Plata, Gueidan, da Silva Canêz, Knight and Ludwig2014), but this was based on a misidentification of one of the species contained in this clade as Campylothelium puiggarii which turned out to represent an undescribed species unrelated to C. puiggarii; we now assume that Campylothelium falls into the Astrothelium clade although the type, Campylothelium puiggarii, has not yet been sequenced. The sister group relationship of Polymeridium s. str. with the Trypethelium tropicum clade was supported, as well as the sister group relationship of Marcelaria and Trypethelium s. str. and the position of the Polymeridium proponens (Dictyomeridium) and the Trypethelium virens (Viridothelium) clades close to Marcelaria and Trypethelium (Fig. 2).
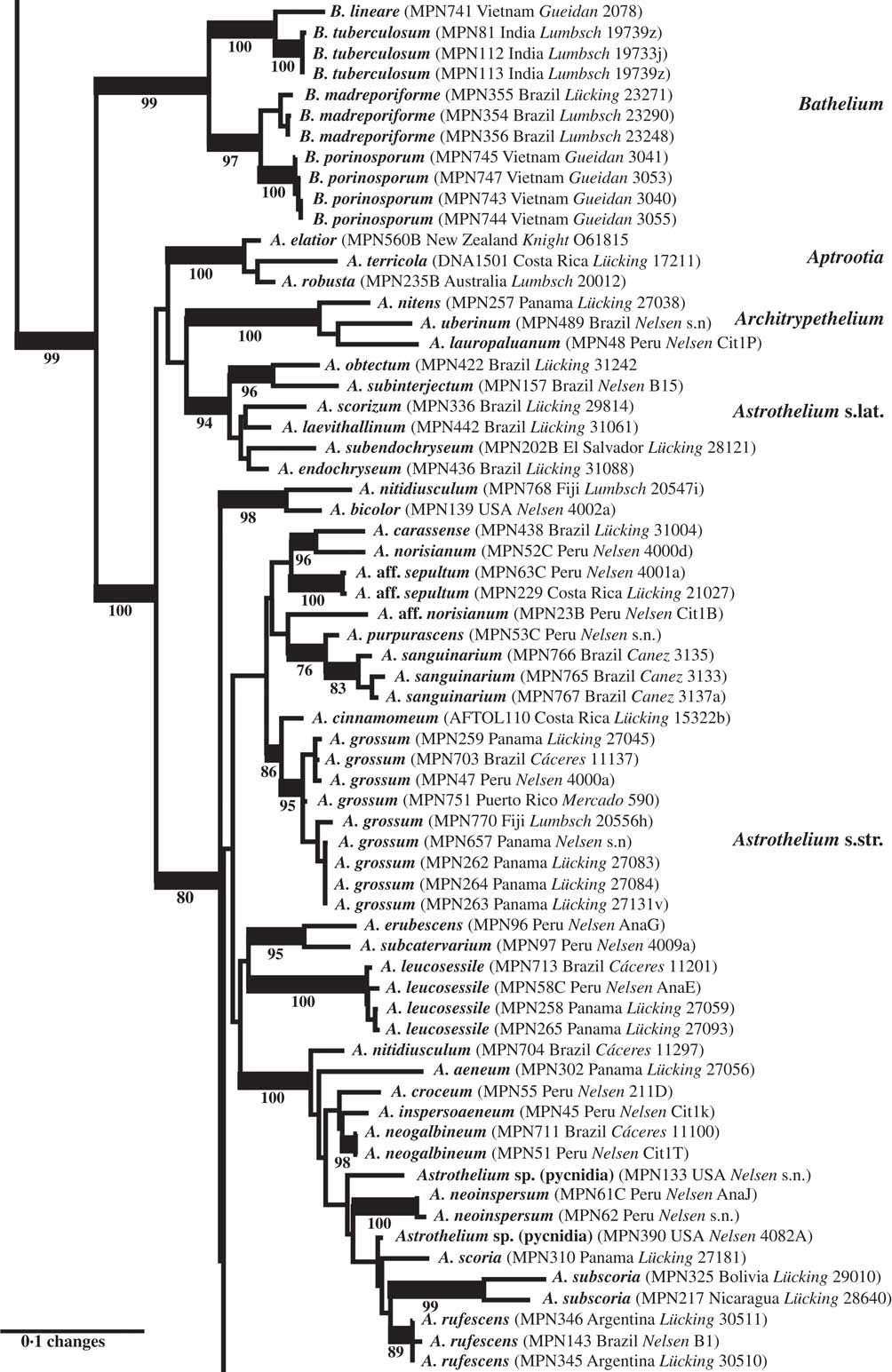
Fig. 3 Combined mtSSU-nuLSU-PICS-Ord tree of Trypetheliaceae (central portion of the tree: Bathelium to Astrothelium p.p.) based on maximum likelihood analysis. Thick lines indicate bootstrap support ≥70% and exact bootstrap support values are given below branches. Accepted genera are in larger font and in bold.
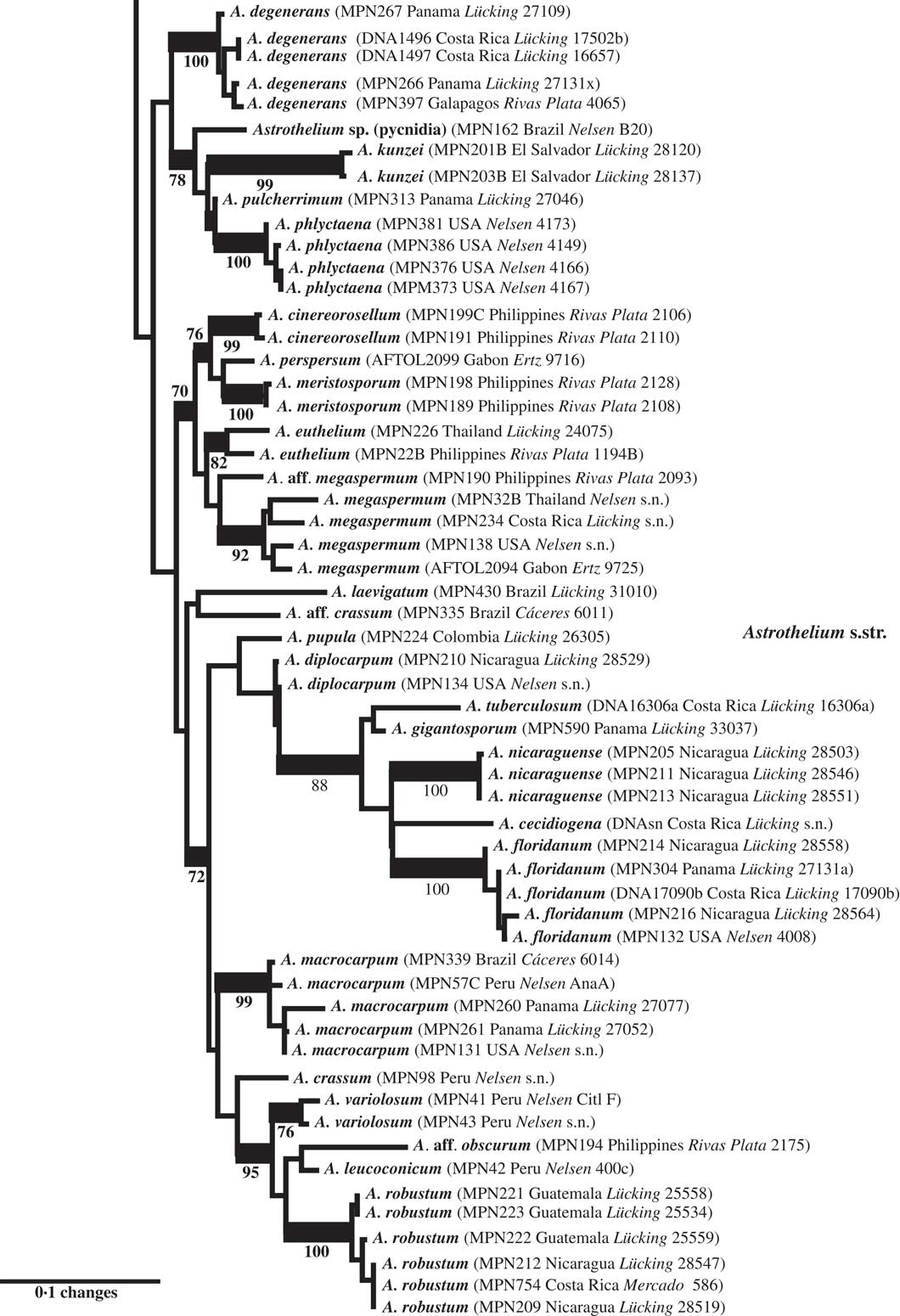
Fig. 4 Combined mtSSU-nuLSU-PICS-Ord tree of Trypetheliaceae (distal portion of the tree: Astrothelium p.p.) based on maximum likelihood analysis. Thick lines indicate bootstrap support ≥70% and exact bootstrap support values are given below branches. Accepted genera are in larger font and in bold.
The second clade included the genera Bathelium s. str., Aptrootia and Architrypethelium as well as a small and a large clade comprising species traditionally classified in the genera Astrothelium, Bathelium, Cryptothelium, Laurera, and Trypethelium, with Astrothelium as the oldest available name (Figs 1, 3 & 4). Bathelium s. str. formed a strongly supported sister group to the remaining clades in this portion of the tree (Fig. 3), but the relationships between the genera Aptrootia, Architrypethelium, and Astrothelium were not supported and not fully resolved, due to the presence of a small clade of species that would morphologically be referable to Astrothelium but clustered, without support, with Aptrootia and Architrypethelium (Fig. 3). The separate mtSSU and nuLSU trees had similar topologies, with a small Astrothelium clade separated from the large Astrothelium clade; however, in the mtSSU tree, Architrypethelium was nested within the small clade and Aptrootia within the large clade (see Supplementary Materials B & C, available online); for both loci, the alternative hypothesis that the two Astrothelium clades form a monophyletic group could not be rejected (SH test, P > 0·05). Therefore, we adopted a conservative solution, retaining Aptrootia and Architrypethelium as separate genera, due to their distinctive features, and treating all other species in this large, terminal clade in a single genus, Astrothelium.
All genus-level lineages as delimited here are characterized by a combination of phenotypic features and we formally recognize 15 genera at this time within Trypetheliaceae (Aptroot & Lücking Reference Aptroot and Lücking2016), excluding Julella (since the phylogenetic position of the type species is unknown) but including Distothelia, which has not yet been sequenced. The lineages at the base of the tree are for the time being recognized in three genera, all with ecorticate thalli, exposed, black ascomata and ascospores of variable types but not astrothelioid (i.e. lacking the endospore causing the diamond-shaped lumina characteristic of most other genera). These are Bogoriella (reinstated for tropical, lichenized species previously placed in Mycomicrothelia; ascospores euseptate, brown), Constrictolumina (for tropical, lichenized species previously placed in Arthopyrenia; ascospores subdistoseptate, often with incomplete septal invaginations, hyaline), and Novomicrothelia (for an additional species previously placed in Mycomicrothelia).
The genus Julella, previously assigned to Arthopyreniaceae or Thelenellaceae (Mayrhofer Reference Mayrhofer1987; Harris Reference Harris1995), has recently been placed in Halojulellaceae and Didymosphaeriaceae, respectively (Hyde et al. Reference Hyde, Jones, Liu, Ariyawansha, Boehm, Boonmee, Braun, Chomnunti, Crous and Dai2013; Ariyawansa et al. Reference Ariyawansa, Tanaka, Thambugala, Phookamsak, Tian, Camporesi, Hongsanan, Monkai, Wanasinghe and Mapook2014) but no molecular data are yet available for the non-lichenized, European type species, J. buxi Fabre. The sequenced material falling within Trypetheliaceae fits the North American temperate species J. fallaciosa, which might or might not be congeneric with J. buxi, so further data are required to resolve this issue; for the time being, we do not formally accept Julella as a genus within Trypetheliaceae. The genus as currently circumscribed might well turn out to be polyphyletic, similar to Arthopyrenia s. lat. and Mycomicrothelia s. lat. A potentially available genus name for the lineage falling within Trypetheliaceae, should J. fallaciosa prove to be unrelated to J. buxi, is Polyblastiopsis Zahlbr. based on P. sericea (A. Massal.) Zahlbr., which appears to be related to, or conspecific with, J. fallaciosa (Purvis et al. Reference Purvis, Coppins, Hawksworth, James and Moore1992; Aptroot & van den Boom Reference Aptroot and van den Boom1995; Harris Reference Harris1995).
Pseudopyrenula is characterized by a morphology similar to Bogoriella and Constrictolumina, but with astrothelioid ascospores with diamond-shaped lumina. In both morphology and position, Pseudopyrenula provides a true transitional genus between the base of the tree and the more derived taxa, combining a primitive morphology with a derived ascospore type.
The genera Polymeridium and Dictyomeridium share the plesiomorphic morphology of ecorticate thalli and largely exposed, black ascomata with the aforementioned genera and their ascospores are also euseptate. Given their supported, nested position within a clade largely characterized by astrothelioid ascospores, the euseptate ascospores of Polymeridium and Dictyomeridium could be a secondary reversal (loss of endospore) due to the ecology of these species occurring mostly in dry forest biomes (Harris Reference Harris1984, Reference Harris1991, Reference Harris1995; Cáceres Reference Cáceres2007; Aptroot et al. Reference Aptroot, Lücking, Sipman, Umaña and Chaves2008, Reference Aptroot, Menezes, Lima, Xavier-Leite and Cáceres2013). However, this needs to be tested with a further expanded dataset, although the thin-walled ascospores of Trypethelium s. str. (see below) would support this notion. Polymeridium was initially described as a section of Arthopyrenia (Müller Reference Müller1883), while Harris (Reference Harris1975) eventually raised the section to a separate genus within Trypetheliaceae (Tucker & Harris Reference Tucker and Harris1980). The morphological distinction between Polymeridium s. str. and Dictyomeridium is not yet fully understood, but the latter appears to include species with lateral ostioles and muriform ascospores. The separate placement of this group was anticipated in earlier works (Tucker & Harris Reference Tucker and Harris1980; Harris Reference Harris1991), and Polymeridium proponens had at some point been assigned to Campylothelium (Tucker & Harris Reference Tucker and Harris1980).
The pantropical Trypethelium tropicum complex is supported sister to Polymeridium s. str. and could be included in the latter based on topology alone, but since it differs in the corticate thallus and astrothelioid ascospores, we prefer to recognize it as a new genus, Nigrovothelium. Nigrovothelium is morphologically distinguished from Astrothelium by the completely exposed, sessile ascomata (at least partly covered by thallus or pseudostromatic in Astrothelium) and from Bathelium by the absence of pseudostromata and the black colour of the ascomata. Marcelaria has been characterized already in a separate paper (Aptroot et al. Reference Aptroot, Menezes, Lima, Xavier-Leite and Cáceres2013) as comprising species with exposed, strongly pigmented pseudostromata, which are somewhat similar to those of Trypethelium s. str. but are not distinctly pseudostromatic and produce muriform ascospores.
Trypethelium s. str. is well delimited by its prominent to sessile pseudostromata with apical ostioles and the subdistoseptate, transversely-septate ascospores with more or less rectangular lumina, different to the astrothelioid ascospores in Astrothelium and other genera. The aforementioned notion that the euseptate ascospores in Polymeridium and Dictyomeridium could represent a secondary reversal from astrothelioid forms is in part supported by ascospore ontogeny in Trypethelium s. str., where the finally subdistoseptate ascospores pass through an astrothelioid stage early in their ontogeny (Sweetwood et al. Reference Sweetwood, Lücking, Nelsen and Aptroot2012). Aptroot et al. (Reference Aptroot, Lücking, Sipman, Umaña and Chaves2008) suggested that the T. eluteriae group might be closely related to Bathelium, a hypothesis that is not supported here. Makhija & Patwardhan (Reference Makhija and Patwardhan1992, Reference Makhija and Patwardhan1993) suggested that Trypethelium s. str. includes subgroups with slightly different pseudostromatal anatomy: those of T. eluteriae and T. sphaerocephalum are separated from the thallus by the absence of cortical, algal and medullary layers, and instead a cortical layer is produced beneath the pseudostroma, and ascomata are surrounded by a single layer, either hyaline or filled with yellow to orange crystals. In contrast, pseudostromata in T. subeluteriae contain a cortical layer both beneath and above them.
The only conceivable morphological difference between Viridothelium and Astrothelium are the subdistoseptate versus astrothelioid ascospores. The ascospores of Viridothelium closely resemble those of Trypethelium s. str., but the two genera differ in ascoma morphology: solitary to diffusely pseudostromatic in Viridothelium and distinctly pseudostromatic in Trypethelium s. str. Possible inclusion of Viridothelium within Trypethelium s. str. is rejected by the topology (SH test, P < 0·01).
The genus Bathelium is partially confirmed here as a monophyletic entity, but several species placed in this genus by Harris (Reference Harris1995) fall into Astrothelium, notably those with small, 3-septate ascospores and with rather low pseudostromata, such as A. degenerans. Bathelium s. str. instead produces strongly prominent to sessile, very conspicuous pseudostromata. This concept coincides in part with that of Trevisan (Reference Trevisan1853) and Massalongo (Reference Massalongo1860). An additional difference is that the ascospores are subdistoseptate in Bathelium and astrothelioid in Astrothelium, for example A. degenerans, a feature best observed in species with transversely septate ascospores.
The two genera Aptrootia and Architrypethelium, originally defined based on their peculiar ascospores (dark brown with a hard outer shell in Aptrootia and large, 3-septate and often dark brown in Architrypethelium), are confirmed as monophyletic and retained here. The ascospores of both genera pass through an astrothelioid stage before producing their characteristic mature structure (Sweetwood et al. Reference Sweetwood, Lücking, Nelsen and Aptroot2012) which supports the close relationship between these genera and Astrothelium.
Finally, Astrothelium as defined here includes the bulk of species in the family, characterized by rather thick, corticate thalli and astrothelioid ascospores. The genera Campylothelium, Cryptothelium, and Laurera are now considered synonyms of this genus and most species previously classified as Trypethelium are also placed here (Aptroot & Lücking Reference Aptroot and Lücking2016). Harris (Reference Harris1995) had already argued that the delimitation of these genera was artificial, and predicted that many species from these genera would eventually be placed in a single genus, although he suggested Laurera (Reichenbach Reference Reichenbach1841) as a potential name, which is, however, younger than Astrothelium (Eschweiler Reference Eschweiler1824). While most of the species in this considerably emended genus have rather thick, corticate, often olive-green thalli with the ascomata usually immersed or covered by a thallus layer, much variation is found in ascoma disposition, being either solitary or aggregate or fused with either apical or lateral ostioles; also, the ascospores range from transversely septate to muriform but are without exception hyaline. While muriform ascospores usually do not display diamond-shaped lumina, their ontogeny passes through an astrothelioid stage (Sweetwood et al. Reference Sweetwood, Lücking, Nelsen and Aptroot2012), explaining the observed close relationship between species with differently septate ascospores, for instance the well-known A. megaspermum with large, muriform ascospores, falling in a small, supported clade together with taxa with small, transversely septate ascospores, A. cinereorosellum and A. perspersum (Fig. 4).
The results presented here also provide a refined understanding of species delimitation in Trypetheliaceae. Characters such as thallus and ascoma morphology, hymenium inspersion, and the presence of lichexanthone, as used in Harris (Reference Harris1984), have been successively neglected when delimiting species, so that more recently, species were almost exclusively defined by pigments and ascospore septation and size (e.g. Harris Reference Harris1991, Reference Harris1995); even ascospore size was at some point considered of limited value, such as in Pseudopyrenula (Harris Reference Harris1998). On the other hand, taxa such as Trypethelium eluteriae and T. subeluteriae have been distinguished by successive workers based on very subtle morphological and chemical differences (Makhija & Patwardhan Reference Makhija and Patwardhan1992; Harris Reference Harris1995; Aptroot et al. Reference Aptroot, Lücking, Sipman, Umaña and Chaves2008). Without independent evidence, arguments for either splitting or lumping might be valid yet remain subjective, but fortunately can be tested using a phylogenetic framework. As an example, the separation of Trypethelium eluteriae and T. subeluteriae was supported by our results (Fig. 2).
More importantly, we repeatedly found that species originally identified with names using available keys were located in different places in the tree, especially in the newly defined genus Astrothelium, with the commonly applied names A. (Trypethelium) aeneum, A. nitidiusculum, and A. ochroleucum (Figs 3 & 4). These morphotaxa apparently comprise many, often unrelated species, such as A. leucosessile, A. kunzei, and A. inspersaeneum (Aptroot & Lücking Reference Aptroot and Lücking2016). Similar results were found in Pseudopyrenula and Nigrovothelium (Fig. 2), including the reinstated Pseudopyrenula endoxanthoides and the newly recognized Nigrovothelium bullatum (Aptroot & Lücking Reference Aptroot and Lücking2016; Lücking et al. Reference Lücking, Nelsen, Aptroot, Benatti, Binh, Gueidan, Gutiérrez, Jungbluth, Lumbsch and Marcelli2016). In these cases, examination of the sequenced material revealed that clades were distinguished by characters such as hymenium inspersion and presence of lichexanthone, but especially by the morphology of thallus and ascomata. Thus, features such as a bullate thallus or the degree of dispersion and aggregation or emergence of the ascomata and pseudostromata appear to be species-specific. This suggests that a much more refined species concept has to be applied within the family, similar to the situation found in Graphidaceae, especially the megadiverse genera Graphis and Ocellularia, where gross morphology had been similarly neglected but was found to be diagnostic (Lücking Reference Lücking2009, Reference Lücking2014, Reference Lücking2015; Lücking et al. Reference Lücking, Archer and Aptroot2009). Based on these findings, the refined species concept laid out in the monographic synopsis of the family (Aptroot & Lücking Reference Aptroot and Lücking2016) increases the number of recognized species in Trypetheliaceae based on names reinstated from prior synonymy by c. 70, in addition to well over 100 new species (Aptroot & Cáceres Reference Aptroot and Cáceres2016; Aptroot et al. 2016 Reference Aptroot, Ertz, Etayo, Gueidan, Mercado-Díaz, Schumm and Weerakoona , Reference Aptroot, Mendonça, Andrade, Silva, Martins, Gumboski, Fraga Júnior and Cáceresb ; Flakus et al. Reference Flakus, Kukwa and Aptroot2016; Lücking et al. Reference Lücking, Nelsen, Aptroot, Benatti, Binh, Gueidan, Gutiérrez, Jungbluth, Lumbsch and Marcelli2016).
Taxonomic Novelties
Constrictolumina Lücking, M. P. Nelsen & Aptroot gen. nov.
MycoBank No.: MB 816872
Differing from Arthopyrenia s. str. in the lichenized thallus and the ascospores with thicker walls, and usually forming secondary endospore thickenings resembling incomplete septa.
Type: Constrictolumina cinchonae (Ach.) Lücking, M. P. Nelsen & Aptroot (holotype).
Thallus not corticate.
Ascomata single, roughly conical, erumpent to prominent and more or less exposed, not in distinct pseudostromata but sometimes fused sideways. Ostiole apical. Hamathecium hyaline, clear, filaments thick at the base, thinner above, not anastomosing. Asci clavate. Ascospores 1–3-septate, rarely submuriform, with irregular endospore formation, sometimes with pseudosepta, often one or two cells with secondary endospore invaginations resembling incomplete septa, smooth or ornamented, hyaline, very rarely becoming brownish, often becoming granular ornamented.
Pycnidia sometimes present.
Notes. This aggregate of tropical, lichenized species is separated here for the first time into a formally described genus, following the realization that this group forms part of the family Trypetheliaceae, unrelated to the non-lichenized representatives of Arthopyrenia s. str. (Nelsen et al. Reference Nelsen, Lücking, Grube, Mbatchou, Muggia, Rivas Plata and Lumbsch2009, Reference Nelsen, Lücking, Mbatchou, Andrew, Spielmann and Lumbsch2011; Hyde et al. Reference Hyde, Jones, Liu, Ariyawansha, Boehm, Boonmee, Braun, Chomnunti, Crous and Dai2013). Details of the species were given primarily by Harris (Reference Harris1975, Reference Harris1995), as part of a variously defined genus Arthopyrenia s. lat. Including the two species sequenced and recombined here, the new genus unites nine tropical taxa treated elsewhere in this issue (Aptroot & Lücking Reference Aptroot and Lücking2016). Constrictolumina exhibits a unique hamathecium structure different from the remainder of the Trypetheliaceae, one of the reasons why this group was not previously suspected to be related to the latter.
Constrictolumina cinchonae (Ach.) Lücking, M. P. Nelsen & Aptroot comb. nov.
MycoBank No.: MB 816878
Verrucaria cinchonae Ach., Synops. Lich.: 90 (1814).—Arthopyrenia cinchonae (Ach.) Müll. Arg., Flora 66: 287 (1883); type: “cort. Cinchonae officinalis” (H-ACH 781B!—holotype).
Constrictolumina planorbis (Ach.) Lücking, M. P. Nelsen & Aptroot comb. nov.
MycoBank No.: MB 816879
Verrucaria planorbis Ach., Synops. Lich.: 92 (1814).—Arthopyrenia planorbis (Ach.) Müll. Arg., Mem. Soc. Phys. Genève 30: 27 (1888); type: “cort. Crotonis Cascarillae” (H-ACH, holotype, not seen).
Dictyomeridium Aptroot, M. P. Nelsen & Lücking gen. nov.
MycoBank No.: MB 816873
Differing from Polymeridium s. str. in the ascomata with laterial ostiole in combination with muriform ascospores.
Type: Dictyomeridium proponens (Nyl.) Aptroot, M. P. Nelsen & Lücking (holotype).
Thallus not corticate.
Ascomata single or a few aggregate, roughly conical to pyriform, erumpent to prominent and more or less exposed, not in distinct pseudostromata. Ostioles eccentric. Hamathecium hyaline, not inspersed. Asci with 2–8 ascospores. Ascospores muriform, hyaline, smooth, often IKI+ violet.
Pycnidia sometimes present.
Notes. This species aggregate is united here as a segregate of Polymeridium, with seven muriform-spored species with lateral ostiole (Aptroot & Lücking Reference Aptroot and Lücking2016). While Dictyomeridium is phylogenetically separate from Polymeridium, the morphological differences are subtle, since the latter includes some species with muriform ascospores or lateral ostioles, but not in combination. The type of Dictyomeridium, D. proponens, representing the most common species of the genus, was for a long time recognized under different names in the genus Campylothelium.
Dictyomeridium proponens (Nyl.) Aptroot, M. P. Nelsen & Lücking comb. nov.
MycoBank No.: MB 816880
Verrucaria proponens Nyl., Bull. Soc. Linn. Normandie, sér. 2 2: 130 (1868).— Polyblastia proponens (Nyl.) Müll. Arg. Flora 65: 402 (1882).—Campylothelium proponens (Nyl.) Müll. Arg., Hedwigia 31: 286 (1892).—Polyblastiopsis proponens (Nyl.) Zahlbr., Catal. Lich. Univ. 1: 351 (1922).—Polymeridium proponens (Nyl.) R. C. Harris, Bol. Mus. Paraense Emílio Goeldi, Ser. Bot., 7: 637 (‘1991’) [1993]; type: New Caledonia, Lifu, Loyalty Islands, Thiebaut. (H-NYL—holotype, not seen).
Nigrovothelium Lücking, M. P. Nelsen & Aptroot gen. nov.
MycoBank No.: MB 816875
Differing from Polymeridium in the corticate thallus and astrothelioid ascospores, from Bathelium in the mostly single, black ascomata and astrothelioid ascospores, and from Astrothelium in the fully exposed, sessile, black ascomata.
Type: Nigrovothelium tropicum (Ach.) Lücking, M. P. Nelsen & Aptroot (holotype).
Thallus corticate.
Ascomata usually single but often crowded and irregularly confluent, sessile, ovoid, not in pseudostromata. Ostiole apical. Hamathecium hyaline, clear, filaments thin, anastomosing paraphysoids, often inspersed with oil. Asci clavate. Ascospores transversely 3-septate, with distinct endospore formation creating diamond-shaped lumina (astrothelioid), hyaline.
Notes. The genus is described here to accommodate Trypethelium tropicum and at least one additional, newly recognized species (Lücking et al. Reference Lücking, Nelsen, Aptroot, Benatti, Binh, Gueidan, Gutiérrez, Jungbluth, Lumbsch and Marcelli2016). While this genus resembles other genera in certain characters, such as Pseudopyrenula and Astrothelium in the astrothelioid ascospores and Bathelium in the exposed, dark ascomata, its unique combination of characters and its phylogenetic position, sister to the morphologically distinct Polymeridium, merit its recognition as a separate taxon.
Nigrovothelium tropicum (Ach.) Lücking, M. P. Nelsen & Aptroot comb. nov.
MycoBank No.: MB 816881
Verrucaria tropica Ach., Lichenogr. Univ.: 278 (1810).—Sagedia tropica (Ach.) A. Massal., Ricerch. Auton. Lich.: 161 (1852).—Pyrenula tropica (Ach.) Trevis., Spighe e Paglie: 17 (1853).—Spermatodium tropicum (Ach.) Trevis., Conspect. Verruc.: 11 (1860).—Pseudopyrenula tropica (Ach.) Müll. Arg., Flora 66: 248 (1883).—Trypethelium tropicum (Ach.) Müll. Arg., Bot. Jahrb. Syst. 6: 393 (1885); type: America, Swartz (H-ACH 707A!—lectotype, designated here; BM-ACH—isolectotype).
Novomicrothelia Aptroot, M. P. Nelsen & Lücking gen. nov.
MycoBank No.: MB 816876
Similar to Bogoriella but forming a separate phylogenetic clade, with the following substitutions in the large subunit nuclear ribosomal DNA (nuLSU; relative positions following Supplementary Material A, available online): 46, 47, 308, 449, 458 (A replaces C); 117, 298, 379, 397, 447, 459 (A replaces G); 32, 68, 81, 109–111, 311, 316, 355 (A replaces T); 45, 70 (C replaces A); 98 (C replaces G); 41, 87, 106, 195, 196, 351, 419, 457, 461 (C replaces T); 36, 383 (G replaces A); 322 (G replaces C); 44 (G replaces T); 83, 266, 307, 321, 361 (T replaces A); 40, 97, 320, 385, 440, 474 (T replaces C); 352, 354 (T replaces G); also differing from most species of Bogoriella in the inspersed hamathecium and ascospore wall invaginations besides the (sub-)median septum.
Type: Novomicrothelia oleosa (Aptroot) Aptroot, M. P. Nelsen & Lücking (holotype).
Thallus not corticate.
Ascomata single, roughly conical, erumpent to prominent and more or less exposed, not in pseudostromata. Ostiole apical. Hamathecium hyaline, inspersed with oil droplets, filaments thin, anastomosing paraphysoids. Asci clavate. Ascospores transversely 1-septate, with irregular endospore formation, becoming ornamented, brown, rather elongated.
Pycnidia sometimes present.
Discussion. The genus is described here to accommodate a single species that was until now united with temperate, non-lichenized fungi in the genus Mycomicrothelia. Novomicrothelia is phylogenetically distinct from the morphologically similar, reinstated genus Bogoriella but the phenotypic distinction between the two genera is not very clear yet; more data are needed for these basal lineages within the family to fully understand their phylogeny and classification. According to Harris (Reference Harris1995), N. oleosa is unique based on its inspersed hamathecium and ascospore wall invaginations similar to those found in Constrictolumina but the small number of species of Bogoriella sequenced so far does not allow us to conclude whether these are consistent differences. Two species currently accepted in Bogoriella have an inspersed hamathecium (Aptroot & Lücking Reference Aptroot and Lücking2016) but these have not yet been sequenced.
Since the phenotypic differences between Novomicrothelia and Bogoriella are not yet clear, but both form distantly related clades for which monophyly as a single clade was rejected, we provided diagnostic molecular features as allowed by the Code. The Code specified in such a case that the differential characters (i.e. relative columns and substitutions) need to be spelled out, and we provide a possible model for this case. It is obvious that further data might change these characters, in particular reduce the number of diagnostic columns, but since this applies analogously to any phenotypic characters when further data are added, it does not make the diagnosis invalid.
Novomicrothelia oleosa (Aptroot) Aptroot, M. P. Nelsen & Lücking comb. nov.
MycoBank No.: MB 816882
Mycomicrothelia oleosa Aptroot, Biblioth. Lichenol. 44: 133 (1991); type: Trinidad, Caroni, north bank road, Britton et al. 869 (NY!—holotype).
Viridothelium Lücking, M. P. Nelsen & Aptroot gen. nov.
MycoBank No.: MB 816877
Differing from Astrothelium in the subdistoseptate ascospores and from Trypethelium s. str. in the absence of well-defined pseudostromata.
Type: Viridothelium virens (Tuck. ex Michener) Lücking, M. P. Nelsen & Aptroot (holotype).
Thallus corticate, often warted.
Ascomata simple or aggregated in pseudostromata, which can be hardly to clearly raised and are usually not of a different structure and colour from the thallus. Ostioles apical or eccentric, simple or fused. Wall hyphal (textura intricata), usually carbonized. Hamathecium inspersed with oil droplets or not, filaments thin, anastomosing paraphysoids. Ascospores subdistoseptate, with thin walls and only slightly thickened septa, hyaline, I– or weakly I+ violet-blue, transversely septate.
Pycnidia occasionally present.
Notes. This new genus accommodates Trypethelium virens, a taxon that has long been considered unique due to its northern temperate distribution and the non-astrothelioid, I+ weakly amyloid ascospores (Aptroot & Lücking Reference Aptroot and Lücking2016; Aptroot et al. 2016 Reference Aptroot, Ertz, Etayo, Gueidan, Mercado-Díaz, Schumm and Weerakoona , Reference Aptroot, Mendonça, Andrade, Silva, Martins, Gumboski, Fraga Júnior and Cáceresb ; Lücking et al. Reference Lücking, Nelsen, Aptroot, Benatti, Binh, Gueidan, Gutiérrez, Jungbluth, Lumbsch and Marcelli2016); several further, tropical species are also included in this clade. Viridothelium is superficially similar to Astrothelium but appears in a distant phylogenetic position; the main difference lies in the subdistoseptate ascospores resembling those of Trypethelium s. str.
Viridothelium virens (Tuck. ex Michener) Lücking, M. P. Nelsen & Aptroot comb. nov.
MycoBank No.: MB 816883
Trypethelium virens Tuck. ex Michener, W. Dard. Fl. Cest. ed. 3: 453 (1853).—Trypethelium eluteriae var. virens (Tuck. ex Michener) Trevis., Flora 44: 20 (1861); type: USA, Arkansas, Dardanelle, Michener, 1853 (FH—holotype, not seen; M!—isotype).
We are grateful to a number of organizations for funding including: NSF-DEB 0715660 “Neotropical Epiphytic Microlichens – An Innovative Inventory of a Highly Diverse yet Little Known Group of Symbiotic Organisms” to The Field Museum (PI Robert Lücking), a grant from the Committee on Evolutionary Biology (University of Chicago) to MN, and the Caterpillar® Company provided funds to study lichens from Panama. The American Society of Plant Taxonomists is also acknowledged for a Graduate Student Research Grant awarded to MN. Additionally, MN’s work was supported the University of Chicago and by a Brown Family Fellowship through the Field Museum, as well as by a William Harper Rainey Fellowship through the University of Chicago. The CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) is thanked for a research grant and field trip funding (Processos 311706/2012-6 and CNPq-Sisbiota Processo 563342/2010-2) to MESC. As part of the Census of Galapagos Biodiversity, the Galapagos Lichen Inventory received funds from several donors (for a detailed list see http://www.darwinfoundation.org/datazone/checklists). The lichen inventory in particular received funds from The Bay and Paul Foundations, the Erwin Warth Stiftung, NSF (grant no. DEB 0841405) and, most recently, the Mohamed bin Zayed Species Conservation Fund, project no. 152510692. This publication is contribution number 2143 of the Charles Darwin Foundation for the Galapagos Islands. We especially thank Galo Quedaza and Victor Carrión from the Galapagos National Park for technical support and specimen export permits for Galapagos material analyzed in this study. Logistical support was provided by the University of Panama (Department of Botany) in the development of two lichen seminars (2009, 2011) under the program Neotropical Epiphytic Microlichens in which the lichens were collected. Thanks also to Park authorities and rangers from Parque Nacional Altos de Campana during the field trips and to the Ministry of the Environment (former A.N.A.M.) for collection and export permits. The Universidad Distrital Francisco José de Caldas in Bogotá, Colombia, is thanked for providing support to BM as part of the program ‘Mobilidad Académica’ for professors. The Natural History Museum in London is thanked for research and travel funds to CG and the Vietnam National Museum of Nature in Hanoi for organizing the fieldwork.
Supplementary Material
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0024282916000505







