Starch, acting as the major energy-yielding component of the daily diet, is the main carbohydrate in mammal (including human) nutrition(Reference Knudsen, Lærke and Steenfeldt1). The glucose release as a source of energy for the body and the timeline of digestion are the major physiological properties of starch(Reference Wiseman2). Previous research has found that the digestibility of starch in the small intestine of mammals can be modified from a rapid digestion to indigestibility(Reference Englyst, Kingman and Cummings3); thus for nutritional purposes, starch has been divided into rapidly digestible starch, slowly digestible starch and resistant starch (RS) to specify its nutritional quality related to physiological response and health effects(Reference Englyst and Hudson4). Rapidly digestible starch leads to a rapid increase in blood glucose and insulin levels(Reference Englyst, Englyst and Hudson5), whereas slowly digestible starch has moderate glycaemic and insulinaemic responses. The same results were observed in pigs by Van der Meulen et al. (Reference Van der Meulen, Bakker and Smits6) and Noah et al. (Reference Noah, Krempf and Lecannu7). However, the latest research from Liu et al. (Reference Liu, Zhang and Bin8) was rather different from the Van der Meulen et al. (Reference Van der Meulen, Bakker and Smits6) and Noah et al. (Reference Noah, Krempf and Lecannu7) research. A worthwhile finding that postprandial blood glucose and insulin levels were higher in pigs fed diets containing rapidly digestible starch than those fed diets containing maize starch (MS) and RS within 4 h was found when our team studied the papers of Van der Meulen et al. (Reference Van der Meulen, Bakker and Smits6), Noah et al. (Reference Noah, Krempf and Lecannu7) and Liu et al. (Reference Liu, Zhang and Bin8). Besides, the degree of digestion and the rate of starch digestion in different segments of the small intestine in pigs are still unclear. Furthermore, there is some debate as to whether slowly digested starch or rapidly digested starch will lead to higher postprandial systemic circulating amino acid (AA) levels. We hypothesised that if pigs consumed their meals every 4 h, and six times within 24 h, higher blood metabolite levels would be obtained, as well as better growth performance. The objectives of the present study were to determine the digestibility of starch by in vitro and in vivo methods, and to evaluate the postprandial systemic circulating levels of blood metabolites (mainly AA) in pigs under a ‘six time intake per d’ feeding procedure.
Materials and methods
Preparation of starches
MS and sticky rice starch (SRS) were commercially available from Changsha food market (Changsha, Hunan, China). RS was purchased from National Starch Specialties (Shanghai) Limited (Shanghai, Jiangsu, China).
Animals, experimental design and diets
The present study involved an in vitro digestibility trial (experiment 1) and an animal experiment (experiment 2). The protocol for the animal experiment was approved by the Animal Care and Use Committee of the Institute of Subtropical Agriculture, The Chinese Academy of Sciences.
Experiment 1: in vitro digestibility trial
The in vitro digestibility of dietary starch was determined based on the previous method described by Englyst et al. (Reference Englyst, Kingman and Cummings3) and Hung & Morita(Reference Hung and Morita9) with minor modification. Briefly, 100 mg sample was incubated with porcine pancreatic α-amylase (no. 7545; Sigma-Aldrich, St Louis, MO, USA) and amyloglucosidase (no. 9913, Sigma-Aldrich) in 4 ml of a 0·1 m-sodium maleate buffer (pH 6·0) in a shaking water-bath (200 strokes/min) at 37°C for 0·5 to about 6 h. After incubation, ethanol (95 %) was added and the sample was then centrifuged at 3000 rpm for 10 min. The glucose content of the supernatant fraction was measured using a CX4PRO Select Biochemistry Analyser (Beckman Coulter Inc., Fullerton, CA, USA). The digested starch content was thus determined from the glucose content in the supernatant fraction. Digestibility is expressed as the ratio of the content of digested starch at each incubation time point to the content of the total starch of the sample.
Experiment 2: animal experiment
Eighteen barrows, weaned at age 21 d with an average initial body weight of 7·04 (sd 0·52) kg, were allocated on the basis of weight and litter of origin to three dietary treatments in a randomised complete block design. Each pig was surgically fitted with a catheter in the jugular vein according to the procedures described by Huang et al. (Reference Huang, Yin and Wang10) and Li et al. (Reference Li, Dai and Yin11). The preparation of catheters and detailed description of pre- and post-operative care were previously described by Li et al. (Reference Li, Dai and Yin11). The pigs were returned to the metabolic crates immediately after surgery. Each crate was equipped with a suspended water line fitted with a low-pressure nipple and wire flooring. During a 3 d recovery period, an antibiotic (penicillin) was administered intravenously to the animal. The catheters were checked for potency by flushing and filling with sodium heparin solution daily. The skin around the catheter was cleaned with lukewarm water daily, dried, and a skin-protecting paste was applied (Stomahesive Paste; ConvaTec, Princeton, NJ, USA). The pigs were trained to adapt to a new feeding procedure. Briefly, all pigs were fed six times daily (04.00, 08.00, 12.00, 16.00, 20.00 and 24.00 hours) and trained to consume their meals within 10 min. Water was freely available. The temperature was kept at 26 ± 2°C, and relative humidity was maintained from 60 to 75 %. Following recovery, the pigs were fed the experimental diets.
Venous blood samples were taken from each pig via a catheter into 5 ml heparin-free vacutainer tubes (Becton Dickinson Vacutainer Systems, Franklin Lakes, NJ, USA) hourly from 08.30 to 15.30 hours on day 7. All samples were centrifuged at 3000 rpm (Heraeus Biofuge 22R Centrifuge; Hanau, Germany) for 10 min at 4°C, and serum samples were immediately separated and placed in test-tubes and stored at − 20°C for later analysis. The pigs were still fed six times daily according to the feeding procedure during the sample collection period. On day 8, all pigs were fed at 08.00 hours and then euthanised at 11.00 hours. Digesta samples were collected from the anterior jejunum, posterior jeunum, anterior ileum and posterior ileum of each pig and stored at − 20°C. When the sampling was completed, all digesta samples were freeze-dried and ground through a 0·10 mm mesh screen for chemical analysis.
Dietary crude protein, nutritional indispensable AA, vitamins and minerals were supplemented to meet or exceed the National Research Council's nutritional requirements for swine(12) with body weight of 5–10 kg. Ingredients and AA composition of the diets are summarised in Tables 1 and 2, respectively.
Table 1 Ingredients and chemical composition of the experimental diets

MS, maize starch; SRS, sticky rice starch; RS, resistant starch.
* Provided by Guangzhou Tianke Industry Co. (Guangzhou, Guangdong, China).
† Supplied (per kg diet): vitamin A, 6 mg; vitamin D3, 8 mg; vitamin E, 30 mg; vitamin K, 3 mg; vitamin B2, 27 mg; vitamin B6, 2 mg; vitamin B12, 30 μg; biotin, 80 μg; folic acid, 8 mg; nicotinic acid, 24 mg, Na (NaCl), 3 g, Zn (ZnSO4), 165 mg; Fe (FeSO4), 165 mg; Mn (MnSO4), 33 mg; Cu (CuSO4), 165 mg, iodine (CaI2), 297 μg; Se (Na2SeO3), 297 μg.
Table 2 Analysed amino acid composition of the experimental diets (%)
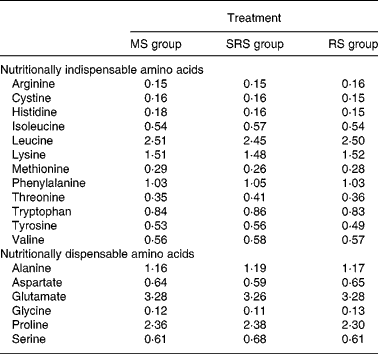
MS, maize starch; SRS, sticky rice starch; RS, resistant starch.
Chemical analysis
DM and crude protein contents were analysed according to AOAC procedures(13). Total starch content was measured by American Association of Cereal Chemists (AACC) method 76·13(14). Serum AA concentrations were determined using a Hitachi L-8800 Amino Acid Analyser (Tokyo, Japan), as previously described by Yao et al. (Reference Yao, Yin and Chu15). AA analyses of the diet and posterior ileum digesta were previously described by Yin et al. (Reference Yin, Liu and Yin16). Titanium oxide concentration was determined according to the method described by Yin et al. (Reference Yin, McEvoy and Schulze17).
The apparent digestibility of dietary starches in different fragments of the small intestine and the apparent digestibility of AA in the digesta of the posterior ileum were calculated using the following equations, as described by Fan et al. (Reference Fan, Li and Yin18) with minor modification:
where SAD is the apparent small intestine digestibility of starch or AA, Sf is the total starch or AA concentration in the diet, Sd is the total starch or AA concentration in the small intestine digesta, TiO2f is the titanium oxide concentration in the diet and TiO2d is the titanium oxide concentration in the digesta.
Statistical analysis
All physico-chemical analyses were performed at least in duplicate. The data on in vitro and in vivo digestibility of starches and apparent posterior ileum digestibility of AA were analysed by one-way ANOVA using the general linear model (GLM) procedure of SAS for a randomised complete block design (SAS Institute, Inc., Cary, NC, USA). The data on serum-free AA concentrations were analysed as a split-plot design for repeated measures using the GLM procedure of SAS. The statistical model included the effect of treatment as the main plot (tested by the animal within treatment variance) and the effects of time and the treatment × time interaction as the subplots. Comparisons among treatments within sampling time were made when a significant F test (P < 0·05) for the treatment × time interaction was observed. Duncan's multiple-comparison test was used to determine differences among the means of treatment groups. P < 0·05 was taken to indicate statistical significance.
Results
In vitro digestibility of dietary starch (experiment 1)
The digestibility of the three dietary starches was increased when the incubation time was extended (Table 3). SRS was hydrolysed more quickly (P < 0·05) than MS and RS, and was almost completely hydrolysed within 4 h. The hydrolysis rate of MS remained at a slow and steady pace, and 95·76 % was hydrolysed within 6 h. The hydrolysis rate of RS was even slower (P < 0·05) than that of MS, and only 52·66 % was hydrolysed when the incubation time was extended to 6 h.
Table 3 In vitro digestibility of dietary starch at different incubation times (%) (n 6 per group)
(Mean values and pooled standard errors of the mean)
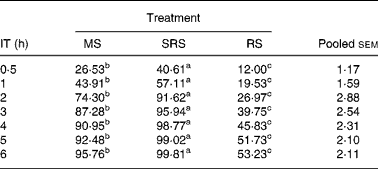
IT, incubation time; MS, maize starch; SRS, sticky rice starch, RS, resistant starch.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
Animal experiment (experiment 2)
In experiment 2, pigs were healthy and consumed their meals. In this experiment, the pigs in the SRS group consumed their meals within 6 min, which was faster than the pigs in the other two groups. Although pigs in the MS and RS groups consumed their diets slowly, they still completed their feed intake within 10 min. All pigs were euthanised on day 8. Examination of the catheter site revealed no abnormalities.
In vivo digestibility of dietary starches
The degree of hydrolysis of SRS in different segments of the small intestine was higher (P < 0·05) than that of MS, and that of MS was also higher (P < 0·05) than of RS in turn (Table 4). Notably, compared with MS and RS, SRS was easily (P < 0·05) hydrolysed, 81·90 % of which was hydrolysed in the anterior jejunum, but not more than half of MS and RS was hydrolysed in the same site of the jejunum. SRS was completely digested in the posterior ileum, and 93·08 % of MS was also hydrolysed in the same site of the ileum. RS was difficult to hydrolyse in the small intestine; only 67·48 % of RS was hydrolysed in the posterior ileum.
Table 4 Digestibility of dietary starches in different segments of the small intestine in piglets (%) (n 6 per group)
(Mean values and pooled standard errors of the mean)
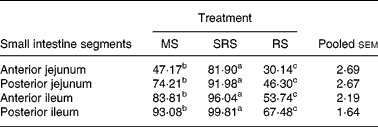
MS, maize starch; SRS, sticky rice starch, RS, resistant starch.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
Apparent posterior ileum digestibility of amino acids
The digestibility of isoleucine, leucine, methionine, phenylalanine, threonine, tryptophan, valine, alanine, aspartate and serine in the SRS group was higher (P < 0·05) than in the MS group (Table 5). All nutritionally indispensable and dispensable AA in the SRS group were higher when compared with those in the RS group (P < 0·05). The digestibility of lysine, phenylalanine, tyrosine, valine, glutamate and proline in the MS group was higher (P < 0·05) than in the RS group.
Table 5 Apparent posterior ileum digestibility of amino acids (%) (n 6 per group)
(Mean values and pooled standard errors of the mean)

MS, maize starch; SRS, sticky rice starch, RS, resistant starch.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
Serum circulating amino acids
The postprandial serum concentration of AA at different time points and variation in postprandial systemic circulating lysine, methionine and tryptophan are summarised in Tables 6 and 7 and Figs. 1–3, respectively. All AA were affected (P < 0·05) by treatment as well as time and treatment × time interaction. Consequently, comparisons of the means among treatments within sampling time were made. At 08.30 hours, concentrations of arginine, cystine, leucine, lysine, threonine, tryptophan, tyrosine, alanine, glycine, proline and serine in the SRS group and leucine and proline in the MS group were higher (P < 0·05) than in the RS group. Threonine and alanine in the SRS group were higher (P < 0·05) than in the MS group. At 09.30 hours, concentrations of all nutritionally indispensable AA, alanine, glycine and proline in the SRS group, as well as lysine and proline in the MS group, were higher (P < 0·05) but concentrations of glutamate in the SRS group and threonine and valine in the MS group were lower (P < 0·05) than in the RS group. Concentrations of most nutritionally indispensable AA, except for lysine, and alanine and proline in the SRS group were higher (P < 0·05) than in the MS group. At 10.30 hours, concentrations of arginine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, tyrosine, valine, alanine, glutamate, glycine and proline in the SRS group were higher (P < 0·05) than in the RS group, and arginine and proline in the MS group likewise. Concentrations of arginine, lysine, methionine, phenylalanine, tyrosine, valine and proline in the SRS group were higher (P < 0·05) than in the MS group. At 11.30 hours, concentrations of lysine, methionine, threonine, tryptophan, tyrosine, valine, alanine, aspartate, glutamate, glycine in the SRS group and lysine, threonine, alanine and glycine in the MS group were higher (P < 0·05) but glutamate in the MS group was lower (P < 0·05) compared with the RS group. Concentrations of isoleucine, methionine, valine, aspartate and glutamate in the SRS group were higher (P < 0·05) than in the MS group. During the second feeding cycle, from 12.30 to 15.30 hours, the variation in postprandial systemic circulating AA was the same as that observed during the first feeding cycle, from 08.30 to 11.30 hours.
Table 6 Serum amino acid concentrations after first feeding (mmol/l) (n 6 per group)
(Mean values and pooled standard errors of the mean)

MS, maize starch; SRS, sticky rice starch, RS, resistant starch.
a,b,c Mean values within a row, within the same sampling time, with unlike superscript letters were significantly different (P < 0·05).
Table 7 Serum amino acid concentrations after second feeding (mmol/l, continued from Table 6) (n 6 per group)
(Mean values and pooled standard errors of the mean)

MS, maize starch; SRS, sticky rice starch, RS, resistant starch.
a,b,c Mean values within a row, within the same sampling time, with unlike superscript letters were significantly different (P < 0·05).

Fig. 1 Variation in postprandial serum systemic circulating lysine in two feeding cycles. (![]() ), Maize starch-fed group; (
), Maize starch-fed group; (![]() ), sticky rice starch-fed group; (
), sticky rice starch-fed group; (![]() ), resistant starch-fed group. Values are means (n 6 per group). a,b Mean values, within the same sampling time, with unlike letters were significantly different (P < 0·05).
), resistant starch-fed group. Values are means (n 6 per group). a,b Mean values, within the same sampling time, with unlike letters were significantly different (P < 0·05).

Fig. 2 Variation in postprandial serum systemic circulating methionine in two feeding cycles. (![]() ), Maize starch-fed group; (
), Maize starch-fed group; (![]() ), sticky rice starch-fed group; (
), sticky rice starch-fed group; (![]() ), resistant starch-fed group. Values are means (n 6 per group). a,b Mean values, within the same sampling time, with unlike letters were significantly different (P < 0·05).
), resistant starch-fed group. Values are means (n 6 per group). a,b Mean values, within the same sampling time, with unlike letters were significantly different (P < 0·05).

Fig. 3 Variation in postprandial serum systemic circulating tryptophan in two feeding cycles. (![]() ), Maize starch-fed group; (
), Maize starch-fed group; (![]() ), sticky rice starch-fed group; (
), sticky rice starch-fed group; (![]() ), resistant starch-fed group. Values are means (n 6 per group). a,b Mean values, within the same sampling time, with unlike letters were significantly different (P < 0·05).
), resistant starch-fed group. Values are means (n 6 per group). a,b Mean values, within the same sampling time, with unlike letters were significantly different (P < 0·05).
Discussion
Starch was once presumed to be almost completely digested in mammals at all ages; however, recent research has found that the digestibility of starches from different sources in the mammal small intestine are different. Starches with a high amount of amylose are hard to hydrolyse, whereas fully gelatinised amylopectin is easily digested, which serves as a source of rapidly digestible starch(Reference Englyst, Veenstra and Hudson19). Thus, the ratio of amylose:amylopectin affects starch digestibility and its physiological response. In the present study, MS contains 23·6 % of amylose and 76·4 % of amylopectin, SRS contains 100 % of amylopectin and RS contains 96·5 % amylose as determined in our preliminary experiment. The present results confirmed that amylopectin is more easily digested than amylose in an in vitro digestibility model, as well as in the pig small intestine again. In vitro digestibility of SRS is significantly higher than that of MS and RS at different time points within 6 h incubation. Furthermore, 81·90 % of SRS was digested in the anterior jejunum, while only 47·17 % of MS and 30·14 % of RS were digested in the same segments of the small intestine. As the digesta flowed into different segments of the small intestine, the digestibilities of MS, SRS and RS were increased but the increasing ranges of SRS were lower than of MS and RS. Human clinical data showed that RS and slowly digestible starch offered the advantage of a slow increase in postprandial blood glucose levels, and sustained blood glucose levels over time compared with rapidly digestible starch, such as SRS, with its fast and high peak and fast decline(Reference Cummings, Beatty and Kingman20–Reference Lehmann and Robin22). Similar results were also observed in pigs by Van der Meulen et al. (Reference Van der Meulen, Bakker and Smits6) and Noah et al. (Reference Noah, Krempf and Lecannu7); thus the present results of dietary starch digestibility in different segments of the small intestine can explain why slowly digestible starch, rapidly digestible starch and RS have different effects on postprandial blood glucose and insulin levels.
The small intestine is an important organ that is responsible for the digestion of dietary starch and protein, as well as the absorption of free glucose, small peptides and free AA(Reference Gary23, Reference Libao-Mercado, Yin and van Eys24). Different fractions of nutritional ingredients in the small intestine would improve or inhibit each other's absorption into enterocytes. In the present study, amylopectin was digested rapidly but amylose was digested slowly and increased the viscosity of digesta(Reference Sasaki, Yasui and Matsuki25). Increasing the viscosity of digesta could inhibit the nutritional ingredients making contact with digestive enzymes, thus decreasing the digestibility of nutritional ingredients(Reference Siddhuraju and Becker26–Reference Owens, Zinn and Kim28), such as protein and AA. Such digestion-resistant effects may be enhanced as the dietary concentration of amylose increases(Reference Åkerberg, Liljeberg and Björck29). This is one of the reasons why the in vivo digestibility of SRS is greater in the anterior jejunum (Table 5) and the apparent posterior ileum digestibility of AA in the SRS group is higher than in the MS and RS groups. Increased digestibility of protein would result in increased absorption of free AA into enterocytes. Although branched-chain AA, aspartate, glutamate, glutamine, proline and arginine are extensively catabolised by enterocytes of post-weaning pigs(Reference Wu, Knabe and Flynn30, Reference Wu31), degradation of other AA is absent or negligible in these cells(Reference Chen, Yin and Jobgen32). Thus, serum concentrations of nutritionally indispensable AA in the SRS group were remarkably higher at 09.30 and 13.30 hours (Tables 5 and 6). Because muscle protein synthesis is very sensitive to the circulating levels of AA in young pigs(Reference Davis, Burrin and Fiorotto33, Reference Frank, Escobar and Hguyen34) via mammalian target of rapamycin (mTOR) and perhaps other signalling pathways(Reference Davis, Nguyen and Suryawan35, Reference Jobgen, Fried and Fu36), the higher levels of serum concentrations of AA would promote protein accretion and thus growth performance in early-weaned pigs.
We have observed that postprandial serum glucose concentration in the SRS group was higher and could be sustained for 3·5 h after the pigs consumed their meals(Reference Yin, Huang and Zhang37). Glucose is an important signal molecule in regulating the AA transporters and stimulating protein synthesis through an mTOR pathway(Reference Roos, Lagerlöf and Wennergren38–Reference Gleason, Lu and Witters40). Higher levels of serum glucose amend the phosphorylation level of mTOR thus increasing the amount of AA absorbed into the systemic circulation to meet the needs of protein synthesis. This is the second reason for the higher digestibility of AA and serum AA levels in the SRS group.
In summary, although the precise mechanisms responsible for affecting postprandial serum AA levels of dietary starches with different digestion rates remains to be explored, it was indicated from our present study that dietary starches digested rapidly in vitro would positively reflect higher digestibility of those starches in the anterior small intestine of pigs. A diet containing higher levels of rapidly digestible starch ameliorates the digestive and absorptive function and keeps the systemic circulating concentrations of most AA higher within 4 h postprandially in pigs.
Acknowledgements
This research was supported by grants from the National Natural Science Foundation of China (NSFC; no. 30671517). The authors are grateful to all other staff in the Laboratory of Animal Nutritional Physiology and Metabolic Process, Institute of Subtropical Agriculture, the Chinese Academy of Sciences for their assistances in the present study.
Y. Y. was in charge of the whole trial. F. Y. conducted the in vitro digestibility trial, animal experiment and wrote the whole of the paper. Z. Z. and J. H. assisted with the animal trial and chemical analyses.
The authors have no conflicts of interest.












