International clinical guidelines recommend multi-component weight management programmes as the treatment of choice in challenging the burgeoning obesity epidemic( 1 – Reference Yumuk, Tsigos and Fried 4 ). Multi-component weight management programmes have been shown to support sustainable weight losses of 5–10 % body weight and associated with clinical improvements in reducing health risk factors( 2 , 3 ). Effective components primarily include a personalised energy deficit diet (EDD) involving a 2514 kJ (600 kcal) reduction in total energy intake per day, support to increase physical activity and behaviour change techniques( 2 , 3 , Reference Loveman, Frampton and Shepherd 5 ).
Adults with intellectual disabilities (defined as a significant impairment in intellectual functioning and adaptive behaviour including practical and social skills)( Reference Schalock, Borthwick-Duffy and Bradley 6 ) have been reported to have a higher prevalence of obesity and associated health inequalities in comparison to the general population( Reference Melville, Hamilton and Hankey 7 – Reference Hsieh, Rimmer and Heller 10 ). Despite adverse effects of obesity on the health of adults with intellectual disabilities, little is known about whether evidenced based approaches to weight management are effective for this population group. The current evidence has identified a paucity of weight management programmes for adults with intellectual disabilities( Reference Hamilton, Hankey and Miller 11 – Reference Spanos, Melville and Hankey 13 ). In a recent review( Reference Spanos, Melville and Hankey 13 ), only eight studies were classified as multi-component and were primarily focused on a health education approach, with only one offering participants an EDD( Reference Saunders, Saunders and Donnelly 14 ). The effect sizes of these studies were small and non-significant, and studies were limited in their reporting of clinical effectiveness. Furthermore, studies were of poor methodological quality, and only one study including a multi-component programme implemented a randomised control trial design( Reference Jackson and Thorbecke 15 ).
To fill the gap in current knowledge, TAKE 5 was developed and piloted( Reference Melville, Boyle and Miller 16 – Reference Spanos, Hankey and Melville 18 ). TAKE 5 is a multi-component weight management programme developed to reflect UK weight management guidelines( 2 , 3 ). TAKE 5 was modelled on the approach used by a weight management service in the UK, the Glasgow and Clyde Weight Management Service (GCWMS)( Reference Morrison, Boyle and Morrison 19 , Reference Logue, Allardice and Gillies 20 ). The approach used by GCWMS, developed for the general population, was adapted for adults with intellectual disabilities and implemented with support from participants’ carers where applicable. Results of the single-stranded study illustrated that TAKE 5 was acceptable to adults with intellectual disabilities and their carers and demonstrated significant reductions in health risk factors including weight, BMI, waist circumference, sedentary behaviour and increased physical activity levels( Reference Melville, Boyle and Miller 16 – Reference Spanos, Hankey and Melville 18 ). Based on these findings and initial support for a multi-component programme utilising an EDD in adults with intellectual disabilities, the aim of this study was to carry out a cluster randomised controlled trial of TAKE 5 in comparison to a health education programme (providing information on diet and physical activity behaviours).
Methods
Study design
This study was designed as a pilot, single-centre, single-blind randomised trial. Treatment as usual (TAU) for adults with intellectual disabilities in the UK, varies from no weight management programmes to community-based health education programmes. Therefore, to provide a standardised TAU, the Waist Winners Too (WWToo) health education programme was used as the control arm of the study( Reference Jones, Melville and Tobin 21 ). Participants were randomised to TAKE 5 or WWToo for a 12-month period; a 6-month weight loss period followed by a 6-month weight maintenance period. The trial was registered before data collection (http://www.isrctn.com/ ISRCTN52903778) and the study protocol has previously been published( Reference Harris, Melville and Jones 22 ). The trial is reported according to CONSORT guidelines for reporting cluster randomised designs( Reference Campbell, Piaggio and Elbourne 23 ) and followed guidelines by the Medical Research Council on developing and evaluating ‘complex’ interventions( 24 , 25 ).
Ethical approval
The study was conducted in accordance with the ethical guidelines of the Declaration of Helsinki and consistent with the principles of Good Clinical Practice. Ethical approval was received from the Scotland A Research Ethics Committee (reference no.: 13/SS/0229) on 16 December 2013. The process of obtaining consent was conducted in accordance with the Adults with Incapacity Act, 2000( Reference Executive 26 ). Written informed consent was obtained from participants who had capacity. In circumstances where a participant did not have capacity, written informed consent was provided by the nearest relative or welfare guardian.
Study population
A multi-point recruitment strategy developed by Foster et al. ( Reference Foster, Brennan and Matthews 27 ) was utilised to recruit participants from multiple organisations in Greater Glasgow & Clyde, Scotland, including specialist intellectual disabilities services, provider organisations and local day centres. Participants were eligible to take part if they were diagnosed with intellectual disabilities (all level of intellectual disabilities were included, mild to profound), adults (≥18 years), obese (BMI ≥30 kg/m2), ambulatory, not currently on a prescribed or restricted diet (e.g. for phenylketonuria or diabetes) and had not intentionally lost weight of >3 kg in the previous 3 months. Participants were excluded if they had the following genetic syndromes; Prader–Willi syndrome, Cohen syndrome or Bardet–Biedl syndrome, taking medication for the purpose of losing weight (either prescribed or over the counter) and individuals who were pregnant or became pregnant during the study.
Randomisation
Cluster randomisation was conducted to minimise potential risk of contamination between programmes, clustering of outcomes and to minimise imbalance between study groups. If participants lived together (in shared tenancies) or were supported by the same group of paid carers they were randomised in a cluster. Participants were stratified by presence of Down’s syndrome, level of intellectual disabilities and number of participants within a cluster. Randomisation was implemented by the researcher (L. H.) using an interactive voice response system. This was hosted by the Robertson Centre for Biostatistics, University of Glasgow. The researcher was blinded to participant group allocation. Participants were enrolled in the study through notification of the principal investigator via email of the group allocation.
Sample size
In the single-stranded pilot study, the sample size and recruitment were based on the referral of adults with intellectual disabilities to specialised intellectual disabilities dietitians( Reference Melville, Boyle and Miller 16 ). The sample size for this study was calculated separately based on the feasibility of conducting a full-scale trial and to provide insight into recruitment and retention rates, which would have a 95 % CI of no more than ±10 %; to determine the probable variance of outcomes to power a larger trial. If fifty participants provide 12-month outcome data, a 90 % CI for each variance estimate would have a width of approximately 70 % of the estimate (i.e. −29 to +41 %); and to allow an attrition rate of 20 %. Before this study, there were no cluster randomised controlled trials of weight management programmes to inform the degree of clustering. Therefore, the sample size was increased by 10 % based on the following assumption of a conservative intra-class correlation coefficient (ICC) of 0·1, and an average of two participants per cluster. Thus, the final samples size was projected to be sixty-six participants (thirty-three participants in each programme).
Weight management programmes
The two weight management programmes under investigation were both made up of a diet, physical activity and behaviour change components. The distinct difference between the two programmes was based on the inclusion of the quantitative EDD in the TAKE 5 programme. However, the diet component in the WWToo programme was based on general advice, with which compliance cannot be quantified. The physical activity component of the TAKE 5 programme is more comprehensive as it included the use of pedometers. Finally, the behaviour change techniques utilised in the TAKE 5 programme were more numerous and used more flexibly to meet the individual needs of participants.
A dietitian and a health professional, trained for the purpose of this study, delivered both programmes. Both had experience in delivering lifestyle programmes to adults with intellectual disabilities. To ensure fidelity and minimise contamination between the programmes, the dietitian and health professional followed a pre-set protocol and manual for each session. Weight management programmes were delivered on a one-to-one basis in the participant’s home or convenient location. The programme sessions were delivered over a 12-month period, nine to twelve sessions in the weight loss phase and six sessions in the weight maintenance phase. The variability in the number of weight loss sessions was to allow appointments to be organised flexibly to maximise the consistent involvement of family and paid carers. Nine weight loss sessions were attended by carers and participants and no additional appointments were requested. Each session was scheduled to last approximately 40–60 min. This was to allow appointments to be organised flexibly to account for individual needs and abilities of participants. Full details of the programmes have been published previously( Reference Jackson and Thorbecke 15 , Reference Spanos, Hankey and Boyle 17 , Reference Jones, Melville and Tobin 21 ).
Carer involvement
Participants were invited to be supported throughout the programmes by family and/or paid carers. Carers were invited to attend sessions to help with communication or where necessary the implementation of behaviour change techniques, for example, goal setting and self-monitoring of the participants’ physical activity or dietary intake. The level of carer involvement was dependent on the individual needs and abilities of participants and ranged from minimal support for example assistance with completing food diaries or encouraging participation in physical activity, to 24-h support in preparing and cooking all meals and actively assisting the participant to go for a walk.
TAKE 5 components
Diet
To achieve a healthy sustainable weight loss of 0·5–1·0 kg/week participants followed a personalised EDD with a deficit of 2510 kJ/d (600 kcal/d)( 2 , 3 ). Daily energy intake was calculated based on total energy requirements minus 2510 kJ/d (600 kcal/d). Participants total energy requirements were calculated based on their BMR (using the Mifflin St. Jeor equation)( Reference Mifflin, St Jeor and Hill 28 ) multiplied by a physical activity level of 1·3( Reference Leslie, Lean and Baillie 29 ). Quantitative dietary intake was from a specified number of portions based on the EatWell Plate ( 30 ) and based on recommendations of a healthy balanced diet. In the weight maintenance phase, a personalised diet was advised based on the estimated energy requirements to maintain body weight.
Physical activity
The physical activity component was based on guidance of the benefits of being physically active and followed consensus guidelines on physical activity programmes for beginners( Reference O’Donovan, Blazevich and Boreham 31 ). Participants were supported to gradually increase their participation in physical activity towards aiming to achieve health recommendations on the duration and intensity of physical activity( 32 , 33 ). This was achieved by setting physical activity goals based on the participants’ current level of physical activity, abilities and their preferred form of physical activity. Physical activity goals were individualised for each participant and focused on three types of physical activity:
-
(1) Lifestyle physical activity: physical activity that could be performed in the home environment such as housework, walking up stairs and following the interactive you can do it DVD.
-
(2) Walking: based on baseline average steps per day, individuals were encouraged to set targets to progressively increase walking behaviour and used pedometers to monitor step counts.
-
(3) Sport and exercise: information was given to each participant on local leisure facilities and clubs with accessible sports and exercise groups/classes( Reference Melville, Boyle and Miller 16 ).
Behaviour change techniques
The most powerful behaviour change techniques shown to support changes in body weight (goal setting, self-monitoring, review of goals and feedback) were used in every session( 2 , 3 , Reference Michie, Abraham and Whittington 34 ). Specific, Measurable, Relevant, and Time-specific (SMART)( Reference Doran 35 ) goals were set relevant to individual dietary habits and physical activity levels. Participants were encouraged to monitor their food intake (to the specified number of portions of the EDD) and physical activity, in diaries with support from carers. Diaries were reviewed and used to monitor progress, identify potential barriers to change and discuss progress to achieve goals.
Weight maintenance
To maintain body weight loss participants were encouraged with support from carers, where appropriate, to maintain the healthy lifestyle habits from phase one. Dietary intake followed the same dietary principles used to support weight loss, without an energy deficit of 2510 kJ/d (600 kcal/d). Instead, dietary plans aimed to ensure a euenergetic dietary prescription and intake. Individuals were encouraged to build on the levels of physical activity they achieved in the first 6 months and continue to aim to meet clinical recommendations. Behaviour change techniques used in the weight loss phase were continued to be used flexibly. Specific approaches, in particular, relapse prevention/coping planning and barrier identification/problem solving were used to prevent large fluctuations in body weight. Weekly self-monitoring of body weight was encouraged as this has been shown to be influential in maintaining body weight.( Reference Wing and Phelan 36 ) Regular self-monitoring also helped to implement this behaviour as part of their routine in order to facilitate weight maintenance after the programme had finished.
Waist Winners Too components
WWToo was developed from the original mainstream Waist Winners weight management programme by a partnership group of health professionals, NHS Dietitians, Learning Disabilities Nursing, Health Improvement and Glasgow Life. For the purpose of this study, the format was adapted from the original community group programme with eight weekly sessions to an individualised programme, delivered on a one-to-one basis. Participants in this comparator programme received the same number of sessions as participants in the TAKE 5.
Diet
The dietary component of the programme focused on a health education approach. This was based on the principles of a healthy balanced diet. Non-quantitative dietary advice was provided based on the EatWell Plate ( 30 ). Food and drink was categorised as ‘healthy’ such as fruit and vegetables, ‘unhealthy’ such as food high in fat and sugar, and other ‘healthy’ foods such as carbohydrates and dairy products which were advised to be consumed in portion-controlled amounts.
Physical activity
Physical activity was discussed based on current public health recommendations on increasing activity and reducing sedentary behaviour( 3 ). At each session, participants reviewed their current participation in physical activity and set new goals to increase physical activity levels.
Behaviour change techniques
The focus of the programme session was to provide educational information on healthy lifestyle behaviours which was achieved by the inclusion of behaviour change techniques. The primary techniques included in each session were goal setting (diet and physical activity goals), self-monitoring (of weight, diet and physical activity) and feedback on performance.
Weight maintenance
To retain participants to the study for the same duration period as those allocated to TAKE 5, a weight maintenance phase for WWToo was developed. Each session focused on the retention of knowledge delivered in the first phase of the programme. Support was also provided for continued monitoring of diet, physical activity and body weight and an opportunity for questions related to maintaining body weight addressed.
Outcome measures
A researcher (L. H.) who was blind to study group allocation was responsible for collecting all outcome measures, completed at baseline, at 6 and 12 months. Level of intellectual disabilities, demographic and health questionnaires were completed at the baseline visit. Level of intellectual disabilities was assessed based on questions on participants’ ability and development in five key areas of functioning: eating and drinking, intimate care, personal safety, communication and decision making. Based on their score participants were categorised as having mild, moderate, severe or profound level of intellectual disabilities. Total scores assessed by the ability and development questionnaire have shown to be highly associated with the Vineland’s Adaptive Behaviour Scale a validated assessment of functioning and ability level( Reference Sparrow, Balla and Cicchetti 37 ) and the questionnaire has been previously used as a measure of level of intellectual disabilities( Reference Melville, Cooper and Morrison 8 , Reference Cooper 38 ). Information on the participant’s health conditions were self-reported as was information on medication use. Medication was classified as obesogenic based on the association with weight gain( Reference Domecq, Prutsky and Leppin 39 ).
Primary outcome
The primary outcome measure was the mean difference in body weight (kg) at 12 months from baseline between the two treatment groups.
Secondary outcomes
Secondary anthropometric outcomes included weight loss of 5 % or more of initial body weight, change in BMI, waist circumference and percentage body fat. A clinically important weight loss was defined as a weight loss of ≥5 % of initial body weight based on clinical recommendations( 2 , 3 ). A weight loss of <3 % was considered a small weight fluctuation following the recommendation by Stevens et al. ( Reference Stevens, Truesdale and McClain 40 ) Successful weight maintenance was achieved if participants maintained ±2·99 % of their weight loss attained at the end of the weight loss phase. Physical activity outcomes included mean percentage time per day spent engaged in moderate–vigorous intensity physical activity, light intensity physical activity, engagement in sedentary behaviour and total metabolic equivalent-min/week. Nutritional outcomes were not assessed primarily due to the difficulties in accurately and reliably assessing dietary intake in adults with intellectual disabilities( Reference Humphries, Traci and Seekins 41 ).
Anthropometric outcomes
Measurements were made with the participant wearing light clothes without shoes. All measurements were made in duplicate and the final value calculated as the mean of the two measurements. Weight in kg was measured to the nearest 100 g, using SECA877 scales (SE approval class III; SECA). Height in metres (m) was measured to the nearest 1 mm using the SECA Leicester stadiometer (SECA). The height (m) and weight (kg) were used to calculate BMI using the formula BMI=weight/height (kg/m2). Waist circumference was measured to the nearest 0·5 cm at the midpoint between the iliac crest and the lowest rib, in full expiration whilst the participant was standing( 42 ). Percentage body fat was calculated using the triceps skinfold thickness (mm) measured to the nearest 1 mm, waist circumference (cm) and age (years) of the participant. Separate regression equations for male and female participants, developed by Lean et al. ( Reference Lean, Han and Deurenberg 43 ) were used to predict body density and percentage body fat.
Physical activity outcomes
Actigraph GT3X+ accelerometers (Manufacturing Technology Inc.) were worn at the hip, attached to a belt worn round the waist for 7 d. Instructions were given to wear the actigraph during all waking hours, except when showering, bathing or swimming. Validity of accelerometer data, the minimum data requirement, was set at 6 h of data, on at least 3 d from 7( Reference Penpraze, Reilly and MacLean 44 ). The accelerometers were set to record activity over 15 s intervals (epochs), with activity counts of four consecutive epochs summed to give activity counts per min. This was then categorised as four levels of activity intensity (sedentary, light, moderate and vigorous) based on cut points from previous studies( Reference Atkin, Gorely and Clemes 45 ). In addition, physical activity levels were also measured subjectively by administering the International Physical Activity Questionnaire-Short (IPAQ-S)( Reference Craig, Marshall and Sjostrom 46 ). In circumstances where participants with intellectual disabilities were regarded by the researcher as unable to answer questions regarding the frequency and time of events, commonly reported as a difficulty for adults with intellectual disabilities( Reference Finlay and Lyons 47 ), carers were invited to assist the participant. To provide an overall measure of physical activity the IPAQ-S variables were combined to calculate total metabolic equivalent-min/week( Reference Jette, Sidney and Blümchen 48 ).
Health-related quality of life
The European Quality of Life-5 dimensions (EQ-5D) youth version was used to measure changes in quality of life (QOL)( Reference Brooks and Group 49 ). Completion of generic measures, such as the EQ-5D, can be difficult for adults with intellectual disabilities because of the level of communication and abstraction required. Therefore, for participants with more severe and profound intellectual disabilities, proxy response of health-related QOL was completed from carers.
Serious adverse events
Serious adverse events (SAE) were defined as an adverse event (an injury or newly diagnosed health condition) that induced hospitalisation or prolonged hospitalisation, resulted in persistent/significant disability or incapacity, or is life threatening or fatal.
Statistical analysis
The primary efficacy analysis was conducted according to the intention to treat (ITT) principle. Mixed linear models were used for continuous outcomes to account for clustering and were adjusted for randomised group, baseline variable, and variables used in the minimisation algorithm (level of intellectual disabilities, number of participants in cluster, and presence of Down’s syndrome). Data are presented as adjusted mean differences and 95 % CI, corresponding P values and ICC. A logistic regression model was fitted for the categorical outcomes (e.g. weight loss of 5 % or more of initial body weight) taking account of clustering and baseline adjustments listed above. Exploratory, per-protocol analysis was also conducted to examine the validity of the ITT results and examine the efficacy of the programmes under ideal conditions (defined as attendance at ≥75 % of sessions). All statistical data were analysed using SPSS 21 IBM statistical package (SPSS IBM).
Results
Participants were recruited between February and October 2014 (Fig. 1). Recruitment rate was on average six participants per month. The recruitment period could have been extended but due to the limited demand capacity of the dietitian and health professional in terms of scheduling participant appointments, enrolment of participants was spaced out over the recruitment period. Further time restrictions to the recruitment period were introduced as this study was undertaken as part of a PhD research project and thus completion of this study was necessary within a set timeframe.

Fig. 1 CONSORT diagram. Flow diagram for the study: outlining the enrolment, screening, allocation and follow-up of participants. Adapted from Schulz et al. ( Reference Schulz, Altman and Moher 50 ).
Baseline characteristics
A total of fifty participants were randomised. Participants had mild (28·0 %), moderate (42·0 %), severe (22·0 %) and profound (8·0 %) level of intellectual disabilities. Eight participants had Down’s syndrome as their diagnosis of intellectual disabilities and two participants were diagnosed with Fragile X Syndrome. Four participants were diagnosed with type 2 diabetes, this was, in general, not treated with medication, except for one participant. Participant characteristics and demographics were comparable between study arms (Table 1). All participants were obese and engaged in low levels of physical activity and a large proportion of time spent in sedentary behaviour (Table 2).
Table 1 Baseline characteristics of participants by randomised group and overall (Mean values and standard deviations; numbers and percentages)

WWToo, Waist Winners Too; SIMD, Scottish Index of Multiple Deprivation.
Table 2 Baseline primary and secondary outcomes by randomised group (Mean values and standard deviations)
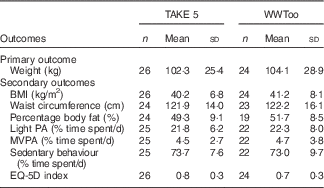
WWToo, Waist Winners Too; PA, physical activity; MVPA, moderate to vigorous physical activity; MET: metabolic equivalent; EQ-5D: European Quality of Life-5 dimensions.
Primary outcome
Based on the ITT analysis there was no significant between group effect on change in body weight post programme at 12 months from baseline (adjusted mean difference −1·90 kg; 95 % CI −4·80, 1·01; P=0·195; Table 3). There was no evidence of clustering for the primary outcome (ICC=0·000). The within group change in body weight at 12 months revealed a significant programme effect for TAKE 5 (−3·55 kg; 95 % CI −5·59, 1·52; P=0·001), however, this was not evident in the WWToo programme (−1·66 kg; 95 % CI −3·69, 0·38 kg; P=0·108).
Table 3 Change in primary and secondary outcomes at 6 and 12 months from baseline (Mean values and 95 % confidence intervals)

WWToo, Waist Winners Too; ICC, interclass correlation coefficient; MVPA, moderate to vigorous physical activity; ED-5D, European Quality of Life-5 dimensions.
* Adjusted for cluster, baseline value and stratification variables (number of participants within a cluster, level of intellectual disability and presence of Down’s syndrome).
Secondary outcomes
Clinically important changes in body weight.
Table 4 illustrates the number of participants achieving a clinically significant weight loss at 6 and 12 months, between the weight management groups. At 12 months, there was a trend for more participants in the TAKE 5 programme (50·0 %) to achieve a clinically important weight loss than the comparator programme (20·8 %) (OR 3·76; 95 % CI 0·92, 15·30; 0·064). During the weight maintenance phase, the majority of participants in both programmes maintained their weight (58·3 %, 68·2 %, TAKE 5 and WWToo, respectively) within ±2·99 % of initial body weight. Seven (29·2 %) participants in the TAKE 5 programme and four participants in the WWToo programme (18·2 %) continued to lose weight and three participants in both programme groups gained weight. There was no significant difference in percentage weight change in the weight maintenance phase between programme groups. Descriptive results on percentage weight change categorised as ≥10 % is presented in the online Supplementary Table S1.
Table 4 Percentage weight change at 6 and 12 months from baseline (Numbers and percentages; odds ratios and 95 % confidence intervals)
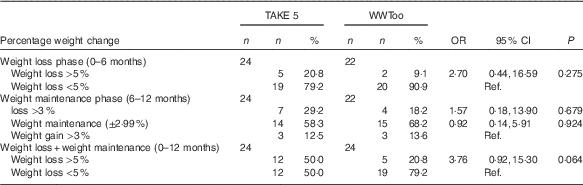
WWToo, Waist Winners Too; Ref. referent values.
* Adjusted for cluster, and stratification variables (number of participants within a cluster, level of intellectual disability and presence of Down’s syndrome).
Anthropometric outcomes
There was no between group changes in anthropometric outcomes. However, TAKE 5 was associated with significant changes in anthropometric outcomes (weight, BMI, waist circumference and percentage body fat) at both 6 and 12 months (Table 3). No significant changes were found in the WWToo programme.
Physical activity
Valid objective accelerometer data were collected for 94 % of participants at baseline. This decreased to 76 and 63 % at 6 and 12 months, respectively. There was no significant between group effect for any of the secondary physical activity outcomes. Results are illustrated in Table 3. Results of the subjective assessment of total physical (IPAQ-S) are not presented due to reliability issues of this measurement.
Health-related quality of life
A high proportion of participants reported to be in a good health state (EQ-5D index for all participants 0·7 (sd 0·3)). There was no change in health-related QOL at 6 or 12 months from baseline in either the TAKE 5 or the WWToo programme (Table 3).
Per-protocol analysis
Exploratory analysis on anthropometric outcomes for individuals defined as completing the programme (per-protocol population), revealed similar effect sizes for within group and between group analysis as the ITT analysis (online Supplementary Table S2).
Serious adverse events
There were no adverse events associated with the trial.
Discussion
This study contributed to the limited evidence of randomised controlled trials of multi-component weight management programme in adults with intellectual disabilities which adhere to clinical guidelines on the treatment of obesity. This study has demonstrated the successful recruitment and retention of adults with intellectual disabilities to a randomised controlled trial and provided evidence on the feasibility of TAKE 5. Key facilitators and barriers to implementing the programme, and process evaluation including the recruitment, reach and fidelity of the programmes( Reference Linnan and Steckler 51 ) will be published separately.
This study overcame the barriers of recruiting adults with intellectual disabilities reported by previous research( Reference Cleaver, Ouellette‐Kuntz and Sakar 52 , Reference Lennox, Taylor and Rey‐Conde 53 ), evidenced by the minimal participation of this population group in research( Reference Cleaver, Ouellette‐Kuntz and Sakar 52 ) and the small sample sizes of previous weight management programmes( Reference Hamilton, Hankey and Miller 11 – Reference Spanos, Melville and Hankey 13 ). Challenges to recruiting adults with intellectual disabilities have been reported including the inability to have direct contact with participants and procedures of taking informed consent( Reference Lennox, Taylor and Rey‐Conde 53 , Reference Iacono 54 ). Effective recruitment in this study was achieved by the identification of a key worker/carer known to the potential participant, and by providing a personal approach to recruitment, for example meeting participants and carers in person, building rapport and aiming to eliminate potential barriers to participating in a research study( Reference Foster, Brennan and Matthews 27 ). Recruitment of participants to the study fell short of the projected final sample size of sixty-six participants( Reference Harris, Melville and Jones 22 ). However, the decision to stop recruitment was justified based on the following reasons: the limited time capacity of the dietitian and health professional to schedule participant appointments, the requirements of the completion of the trial within the researchers PhD timeframe, and the study had successfully provided sufficient insight into the feasibility of the recruitment and retention rates which would inform a full-scale trial.
The results of this study extend the findings of the single-stranded studies by Melville and colleagues( Reference Melville, Boyle and Miller 16 , Reference Spanos, Hankey and Melville 18 ) by providing further support for the acceptability and feasibility of a multi-component weight management programme involving an EDD and tailored to meet the individual needs of adults with intellectual disabilities. The exploratory efficacy analysis in this study revealed that TAKE 5 achieved a slightly lower absolute weight loss and clinically important weight loss of 5–10 % of initial body weight (−2·93 kg, 20·5 % v. −4·47 kg, 36·2 %, respectively) in comparison to the single-stranded study( Reference Melville, Boyle and Miller 16 ). However, adhering to clinical recommendations, the efficacy of a weight management programme should be assessed at 12 months or more( 2 , 3 ). At the end of the weight maintenance phase and end of the programme, participants in TAKE 5 in this study had continued to lose weight (overall weight change from baseline at 12 months: −3·55 kg). This may reflect the design of this study. If participants had not achieved a clinically significant weight loss of 5 % at 6 months they were offered the option to continue to lose weight for a further 3 months, followed by 3 months of weight maintenance. The weight loss in this study compares favourably with the changes in body weight in the single-stranded weight maintenance phase in which participants did not maintain a significant reduction in body weight, −0·6 kg. Based on the definitions by Stevens et al. ( Reference Stevens, Truesdale and McClain 40 ), 58 % of participants maintained their weight loss in this study in comparison to 50 % of participants in the single-stranded study( Reference Spanos, Hankey and Melville 18 ). Moreover, in this study fewer participants gained weight (13 v. 29 %) and a higher percentage of participants continued to lose weight (29 v. 21 %). Although, these results should be interpreted cautiously due to the differences in the duration of the weight maintenance periods (12 months( Reference Spanos, Hankey and Melville 18 ) in comparison to 6 months weight maintenance phase in this study), overall the results suggest that TAKE 5 can achieve sustainable clinically important changes in body weight.
Clinically important weight loss
An important finding of this study is that the degree of weight loss achieved in TAKE 5 is comparable to the weight loss achieved in the general population on completion of the GCWMS programme( Reference Logue, Allardice and Gillies 20 ). Mean weight change at 12 months for the general population was −3·6 kg (95 % CI −3·9, −3·3) in comparison with −3·6 kg (95 % CI −5·6, −1·5) of participants in TAKE 5. Furthermore, the proportion of participants achieving a clinically important weigh loss was greater in the TAKE 5 programme (50 %) in comparison to the participants in the GCWMS (24 %). This illustrates that adults with intellectual disabilities, can access evidence-based weight management services which are tailored to their needs and achieve clinically important weight losses, comparable with the general population.
Programme components
The study design of this trial facilitated the direct comparison of programme components between the two weight management programmes. As both programmes were similar except for the differences in the diet component (TAKE 5: quantitative EDD; WWToo: non-quantitative dietary advice) the potential efficacy of TAKE 5 can be attributed to the EDD. Although, physical activity plays an integral role in weight loss and the maintenance of body weight, the physical activity component in both weight management programmes( 2 , 3 ) failed to elicit an effect on increasing physical activity or reducing sedentary behaviour. Adults with intellectual disabilities experience significant barriers to engaging in physical activity including; a reduced cognitive ability to understand the benefits of physical activity, a lack of awareness of physical activity options, limited time capacity and support from carers to assist engagement in physical activity and limitations in transportation( Reference Hawkins and Look 55 , Reference Bodde and Seo 56 ). Possible explanations for participants not achieving their physical activity goals were associated with the environment and concerns over safety in performing physical activity outside the home. In all, 52 % of participants lived in the most deprived areas of Glasgow and therefore may have limited opportunities to increase physical activity. This is in agreement with previous research investigating the barriers and facilitators to physical activity( Reference Mitchell, Melville and Stalker 57 , Reference Melville, Mitchell and Stalker 58 ). Melville et al. ( Reference Melville, Mitchell and Stalker 58 ) attributed the ineffectiveness of their walking programme in part to the unsupportive environment. Moreover, participants engaged in high levels of sedentary behaviour, and previous research has shown that individuals have low levels of cardiorespiratory fitness( Reference Rimmer, Heller and Wang 59 , Reference Oppewal, Hilgenkamp and van Wijck 60 ). Therefore, participants may not have been able to tolerate physical activity levels required to improve health( 32 , 33 ). Another potential explanation which could in part explain the limited changes in physical activity could also be related in the challenges in adapting complex programmes for the needs of this population group( 61 ). Behaviour change techniques implemented to support increased physical activity were feedback, goal setting and self-monitoring. Although shown to be effective in changing dietary habits, these may not have been of significant magnitude to change levels of physical activity. Future implementation of the programme should refine the physical activity component to focus on feasible home-based activities, interrupting sedentary behaviour and engaging carers in the implementation of the programme. The following strategies, shown to be effective in the general population, should also be utilised to support a change in physical activity behaviour; incorporating the practice of physical activity into the sessions, providing prompts cues to stimulate increased physical activity between sessions, and encourage peers, family and friends to engage in this lifestyle behaviour( Reference Dombrowski, Sniehotta and Avenell 62 , Reference Olander, Fletcher and Williams 63 ). Enhancement of these programme components, in particular the support to increase physical activity, in addition to the already positive changes in diet, could provide a larger effect size on weight loss.
Comparison with previous literature
Comparison of the results of this study with previous weight management programmes based on a standard health education approach( Reference Hamilton, Hankey and Miller 11 – Reference Spanos, Melville and Hankey 13 , Reference Jones, Melville and Tobin 21 ) confirms that a health education approach to weight management is not effective. No significant improvements in outcomes were found at 6 and 12 months. Although a proportion of the participants in WWToo achieved a clinically important weight loss of 5 %, this may be explained by the increased intensity of the WWToo programme in comparison with previous health education programmes. The number of sessions, duration of the programme and weight maintenance component were added to control for contact time and do not reflect clinical health education approaches. This is consistent with clinical recommendations in the general population( 2 , 3 ), which do not support generalised health education approaches for the treatment of obesity, as these are not prescriptive in energy intake and therefore more open to interpretation by the individual.
The results of this study are in agreement with the available literature demonstrating that multi-component weight management programmes including an EDD,( Reference Saunders, Saunders and Donnelly 14 , Reference Melville, Boyle and Miller 16 , Reference Spanos, Hankey and Melville 18 , Reference Martinez-Zaragoza, Campillo-Martinez and Ato-Garcia 64 , Reference Ptomey, Saunders and Saunders 65 ) and specifically tailored to the needs of adults with intellectual disabilities are acceptable and support clinically meaningful weight loss. For example, a recent study by Ptomey et al. ( Reference Ptomey, Saunders and Saunders 65 ) compared an enhanced stop light diet based on pre-prepared portion-controlled meals to a conventional EDD (2092–2929 kJ/d deficit (500–700 kcal/d deficit)). In all, 51 % of the participants in the conventional EDD group achieved a clinically important weight loss at 18 months, which is similar to the findings of this study (50 % of participants in TAKE 5).
Strengths and limitations
This study adds to the limited evidence of weight management in adult with intellectual disabilities by examining the efficacy of the programmes using a randomised controlled trial design. The results of this study provide a more valid and reliable estimate of the effect of the programme, by aiming to reduce methodological errors often associated with observation studies( 25 ) such as imbalances between participant characteristics under investigation, un-measurable confounding factors and reverse causality. Furthermore, this study included all adults with varying severity of intellectual disabilities. This is the first study to provide weight management to adults with all levels of intellectual disabilities (mild to profound). This highlights with support from carers to implement the programme, this population group irrespective is severity of intellectual disabilities can achieve improvements in body weight and composition.
Reliability of self-report outcomes measures was a limitation of this study. For this reason, data on participant’s dietary intake was not assessed. Although, food diaries were used as a behaviour change stagey to self-monitor dietary intake, this was not included as an outcome and therefore prevented insight into compliance with the EDD. Self-report measures of physical activity and health-related QOL were included in this study. Self-report measures of physical activity by the IPAQ-S are dependent on memory to recall physical activity levels over a 7-d period and thought to be close to the limits of the cognitive abilities of many adults with intellectual disabilities( Reference Finlay and Lyons 66 ). Furthermore, comprehension of information on the intensity of physical activities and quantifying amounts of time spent in physical activity was shown to be challenging for some adults with intellectual disabilities. For some participants, support from carers was encouraged to assist in completion of the IPAQ-S. However, issues identified in administering the IPAQ-S were the high turnover of carers which meant that some carers were not aware of activities performed over the 7 d. Moreover, participants often attended day centres or clubs and therefore engagement in physical activity out with the home environment was unknown. Participants also found completion of the EQ-5D visual analogue scale, a measure of health-related QOL, challenging and therefore, results were presented solely for the EQ-5D index. The reliability of the proxy response of EQ-5D of carers is also unknown as research on the use of this questionnaire is limited( Reference Cooper, Morrison and Allan 67 ) and similarly, a lack of consistency in carers at outcome measure appointments meant that measures were not always completed by the same carer. As proxy response is an integral component to conducting research, and in clinical practice involving adults with intellectual disabilities( Reference Finlay and Lyons 66 ), it is important that future research investigates valid methodologies to accurately and reliably measure self-report physical activity levels and health-related QOL of adults with intellectual disabilities.
Research and clinical recommendations
This study contributes to the evidence by closing the gap between research and providing accessible weight management services for this population group. This study provides evidence that an EDD is feasible and an acceptable approach to the treatment of obesity. Due to the negative health risks associated with obesity, clinicians and health professionals need to recognise that in order to achieve weight loss with clinically important benefits, current programmes based on a health education approach are ineffective. A full-scale trial is warranted to establish the effectiveness of TAKE 5 modified on the results of this study.
Conclusion
This study provides evidence on the feasibility of conducting a full-scale trial of a multi-component weight management programme in adults with intellectual disabilities. The multi-point recruitment strategy was shown to be successful in overcoming barriers in conducting research in adults with intellectual disabilities and was able to recruit adults with all levels of intellectual disabilities. This study illustrates the acceptability and feasibility of an EDD approach to weight management in adults with intellectual disabilities and illustrates that current service provision based on health education is not effective in the treatment of obesity. A future full-scale randomised controlled trial is warranted to confirm these findings and provide evidence on the optimum approach to weight management in this population group.
Acknowledgements
The authors would like to thank the participants and family and paid carers.
This work was supported by the Scottish Government – Equally Well Fund.
L. H., C. M. and C. H. led the drafting and editing of the manuscript. L. H. acquired and analysed the data. N. J. was the dietitian on the study. C. P., S. B., H. M. and J. T. were all involved in the original application and the design in the study. All authors read and approved the final manuscript.
None of the authors has any conflicts of interest to declare.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114517001933







