People with severe mental illness (SMI) die approximately 15 years earlier than individuals in the general population,Reference Walker, McGee and Druss1 identified as a significant human rights issue and major source of inequity.Reference Thornicroft2 The vast majority of these earlier deaths are attributable to physical health conditions, primarily cardiovascular disease.Reference Correll, Solmi, Veronese, Bortolato, Rosson and Santonastaso3 Understanding modifiable factors that may diminish or prevent the ‘scandal of premature mortality’ is essential.Reference Thornicroft2 In the general population, there is robust evidence that excessive energy intake and poor diet quality is associated with adverse physical health including cardiovascular disease and premature mortality.Reference Gakidou, Afshin, Abajobir, Abate, Abbafati and Abbas4 This evidence supports population-level state-sanctioned strategies that focus on nutrition as a cornerstone of health outcome determination.
For many people experiencing SMI, antipsychotic, antidepressant and mood-stabilising medications may be essential components of treatment.Reference Lieberman, Stroup, McEvoy, Swartz, Rosenheck and Perkins5, Reference Galletly, Castle, Dark, Humberstone, Jablensky and Killacky6 Many of these medications are associated with substantial weight gain, obesity and associated cardiometabolic abnormalities.Reference Álvarez-Jiménez, González-Blanch, Crespo-Facorro, Hetrick, Rodriguez-Sánchez and Pérez-Iglasias7–Reference Vancampfort, Stubbs, Mitchell, De Hert, Wampers and Ward9 It has been suggested that one of the key factors underlying these abnormalities are the effects of antipsychotic medication on dietary intake and eating behaviours.Reference Elman, Borsook and Lukas10 People receiving antipsychotic medication report increased appetite, decreased satiety and increased cravings for sweet foods and beverages.Reference Blouin, Tremblay, Jalbert, Venables, Bouchard and Roy11 A range of lifestyle interventions have attempted to mitigate the obesogenic effects of these medications; however, a clear understanding of the dietary intake in people experiencing psychotic illness is lacking. A key limitation in a previous systematic review of dietary patterns in schizophreniaReference Dipasquale, Pariante, Dazzan, Aguglia, McGuire and Mondelli12 was lack of evaluation and critique of the quality of dietary intake assessment methods. Given the vast majority of dietary-assessment methods are subjective, the strength of methodology in collecting, interpreting and analysing dietary intakes is a crucial determinant for obtaining meaningful conclusions. Thus, a comprehensive systematic review and meta-analysis of the dietary intake of people with SMI, taking into account dietary-assessment methodology, is warranted to identify possible dietary treatment targets for interventions to improve the physical health of this vulnerable population.
Method
Design
This study was pre-registered on the PROSPERO database (CRD42016048833) and conducted in accordance with the PRISMAReference Stroup, Berlin, Morton, Olkin, Williamson and Rennie13 and MOOSE statementsReference Moher, Liberati, Tetzlaff, Altman, Altman and Antes14 (see supplementary Files 1 and 2 available at https://doi.org/10.1192/bjp.2019.20 for PRISMA and MOOSE checklists).
Search strategy
An online search strategy was undertaken to identify studies published in the English language from 1975 to August 2017 through librarians within the University of Newcastle, Callaghan Campus. Cumulative Index to Nursing and Allied Health Literature (CINAHL), Cochrane, Embase, Medline, PsycINFO and Scopus databases were searched using common psychiatric and nutritional MeSH terms (feeding behavior OR eating OR food intake OR diet* OR nutrition* OR coffee OR caffeine OR beverages AND schizophrenia OR psychotic disorders OR bipolar disorder OR bipolar*; see supplementary File 3 for comprehensive search list). Electronic searches were supplemented with manual cross-checking of the reference lists of relevant publications.Reference Dipasquale, Pariante, Dazzan, Aguglia, McGuire and Mondelli12 Cross-sectional and cohort studies in adults were included.
After the removal of duplicates, stage two involved the assessment of titles and abstracts of identified studies by two independent reviewers (S.B.T. and T.L.B.), with disagreements resolved by further discussion. A priori inclusion/exclusion criteria were applied to determine the eligibility of each publication for inclusion in the review, as per the following inclusion criteria.
Inclusion and exclusion criteria
Population
Adult populations (age ≥18 years or ‘adults’ depending on the database searched), DSM or ICD diagnosis of a severe mental illness (schizophrenia spectrum disorders, bipolar affective disorder, depression with psychosis, or other psychotic illness) or clinician-diagnosed first-episode of psychosis. There were no limitations employed for rates of psychotropic medication prescription, as reporting of this was infrequently included.
Intervention
Types of studies included cross-sectional, cohort and longitudinal designs.
Control
There were no limits on comparison groups, although only studies with matched controls were eligible for meta-analysis.
Outcome
One or more nutritional outcomes, including energy, macronutrients, micronutrients, fat subgroups, fibre, diet quality, food groups and caffeine. We excluded animal studies, studies of people with high-prevalence mental illness (depression and anxiety), or those with eating disorders (anorexia and bulimia nervosa), case studies, letters to the editor, intervention studies and studies with eating behaviour outcomes that lacked specific dietary intake outcomes. Alcohol was excluded as an outcome as we did not include substance use in our search terms. Excluded articles are summarised in Fig. 1.
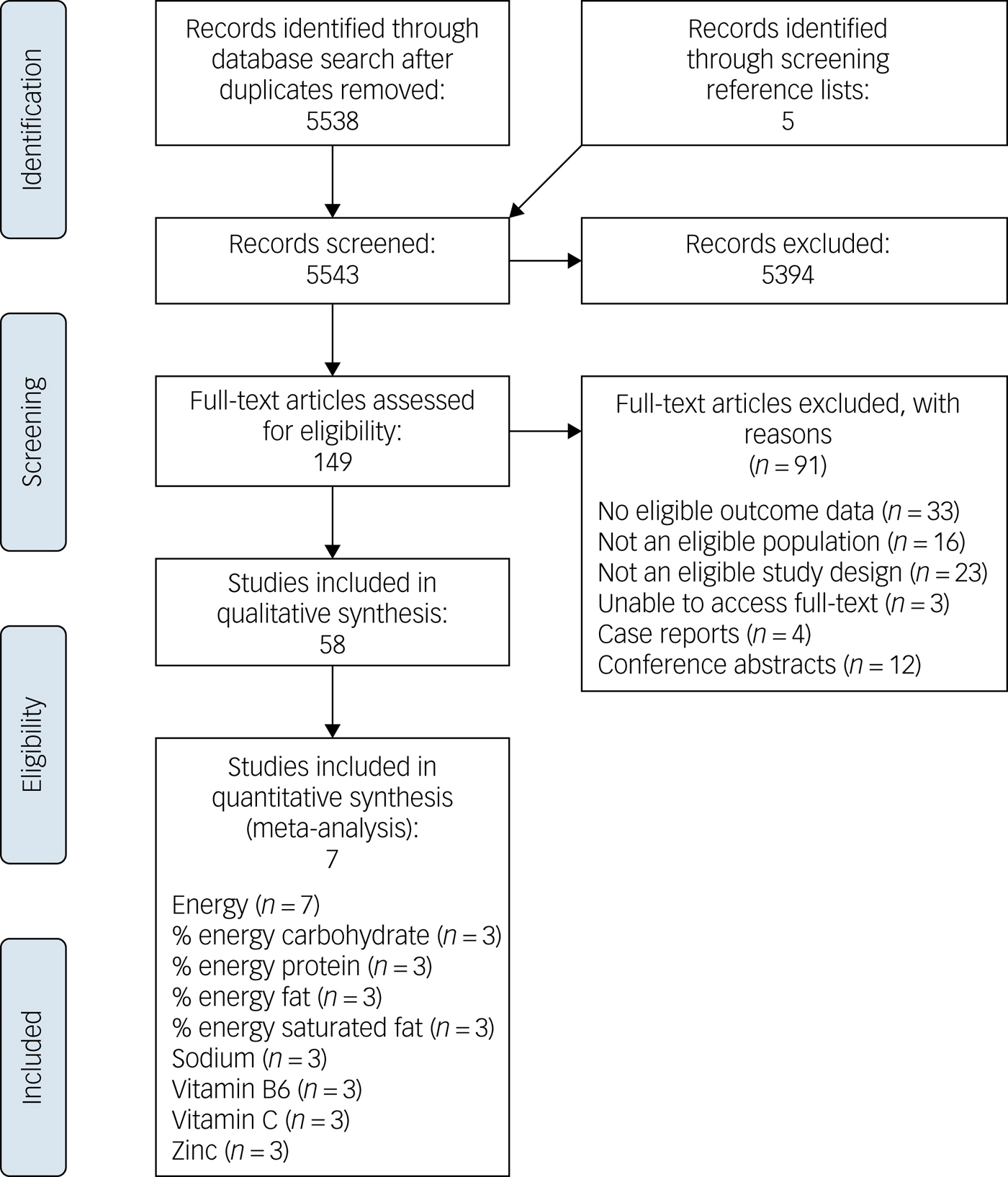
Fig. 1 PRISMA flow chart.
Data extraction
Where necessary, corresponding authors of included studies were contacted for additional data for inclusion in meta-analysis. A follow-up email was sent 3 weeks later if corresponding authors did not reply to the initial request.
Data were extracted using standardised tables developed for this review and included study design, population demographics and dietary intake assessment methods. In cases of uncertainty regarding quality assessment, or data extraction, a third independent reviewer was consulted, until consensus was reached.
Study quality
Study quality was assessed twice for each individual study using a standardised tool from the American Academy of Nutrition and Dietetics.15 Two reviewers scored each study independently. The lead author resolved discrepancies between the different scorers. Scoring included ten quality criteria that were rated as being absent, present or unclear in each study. This included the assessment of population bias, study masking, a description of the assessment tool, statistical methods and study funding. An overall quality rating was assigned, with each study being rated as: (a) negative, if six or more answers to the validity questions are ‘no’, (b) neutral, if answers to validity criterion 2, 3, 6 and 7 do not indicate the study was strong, or (c) positive, if most of the answers to validity criteria were ‘yes’ (including criterion 2, 3, 6 and 7 plus one extra criterion). No studies were excluded based on quality ratings.
Data analysis
Studies were eligible for meta-analysis if they; (a) used a recognised dietary-assessment method (i.e. 24 h recall) or cited a validation study for the tool used, (b) had a matched control group who were assessed at the same time as the target group, and (c) reported outcomes in a compatible metric/measure. Comparisons against national health surveys and other population data sources were not eligible for meta-analysis.
All meta-analyses were performed using Comprehensive Meta-Analysis 2.0.Reference Borenstein, Hedges, Higgins and Rothstein16 To account for expected heterogeneity between studies, a random-effects model was used throughout.Reference DerSimonian and Laird17 First, for studies that used validated, or recognised and acceptable, dietary-assessment tools, comparative meta-analyses were performed to calculate a pooled mean difference (and 95% confidence intervals) between participants with SMI and healthy controls in daily total energy intake, the primary outcome of interest, measured in kilojoules per day (kJ).
As secondary outcomes, we also compared SMI and control groups on daily mean intake of each macronutrient and micronutrient examined in a sufficient number of studies to justify meta-analysis (>2), using the standard units of measurements for these nutrients. If non-standard means were used, we calculated means and standard deviations from the data of the studies where possible. Along with examining differences using standard nutrient measurement values, the overall difference between SMI and control groups for daily intake of each macro/micronutrient was computed as Hedge's g, with resultant effect sizes classified as small (<0.2), moderate (>0.2 to <0.8) or large (>0.8). Statistical significance was set at P<0.05. For all analyses, the variance between studies was assessed using Cochran's Q and reported as I 2, which quantifies the degree of variance resulting from between-study heterogeneity, rather than by chance.
For the primary outcome, we also applied several tests to measure and adjust for publication bias: (a) Egger's regression test was used to quantify the risk of publication bias influencing findings, (b) the ‘fail-safe N’Reference Orwin18 was calculated to determine the number of unpublished null studies that would invalidate the findings, and (c) Duval & Tweedie's trim-and-fill analysis was used to re-calculate the pooled difference after adjusting for any studies potentially reflecting publication bias.
Results
Identification and selection of studies
Electronic database searches identified 5538 unique titles after accounting for duplicates, as summarised in Fig. 1. Five additional titles were sourced by screening a relevant references.Reference Dipasquale, Pariante, Dazzan, Aguglia, McGuire and Mondelli12 A review of titles and abstracts led to the exclusion of 5394 titles. Full-texts were assessed for the remaining 149 titles, of which 91 were excluded for reasons detailed in Fig. 1. In total, 58 studies were identified for critical appraisal and included in this review.Reference Adolfo, AhnAllen and Tidey19–Reference Noguchi, Hiraoka, Watanabe and Kagawa76
The studies were conducted in 17 countries, with the majority conducted in the USA (14 studies, n = 4885 participants), UK (10 studies, n = 575), Spain (6 studies, n = 2637) and Australia (6 studies, n = 1899). The majority of studies were cross-sectional in design (48 studies, n = 33 915), and smaller numbers of cohort (6 studies, n = 924) and case–control (2 studies, n = 275) studies were included, and 1 study each for longitudinal (n = 352) and cross-cue reactivity (n = 15).
Characteristics of included studies
Participants
The 58 studies included a total of 35 481 people with SMI and 5465 non-psychiatric controls. Diagnoses within the studies were: (a) limited to schizophrenia spectrum disorders (27 studies, 47%, n = 26 230), (b) mixed SMI diagnoses (20 studies, 34%, n = 8301), (c) limited to bipolar affective disorder (7 studies, 12%, n = 673), and (d) limited to first-episode psychosis (4 studies, 7%, n = 277).
The majority of studies described participants as out-patients or community-dwelling (40 studies, n = 6944), followed by in-patients (8 studies, n = 886) and mixed settings (2 studies, n = 22 072). Eight studies did not report participant setting. A total of 19 studies (33%) used controls (n = 5465) and 20 studies (34%) used population data as a comparator group.
Participants were receiving a range of psychotropic medications including antipsychotics, mood stabilisers, antidepressants and benzodiazepine medications. Medications were reported in a range of formats including (a) chlorpromazine equivalents, (b) per cent on psychotropic medications as a whole, (c) per cent prescribed an antipsychotic medication, (d) per cent receiving antipsychotic polypharmacy, (e) per cent prescribed a mood stabiliser medication, and (f) detailed description of the specific antipsychotic and mood stabiliser medications prescribed. There were 17 studies (29%) that did not describe medication prescription. See supplementary File 4 for complete details.
Dietary intake assessment methods
Seventeen different types of dietary-assessment methods were identified. Only 12 studies (21%) cited validation studies for the nutrition assessment method used. No study cited validation of the dietary-assessment tool used in a SMI population. One study reported piloting their food frequency questionnaire in 15 people with SMI, however, these data were unpublished. A further 22 studies (38%) used recognised acceptable dietary-intake assessment measures such as 24 h recall; however, only 11 studies (50%) reported that dietitians or other trained interviewers completed dietary intake assessment and analysed it using nutrition data-analysis software, and only four studies used multiple, non-consecutive recalls. Weighed-food records, a more objective measure of dietary intake, was used for two of these studies. In these two studies, one study assessed 7-day dietary intake, and the other 2-day dietary intake, both assessed by a dietitian/nutritionist. One study used a 3-day photographic food record assessed by a dietitian, also considered a more objective measure of dietary intake. For the remaining studies for which validity of the assessment tool was unclear, 14 studies (24%) reported the assessment tool very broadly, such as ‘standardised questionnaire’ or ‘verbal questions’ and 9 studies (16%) report using food frequency questionnaires without citing validity, or questions taken from national health surveys.
Dietary outcomes
A wide variety of dietary outcomes were assessed. The most common dietary outcome measures were energy intake (22 studies), macronutrients (carbohydrate, protein, fat) (20 studies), individual fatty acids or fat subgroups (20 studies), fibre (16 studies), food groups/categories (14 studies), caffeine or coffee intake (12 studies), overall dietary patterns (9 studies) and micronutrients (9 studies). One study also reported health (dietary) knowledge as a secondary outcome. Dietary outcomes were reported in a range of different metrics. For these reasons it was difficult to conduct direct comparisons.
Quality of studies
In total, 39 studies (67%) received a neutral score, 13 studies (22%) received a negative score and 6 studies (10%) received a positive score. Key areas of weakness included: (a) lack of concurrent controls, and comparability of groups on important confounding factors, (b) lack of use of independent assessors and masking for data collectors (when concurrent comparator groups were used), (c) measurements not being based on standard, valid and reliable methods and procedures, (d) measurements implemented at unclear level of precision, and (e) inconsistent measures used across groups. Individual study quality data are outlined in supplementary File 5.
Quantitative analysis
In two studies that only reported median averages,Reference Jacka, Pasco, Mykletun, Williams, Nicholson and Kotowicz46, Reference Konarzewska, Stefańska, Wendolowicz, Cwalina, Golonko and Malus50 medians were used as an imputed mean and standard deviations were estimated from the pooled standard deviations across all other studies. For the one study that did not report standard deviations for controls,Reference Nenke, Hahn, Thompson, Liu and Galletly54 standard deviations was imputed from a study of similar sample size.Reference Haruyuki, Kumagai, Kimura, Kolke and Shimizu42
Energy
Seven studies (n = 1448) reported energy intakes for both people with SMI and controls.Reference Chang, Assari, Prossin, Stertz, McInnis and Evans27, Reference Evans, Ringrose, Harrington, Mancuso, Burant and McInnis32, Reference Jacka, Pasco, Mykletun, Williams, Nicholson and Kotowicz46, Reference Jahrami, Faris, Saif and Hammad47, Reference Konarzewska, Stefańska, Wendolowicz, Cwalina, Golonko and Malus50, Reference Manzanares, Monseny, Ortega, Montalvo, Franch and Gutliérrez-Zotes51, Reference Nunes, Eskinazi, Cambolm Rockett, Delgado and Schwelgert Perry55 Mean energy intake among individuals with SMI was 1332 kJ/day higher than among the controls (95% CI 487–2178 kJ/day, P = 0.002), with a moderate effect size for the difference between groups (7 studies, g = 0.463 (95% CI 0.159–0.767), P = 0.003). Although there was heterogeneity across the study data (Q = 25.7, P < 0.001, I 2 = 76.7%), there was no evidence of publication bias (P = 0.328 for Egger's regression test), and the fail-safe N was 67 (estimating that 67 unpublished ‘null’ studies would need to exist for the actual P-value to exceed 0.05). A trim-and-fill analysis did not identify any outliers, and the random-effects point estimate remained at g = 0.463. These findings were in line with the two studies that used weighed-food records that found: (a) energy intake was significantly higher in the schizophrenia group compared with the general population,Reference Stokes and Peet64 and (b) energy intake increased with the commencement of olanzapine, in line with the weight gain observed.Reference Gothelf, Falk, Singer, Kairi, Philliip and Zigel35
Subgroup analysis found the mean difference in energy intake for the schizophrenia spectrum cohorts compared with controls was +1695 kJ/day (95% CI 380–3010 kJ/day), P = 0.012, and the mean difference between energy intake in bipolar disorder-only cohorts compared with controls was +827 kJ/day (95% CI 146–1508 kJ/day), P = 0.017. The between-group difference in energy intake for bipolar disorder-only and schizophrenia spectrum-only cohorts was not statistically significant (Q = 1.32, P = 0.251). These are illustrated in Fig. 2.
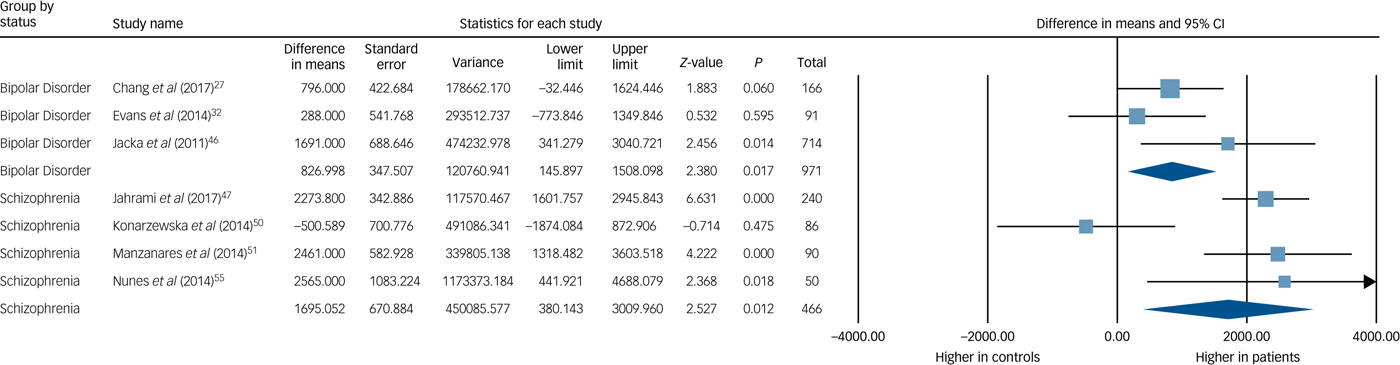
Fig. 2 Meta-analysis of energy intake in bipolar disorder and schizophrenia.
Additional nutrients
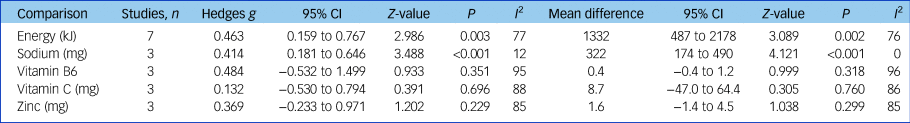
Three studies (n = 387) reported data able to be pooled for meta-analysis for sodium, vitamin B6, vitamin C and zinc.Reference Jahrami, Faris, Saif and Hammad47, Reference Konarzewska, Stefańska, Wendolowicz, Cwalina, Golonko and Malus50, Reference Nunes, Eskinazi, Cambolm Rockett, Delgado and Schwelgert Perry55 Sodium intake was significantly higher in the SMI group (mean difference 332 mg, 95% CI 174–490 mg, Z = 4.121, P < 0.001, I 2 = 12%, g = 0.414). There was no significant difference in pooled intakes of vitamin B6, vitamin C and zinc between people with SMI and controls (all P > 0.2). See Table 1 for meta-analysis results for energy and nutrient intakes.
Table 1 Meta-analysis of dietary energy and nutrient intakes
Qualitative synthesis
Dietary patterns and diet quality scores were assessed in eight studies and two studies, respectively. The SMI group was found to have less healthy dietary patterns in eight studiesReference Gupta and Craig36, Reference Jacka, Pasco, Mykletun, Williams, Nicholson and Kotowicz46–Reference Kilian, Becker, Krüger, Schmid and Frasch49, Reference Roick, FritzWieacker, Matschinger, Heider, Schindler and Riedel-Heller58, Reference Noguchi, Hiraoka, Watanabe and Kagawa76 and women had lower diet quality in one study that assessed diet quality,Reference Amani20 and a mean diet score within the ‘unhealthy’ category for the other study assessing diet quality.Reference Simonelli-Muñoz, Fortea, Salorio, Gallego-Gomez, Sánchez-Bautista and Balanza63 No included study found healthier dietary patterns for the SMI group when compared with the control or population data.
Four studies reported a relationship between dietary patterns and SMI. A higher ‘Western’ and ‘modern’ dietary pattern was positively associated, and ‘traditional’ dietary pattern negatively associated with bipolar disorder.Reference Jacka, Pasco, Mykletun, Williams, Nicholson and Kotowicz46 A ‘cereal’ dietary pattern (bread, rice, confectionary etc.) was positively associated with schizophrenia whereas a ‘vegetable’ dietary pattern was not.Reference Tsuruga, Sugawara, Sato, Saito, Furukori and Nakagami72 Higher energy intake, and lower protein intake, were positively associated with general symptom severity in early psychosis in one study,Reference Manzanares, Monseny, Ortega, Montalvo, Franch and Gutliérrez-Zotes51 whereas life stress was positively associated with increased refined sugar intake in people experiencing psychosis but negatively associated with refined sugar intake in both high-risk for psychosis and healthy participants, in the same study. A fourth study in Japan found men who had infrequent intakes of vegetables, mayonnaise, potatoes, soy products, seaweed and fish products had more pronounced psychiatric symptoms, although this correlation was not found in women.Reference Noguchi, Hiraoka, Watanabe and Kagawa76
Fruit and vegetable intake was found to be lower in the SMI group compared with controls/population data in three studiesReference Kilbourne, Rofey, McCarthy, Post, Welsh and Blow48, Reference McCreadie52, Reference Samele, Patel, Boydell, Leese, Wessely and Murray62 and less than country/region-specific recommendations in nine studiesReference Brown, Birtwistle, Roe and Thompson26, Reference Fusar-Poli, De Marco, Cavallin, Bertorello, Nicolasi and Politi34, Reference Hahn, Galletly, Foley, Mackinnon, Watts and Castle39, Reference Hardy and Gray41, Reference Heald, Pendlebury, Anderson, Narayan, Guy and Gibson43, Reference McCreadie52–Reference Nenke, Hahn, Thompson, Liu and Galletly54, Reference Simonelli-Muñoz, Fortea, Salorio, Gallego-Gomez, Sánchez-Bautista and Balanza63 and higher compared with the general population in one study.Reference Archie, Goldberg, Akhtar-Danesh, Landeen, McColl and McNiven77 Low intakes, or lower intakes than comparison groups, were found for fishReference Nenke, Hahn, Thompson, Liu and Galletly54, Reference Simonelli-Muñoz, Fortea, Salorio, Gallego-Gomez, Sánchez-Bautista and Balanza63 and nuts and vegetable oils.Reference Amani20 Large intakes, or higher intakes than comparison groups, were found for carbonated beverages,Reference Amani20 sweetened beverages,Reference Elmslie, Mann, Silverstone, Williams and Romans30 soft drinks,Reference Sugawara, Yasui-Furukori, Yamazaki, Shimoda, Mori and Sugai69 cakes and other sweets,Reference Sugawara, Yasui-Furukori, Yamazaki, Shimoda, Mori and Sugai69 white bread,Reference Heald, Pendlebury, Anderson, Narayan, Guy and Gibson43 hydrogenated oilsReference Amani20 and fast food/takeaway foods.Reference Heald, Pendlebury, Anderson, Narayan, Guy and Gibson43, Reference Samele, Patel, Boydell, Leese, Wessely and Murray62 In addition, one study found poor diet literacyReference Hardy and Gray41 and one study found difficulties obtaining and/or cooking foodReference Kilbourne, Rofey, McCarthy, Post, Welsh and Blow48 for people with SMI.
Results for macronutrients and micronutrients were mixed when compared with reference groups and recommended intakes, with no clear findings emerging. There were trends for studies to find lower mono- and polyunsaturated fats,Reference Bly, Taylor, Dalack, Pop-Busui, Burghardt and Evans24, Reference Clayton, Hanstock, Hirneth, Kable, Garg and Hazell28, Reference Ellingrod, Taylor, Brook, Evans, Zölliner and Grove29, Reference Nunes, Eskinazi, Cambolm Rockett, Delgado and Schwelgert Perry55 and higher intakes of total and saturated fatReference Manzanares, Monseny, Ortega, Montalvo, Franch and Gutliérrez-Zotes51, Reference Nenke, Hahn, Thompson, Liu and Galletly54, Reference Ratliff, Palmese, Reutenauer, Liskov, Grilo and Tek57, Reference Stokes and Peet64, Reference Strassnig, Singh Brar and Ganguli65, Reference Tsuruga, Sugawara, Sato, Saito, Furukori and Nakagami72 and trans-fatsReference Nunes, Eskinazi, Cambolm Rockett, Delgado and Schwelgert Perry55 in the SMI groups when compared with a comparator (control or population data). In addition, higher sugar intake was found in two studies.Reference Ratliff, Palmese, Reutenauer, Liskov, Grilo and Tek57, Reference Tsuruga, Sugawara, Sato, Saito, Furukori and Nakagami72
Results for fibre intakes were mixed when compared with controls or population data, although the SMI group consumed less than the national/region recommendations in nine studiesReference Bly, Taylor, Dalack, Pop-Busui, Burghardt and Evans24, Reference Ellingrod, Taylor, Brook, Evans, Zölliner and Grove29, Reference Haruyuki, Kumagai, Kimura, Kolke and Shimizu42, Reference Henderson, Borba, Daley, Boxill, Nguyen and Culhane44, Reference Jahrami, Faris, Saif and Hammad47, Reference Konarzewska, Stefańska, Wendolowicz, Cwalina, Golonko and Malus50, Reference Nenke, Hahn, Thompson, Liu and Galletly54, Reference Strassnig, Singh Brar and Ganguli65, Reference Sugawara, Yasui-Furukori, Sato, Saito, Furukori and Nakagami68 and adequate intake in one study.Reference Nunes, Eskinazi, Cambolm Rockett, Delgado and Schwelgert Perry55 For caffeine intake, five studies found higher caffeine intakes, or high caffeine intakes (≥200 mg/day), to be more frequent in the SMI group compared with the reference groupReference Adolfo, AhnAllen and Tidey19, Reference Gurpegui, Aguilar, Martinez-Ortega, Diaz and de Leon37, Reference Henderson, Borba, Daley, Boxill, Nguyen and Culhane44, Reference Strassnig, Singh Brar and Ganguli65, Reference Strassnig, Singh Brar and Ganguli66 and one study found no difference.Reference Arrojo-Romero, Armas Barbazán, López-Moriñigo, Ramos-Rios, Gurpegui and Martinez-Ortega22 One study found people with psychosis to have a higher frequency of ‘high’ coffee consumption (≥5 cups/day) compared with other mental illnesses including depression and alcohol use disorder.Reference Winstead75 Seven studies found smokers in the SMI groups to have the highest caffeine intakes.Reference Adolfo, AhnAllen and Tidey19, Reference Arrojo-Romero, Armas Barbazán, López-Moriñigo, Ramos-Rios, Gurpegui and Martinez-Ortega22, Reference Baethge, Tondo, Lepri and Baldessarini23, Reference Bobes, Arango, Garcia-Garcia and Rejas25, Reference Gurpegui, Aguilar, Martinez-Ortega, Diaz and de Leon37, Reference Strassnig, Singh Brar and Ganguli65, Reference Strassnig, Singh Brar and Ganguli66 Differences in reporting of caffeine and caffeinated drinks as outcomes meant that data could not be pooled for meta-analysis.
Discussion
Main findings
This is the first meta-analysis of dietary intake in people with either psychosis or bipolar disorder and showed significantly higher total energy and sodium intakes compared with controls. This study also found consistent reports of less healthy dietary patterns including low intakes of fruit and vegetables, and high intakes of takeaway and other convenience foods, and sugar-sweetened beverages. A previous qualitative synthesis in schizophrenia and schizoaffective disorderReference Dipasquale, Pariante, Dazzan, Aguglia, McGuire and Mondelli12 suggested poorer dietary patterns in this population. Poorer dietary patterns are not limited to schizophrenia and schizoaffective disorder and included all those with a SMI diagnosis, although energy intake appears to be highest for those with psychotic disorders. This was also the first study to systematically review dietary intake assessment methods and strength of reporting, highlighting a need to improve scientific rigour in this area.
Interpretation of our findings and comparison with findings from other studies
The strongest evidence was found for higher energy and sodium intakes in SMI populations, with statistically significant differences compared with controls (+1332 kJ/day and +322 mg/day higher in those with SMI, respectively) when pooled intakes obtained from validated assessment tools were compared with matched controls. These findings are consistent with the two studies using weighed-food records (considered the most accurate method) that found: (a) a significant increase in caloric intake coinciding with weight gain,Reference Gothelf, Falk, Singer, Kairi, Philliip and Zigel35 and (b) a schizophrenia group that consumed more energy, sugar and fat compared with the general population.Reference Stokes and Peet64 Both meta-analyses revealed moderate effect sizes (g = 0.46 and g = 0.41, respectively), and are of considerable clinical relevance, particularly an increased energy intake of 1332 kJ per day, given that: (a) the general population is already overconsuming energyReference Duffey and Popkin78 and salt,Reference Appel, Frohlich, Hall, Pearson, Sacco and Seals79 and (b) this is compounded with high levels of sedentary behaviourReference Vancampfort, Firth, Schuch, Rosenbaum, Mugisha and Hallgren80 and low cardiorespiratory fitness,Reference Vancampfort, Rosenbaum and Schuch81 which helps to explain the alarming rates of obesity, metabolic syndrome, diabetes, cardiovascular disease and premature mortality in people with SMI.Reference Correll, Solmi, Veronese, Bortolato, Rosson and Santonastaso3
The weight change dynamics paradigm of Hall and coworkers’Reference Hall, Heymsfield, Kemnitz, Klein, Schoeller and Speakman82 predicts that every 100 kJ intake excess will have an eventual body weight change of 1 kg. Applied to the findings from this study, someone with SMI would weigh on average 13 kg more than the general population (mean 8 kg and 17 kg in the bipolar disorder and schizophrenia groups, respectively) from dietary factors alone.
Increased energy and sodium intakes are likely explained by increased hunger and preference for ‘discretionary foods’ such as sweetened beverages and convenience foods, which are high in sugar, salt and fat (and therefore energy) and low in beneficial nutrients such as fibre, vitamins and minerals. Reasons for increases in appetite remain to be clarified. A wide variety of neuroreceptor and neuroendocrine factors regulate eating behaviour and appetite in SMI.Reference Werneke, Taylor and Sanders83 Dopamine, serotonin, muscarinic and histamine receptors have all been implicated in antipsychotic-induced increases in hunger, with drugs with high affinity for 5HT2c and muscarinic receptors associated with the greatest risk of weight gain.Reference Lett, Wallace, Chowdhury, Tiwari, Kennedy and Müller84, Reference Reynolds and Kirk85 Compounded with the lower levels of physical activity among people with SMI,Reference Vancampfort, Firth, Schuch, Rosenbaum, Mugisha and Hallgren80 this helps to explain the stark difference in weight and BMI status between people with SMI and the general population.Reference Mitchell, Vancampfort, Sweers, van Winkel, Yu and De Hert86
Intakes of the micronutrients vitamin B6, vitamin C and zinc were not significantly different from controls in our analyses, although these were limited to the few studies that included such data. Given a previous analysis found blood levels of micronutrients were significantly lower in SMI compared with healthy controls,Reference Firth, Carney, Stubbs, Teasdale, Vancampfort and Ward87 more well-designed dietary intake studies should investigate micronutrients, particularly those with a close relationship to mental health, such as folate.
Strengths and limitations
Search strategy
The search strategy was limited to articles written in English and therefore articles written in another language were not reviewed. In addition, grey literature was not searched for this review. The study aimed to focus on naturalistic cohort data of real-world patients’ dietary consumption such that the results would reflect clinical reality, hence the inclusion of cohort/cross-sectional studies, and the exclusion of randomised controlled trial data.
Medication
Attempts were made to disentangle the effects of antipsychotic and mood-stabilising medication; however, because of insufficient reporting, limited conclusions could be made from this review. Given the differing effects on metabolic health of antipsychotics and mood-stabilising medications, more research is needed to explore the specific effects of individual medications on dietary intake and eating behaviours.
Dietary-assessment methods and tools
Qualitative synthesis found a large range of dietary-assessment and analysis methods and outcomes were employed, a clear challenge for interpreting dietary intake. A large proportion (37%) either used an unvalidated tool or did not report whether the tool had undergone validation, which limits generalisability of any findings. Those studies that used validated assessment or recognised, acceptable methods were often not specific to mental health populations; that is, it is unknown if the tools perform accurately or individuals with mental health diagnoses can accurately report on dietary intake. However, the two studies using weighed-food records and one study using a photographic food diary (i.e. more objective measures of dietary intake), found results in line with the results obtained in the current review. The overall strength of reporting in many studies was also limited, with the majority considered to be neutral (i.e. neither strong nor weak, n = 40).
The dietary intake methods used in studies included in this meta-analysis were based on self-report, so may reflect subjective bias. This review comprehensively and systematically reviewed all published studies, providing a best-guess insight into dietary intake in people with SMI. Misreporting is a common issue in the general population, with an average energy underreporting of approximately 20%, and higher in people who are obese (~30%). Given that people with SMI commonly experience additional barriers, including cognitive impairment, lack of motivation and poor memory, misreporting could be expected to be more common, and to have a larger impact, in this population suggesting the findings on energy intake may be an underestimation. Comparisons against population data can be misleading as the population data can be captured years or even decades earlier, are generally unmatched to the target group and may have used a different nutrition assessment method to the target group. These provide potential explanations for conflicting results, which have been reported.Reference Jahrami, Faris, Saif and Hammad47, Reference Williamson, Kilner and Clibbens74
Results from this review suggest that no dietary-assessment method or tool has been thoroughly validated in SMI. There is a clear need for subjective measures of dietary assessment to be compared with objective measures, such as biomarkers, to assess the accuracy of self-report measures in people with SMI. Biomarkers, such as carotenoids which are reflective of fruit and vegetable intake,Reference Burrows, Williams, Rollo, Wood, Garg and Jensen88 or doubly labelled water which is reflective of total energy intake,Reference Hill and Davies89 are validated specific measures of dietary intake in the general population. Although direct relationships between diet and objective biomarkers likely also exist in mental illness, consideration of certain factors is needed when interpreting results. For example, inflammation associated with mental illness may reduce the levels of these biomarkers. Given cognitive impairment, poor memory, motivation difficulties and potential recall bias, those assessing dietary intake need expertise or training in dietary-assessment method selection and implementation, to ensure appropriate use of particular methods. Given short-term, ‘snapshot’ assessments of dietary intake were commonly used (such as the use of 24 h recalls), long-term dietary intake in SMI may require further investigation. Meta-analyses in this review were also limited because of the range of metrics used to report outcomes, placing greater importance on complementary qualitative synthesis.
First-episode psychosis
As the pooled prevalence of recovery in first-episode of psychosis is 38%,Reference Lally, Ajnakina, Stubbs, Cullinane, Murphy and Gaughran90 and weight gain and metabolic decline are most rapid in first-episode psychosis in the earlier stages of psychotropic medication treatment,Reference Álvarez-Jiménez, González-Blanch, Crespo-Facorro, Hetrick, Rodriguez-Sánchez and Pérez-Iglasias7 it was deemed imperative to review studies that included clinician-diagnosed first-episode psychosis. The four identified studies in first-episode psychosis found unfavourable dietary intakes,Reference Ryan, Flanagan, Kinsella, Keeling and Thakore59, Reference Ryan, Collins and Thakore60, Reference Samele, Patel, Boydell, Leese, Wessely and Murray62, Reference Williamson, Kilner and Clibbens74 in line with the results for established, enduring SMI. This is particularly important, since increased food intake and the majority of associated weight gain is believed to begin following initiation of antipsychotic treatment.Reference Blouin, Tremblay, Jalbert, Venables, Bouchard and Roy11, Reference Fountaine, Taylor, Mancuso, Greenway, Byerley and Smith91 Furthermore, first-episode psychosis has been identified as a ‘critical period’ for targeting lifestyle behaviours in order to prevent obesity and metabolic dysfunction from arising later in life.Reference Teasdale, Ward, Rosenbaum, Samaras and Stubbs92
Impact of diet on brain health
There was limited information obtained from the systematic review for the impact of diet on brain health and mental illness symptomatology; however, evidence in this area is growing. There appears to be a bidirectional relationship between diet quality and depression,Reference Quirk, Williams, O'Neil, Pasco, Jacka and Housden93 with emerging randomised controlled trial evidence finding that improvements in diet quality correlate with improvements in depressive symptoms.Reference Jacka, O'Neil, Opie, Itsiopoulos, Cotton and Mohebbi94, Reference Opie, O'Neil, Jacka, Pizzinga and Itsiopoulos95 Additionally, dietary intake appears to be a factor in brain health, which may be of particular relevance given the neurodegeneration involved with SMI. High blood sugar and a Western diet, which is high in processed, non-nutritious foods, and low in commonly recommended foods of a healthy diet, are associated with smaller hippocampal volume.Reference Cherbuin, Sachdev and Anstey96, Reference Jacka, Cherbuin, Anstey, Sachdev and Butterworth97 Further, a meta-analysis has also demonstrated the potential preventative action of diet on the development of a series of brain ailments including cognitive impairment (eight studies, relative risk (RR) = 0.60, 95% CI 0.43–0.83) and depression (nine studies, RR = 0.68, 95% CI 0.54–0.86).Reference Psaltopoulou, Sergentanis, Panagiotakos, Sergentanis, Kosti and Scarmeas98
Future recommendations
Future recommendations include:
(a) use of technology to assist people with mental illness to record/remember foods consumed;
(b) use of food models/images and a food checklist to assist people with remembering food and beverage items and to estimate portion sizes;
(c) using a trained interviewer or diet expert such as dietitian;
(d) using an online dietary assessment primer or published reviewReference Shim, Oh and Kim99 to choose the most appropriate assessment method; and
(e) using relevant guidelines such as Strengthening the Reporting of Observational Studies in Epidemiology – nutritional epidemiology (STROBE-nut)Reference Lachat, Hawwash, Ockè, Berg, Forsum and Hörnell100 to enhance reporting of dietary intake studies.
Additionally, further research should be dedicated to the following areas:
(a) comprehensive evaluation of dietary intake relative to psychotropic medications;
(b) dietary intake in the early stages of illness as targets for preventative intervention;
(c) dietary intake pre-illness onset to observe if unhealthy dietary intake precedes illness onset (i.e. at-risk mental state), and whether any dietary factors may indicate the onset of illness;
(d) the effect of dietary patterns on psychiatric symptoms, and characteristic of illness such as cognitive impairment, in people with SMI; and
(e) evaluating the validity and reliability of dietary-assessment methodologies in people with SMI to determine appropriate methods for future use, or facilitate the development of new methods. Validation should be completed using energy equations to determine accuracy and where possible use objective biomarkers to validate.
Implications
Overall, the findings of this study are clinically important, as poor dietary intake, particularly low intakes of fruit and vegetables, and high intakes of fast food and other convenience foods, and sweetened beverages, may lead to greater risk for future cardiometabolic illness. Dietary interventions should be a standard part of care for people with SMI, to help mitigate the physical health disparities in this population compared with the wider population.
Funding
J.F. is supported by a Blackmores Institute Fellowship and an MRC Doctoral Training Grant (P117413F07). B.S. holds a Clinical Lectureship supported by Health Education England and the NIHR Integrated Clinical Academic (ICA) Programme (ICA-CL-2017-03-001). B.S. is also partly supported by the Maudsley Charity and the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care South London at King's College Hospital NHS Foundation Trust. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care. T.B. is supported by a Brawn Research Fellowship, University of Newcastle.
Acknowledgements
The authors thank Janelle Skinner and Rebecca McKenna for assisting with data extraction, and Michelle Hsu, Tegan Hayes and Cin Yee Hsia for assisting with study quality checks.
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bjp.2019.20.





eLetters
No eLetters have been published for this article.