Refeeding syndrome can be defined as severe electrolyte and fluid shift associated with metabolic abnormalities in patients with malnutrition undergoing realimentation, whether orally, enterally, or parenterally (Reference Crook, Hally and PanteliCrook et al, 2001). Historically, refeeding syndrome was first described in starving wartime prisoners and victims of famines (Reference Schnitker, Mattman and BlissSchnitker et al, 1951). More recently this syndrome has been well documented in surgical and oncology patients undergoing parenteral nutrition (Reference Crook, Hally and PanteliCrook et al, 2001; Reference HearingHearing, 2004). In psychiatry, refeeding syndrome occurs in people with eating disorders and alcoholism (Reference Cumming, Farquhar and BouchierCumming et al, 1987), but it is often missed in other psychiatric patients where malnutrition is relatively common. Up to 50% of patients admitted to acute psychiatric wards are at risk of malnutrition (Reference Abayomi and HackettAbayomi & Hackett, 2004). This can be extreme in the context of self-neglect, alcohol and drug dependency, depression, schizophrenia and dementia (Reference Gray and GrayGray & Gray, 1989). The consequences of missing a diagnosis of refeeding syndrome in these patients may be serious, as complications range from neurological disability (for example Wernicke-Korsakov syndrome) to metabolic complications (for example hypophosphataemia) and death (Reference Crook, Hally and PanteliCrook et al, 2001). We describe refeeding syndrome in two patients routinely admitted to a general psychiatric ward and illustrate the potential pitfalls in the prevention, diagnosis and management of severe malnutrition and refeeding complications in patients with mental illness.
Case report 1
A 60-year-old White man was transferred from a medical ward after being admitted with severe dehydration and malnutrition associated with a major depressive episode and suicidal ideation. He had stopped eating completely for 3 weeks after about 4 months of eating poorly, but had continued to drink water up to a few days before he was found unconscious at home by a neighbour. Rehydration and refeeding were begun and he was started on vitamin supplementation and potassium chloride. He was admitted to a general psychiatric ward on the fifth day when the depression was treated with 75 mg venlafaxine daily. Physical examination revealed diplopia, ataxia with severe muscle weakness and peripheral oedema. Despite a weight of 81 kg on admission (BMI=24) he stated that he had lost 20 kg in 4 months. During his stay in hospital his weight increased to 89 kg in 2 weeks (BMI=27). An electrocardiogram on the third day of hospitalisation showed a tachycardia of 98 beats per minute and was low voltage. The phosphate level fell to 0.36 mmol/l, and the haemoglobin fell to 8.4 g/dl (see Fig.1). A specific diet for hypophosphatemia was instigated and the patient was screened for possible gastrointestinal bleeding - faecal occult blood and full endoscopy were normal. His recovery was uneventful.
Case report 2
A 54-year-old African-Caribbean man was transferred to a general psychiatric ward following medical admission for collapse at home. He had systematised persecutory delusions which had prevented him from eating for 5 weeks. He had no past psychiatric history. He had a past history of muscle pain for which he took simple analgesics. On admission he weighed 57 kg (BMI=19). Following realimentation he developed diplopia associated with bilateral horizontal nystagmus and palsy of the right sixth cranial nerve. Generalised weakness and ataxic gait were noticed. Examination of the abdomen revealed mild hepatomegaly and tenderness in the right hypocondrium. Diagnoses of delusional disorder and Wernicke's encephalopathy were made. He was treated with parenteral thiamine for 7 days and 5 mg olanzapine daily. He had been cooperative and was allowed to start refeeding without any particular dietary restriction. On admission, routine blood tests were normal but on the fourth day the phosphate levels were markedly reduced at 0.26 mmol/l. The phosphate levels returned to normal without adverse medical complications, except for an acute anaemia - haemoglobin levels fell from 13.3 to 10.1 g/dl (see Fig. 1). The patient refused further investigations for the anaemia.
Discussion
Psychiatric training has traditionally emphasised the importance of identifying and effectively treating Wernicke's encephalopathy in patients with alcohol dependency. However, it is important to be aware of other serious consequences of refeeding in patients with malnutrition, such as electrolyte imbalance, in particular hypophosphataemia, and acute anaemia.
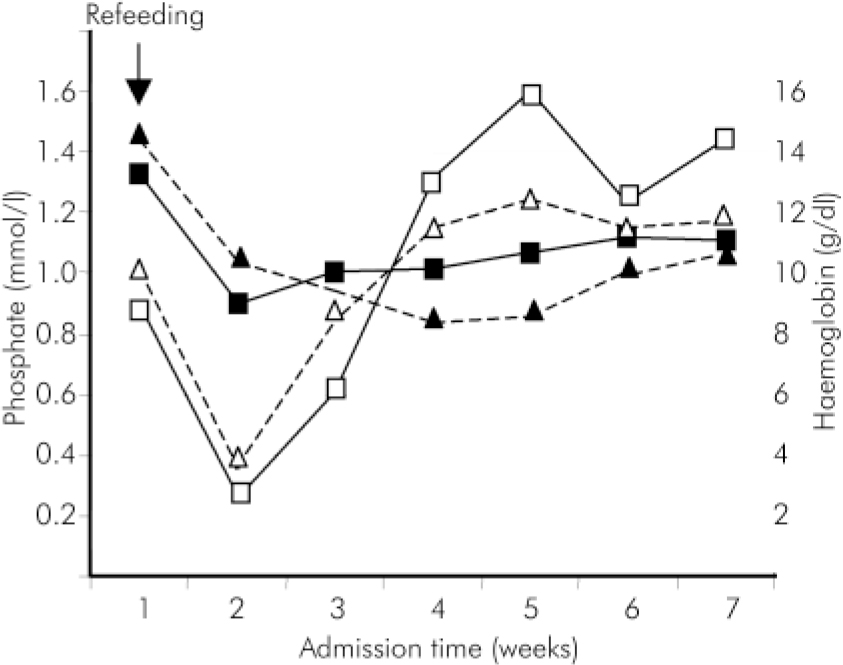
Fig. 1. Variations in phosphate and haemoglobin levels during the admission (- - -▵- - - Case 1, phosphate; - - -▴- - - Case 1, haemoglobin; —□— Case 2, phosphate; —▪— Case 2, haemoglobin).
During refeeding, a shift from fat to carbohydrate metabolism occurs (see Fig. 2; Reference Crook, Hally and PanteliCrook et al, 2001). A glucose load stimulates insulin release, causing increased cellular uptake of glucose, phosphate, potassium, magnesium and water, and protein synthesis. Severe hypophosphataemia may cause a deficit in adenosine triphosphate (ATP) and 2,3 diphosphogycerate synthesis with widespread neuromuscular and haematolgical consequences (Reference MalletMallet, 2002). Thiamine deficiency occurs due to increased cellular utilisation of thiamine in response to carbohydrate refeeding and is associated with the precipitation of Wernicke's encephalopathy, and the potential development of Korsakov syndrome with its attendant lifelong neuropsychiatric disability.
As in the two case reports presented a range of clinical events may go unrecognised. (See Fig. 3 for clinical representations of refeeding syndrome.) Severe hypophosphataemia is a prime feature, and usually this is what alerts the clinician. The other facets to the metabolic disorder include thiamine and other vitamin deficiencies, hypokalaemia, hypomagnesaemia, as well as glucose and fluid balance abnormalities (Reference Crook, Hally and PanteliCrook et al, 2001). The severe hypophosphataemia may present with rhabdomyolysis and cardiomyopathy. It may cause epilepsy, delirium and paraesthesia (Reference KnochelKnochel, 1981). Haemolysis and platelet dysfunction are rare complications that usually follow severe hypophosphatemia (Reference Jacob and AmsdenJacob & Amsden, 1971; Reference KnochelKnochel, 1981), although in the two cases we described haemolytic anaemia followed moderate reduction of phosphate levels. The exact mechanism is unknown but probably is due to increased rigidity of membranes (Reference Jacob and AmsdenJacob & Amsden, 1971) and metabolic acidosis induced by reduced levels of ATP (Reference KnochelKnochel, 1981). Hypokalaemia may be associated with the development of cardiac arrhythmias, hypotension, and cardiac arrest. It may also cause ileus, weakness, paralysis, delirium and rhabdomyolysis. Hypomagnesaemia when severe may cause potentially fatal cardiac arrhythmias as well as neuromuscular symptoms, ataxia, epilepsy and delirium. Fluid intolerance in refeeding syndrome may result in cardiac failure, dehydration or fluid overload, hypotension, pre-renal failure and sudden death. Abnormal glucose and lipid metabolism can potentially trigger hyperglycaemia and hypercapnic respiratory failure (Reference Crook, Hally and PanteliCrook et al, 2001).
The lack of understanding and recognition of refeeding syndrome may result in psychiatrists and psychiatric nurses considering physical complications of this syndrome such as fatigue, ataxia, paresthesias and delirium to be a manifestation of mental illness. Blood tests should be taken before and after refeeding to monitor haemoglobin, plasma electrolytes (in particular sodium, potassium, phosphate, and magnesium), glucose, alanine aminotransferase, aspartate aminotransferase, creatinine phosphokinase. Laboratory findings such as raised creatinine phosphokinase and hypophosphataemia, if detected, can be wrongly attributed to neuroleptic malignant syndrome (Reference HarschHarsch, 1987).
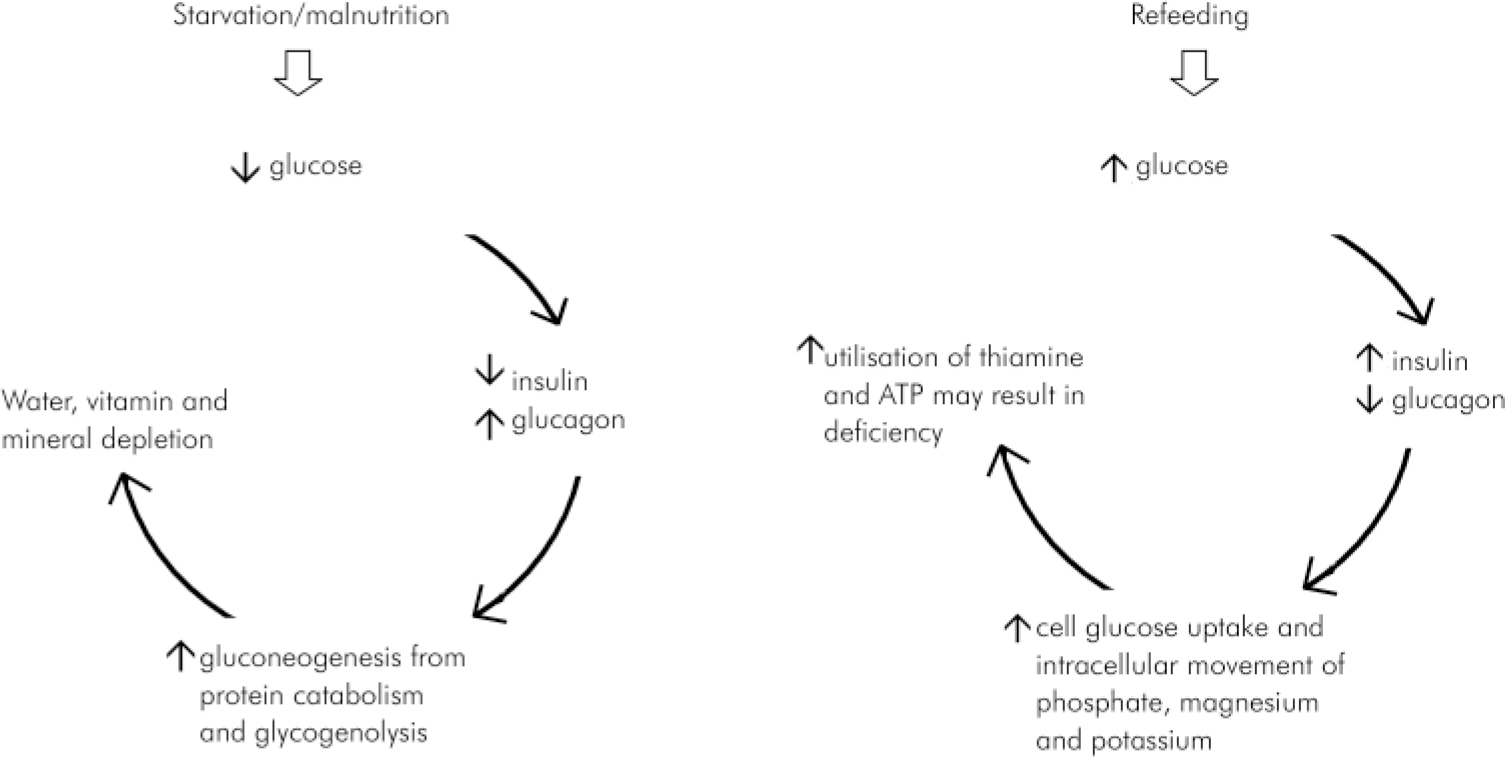
Fig. 2. Basic pathogenetic mechanisms involved in refeeding syndrome. Reprinted from Crook et al (Reference Crook, Hally and Panteli2001) The importance of the refeeding syndrome. Nutrition, 17, 632-637, with permission from Elsevier.
Box 1. Practice points in the management of refeeding syndrome in patients with mental illness
-
• Malnourishment is common in subjects with anorexia and alcoholism but also in depression, schizophrenia and elderly patients with cognitive impairment
-
• An increase in appetite is usually considered a good prognostic factor in patients with psychiatric disorders who are malnourished. For this reason nutritional repletion is usually encouraged in acute psychiatric wards and other psychiatric settings. However, it should be planned with the help of a dietician
-
• Refeeding syndrome is a largely unrecognised cause of Wernicke's encephalopathy, epileptic seizures, and cardiac and respiratory failure in psychiatric patients with low body weight. It has the potential to cause sudden death
-
• Refeeding syndrome may start through the positive impact of psychotropic medication and the ward milieu before the ward staff have properly considered the potential development of refeeding syndrome
-
• The clinical manifestations of mental illness and refeeding syndrome overlap; emergent symptoms of refeeding syndrome may be misattributed to mental illness
-
• Blood tests may be normal on admission, and should be taken serially
-
• Psychotropic medications may contribute to complications of refeeding syndrome such as rhabdomyolysis, hypotension, arrhythmia, seizures, and hypophosphataemia
-
• The management of refeeding syndrome in psychiatric patients may be challenging through their potential non-cooperation with blood tests, intravenous infusions or nutritional restrictions; prevention is therefore paramount
-
• Standardised management protocols that take into account the special circumstances of patients with psychiatric disorders are required
Psychotropic drug treatment, which can stimulate appetite, may increase the risk of refeeding syndrome being precipitated and aggravate electrolyte imbalance.
The management of refeeding syndrome has been described using intravenous phosphate regimens and parenteral thiamine (Reference Faintuch, Soriano and LadeiraFaintuch et al, 2001; Reference HearingHearing, 2004). However there is a lack of consensus as to who should be treated, how, and where, but it would seem important that hospital nutrition teams should be involved early (Reference HearingHearing, 2004). Patients with mental illness may refuse physical investigation, and fail to adhere to dietary restrictions. Close liaison between medical and psychiatric teams is required.
It is not uncommon for psychiatric in-patients not to be weighed, and for the significance of nutritional assessment not to be understood. However the first case we present illustrates that a refeeding syndrome can occur with an apparently normal body weight and highlights the importance of taking a detailed history.
Psychiatrists and mental health workers require specific training in this area (see Box 1), and textbooks of psychiatry and guidelines will need to include reference to refeeding syndromes in psychiatric patients who are malnourished.
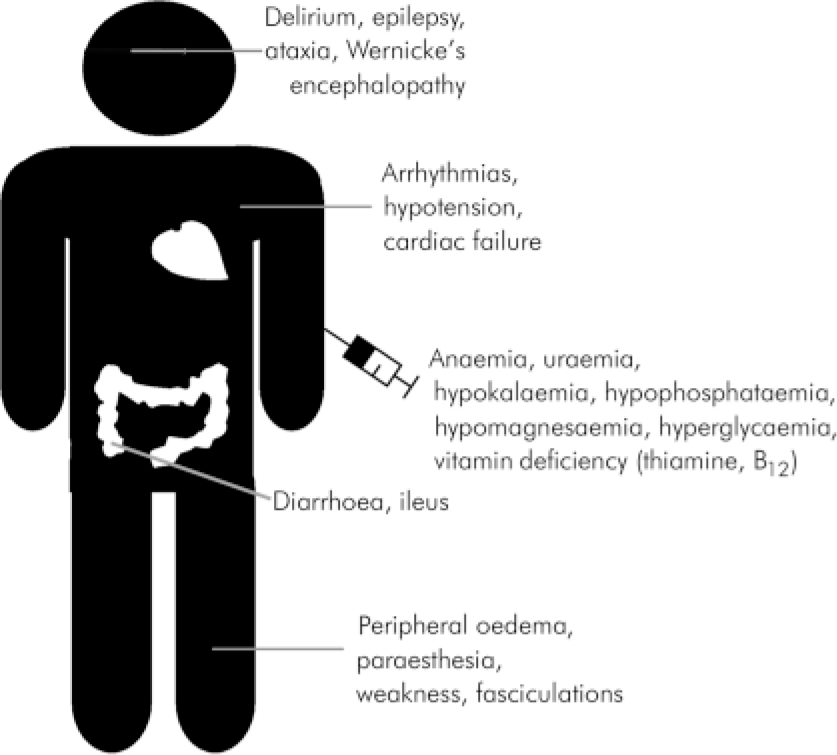
Fig. 3. Clinical presentations of refeeding syndrome.
Declaration of interest
None.






eLetters
No eLetters have been published for this article.