Obesity continues to be a major worldwide health problem, despite the efforts of the medical community. At least 2·8 million adults die from obesity-related causes each year, and 65 % of the worldwide population lives in countries where obesity causes more deaths than underweight( 1 ). Although it is a difficult task, intensive lifestyle interventions can achieve weight loss that is sustained over the long term, as shown by the findings of a recent large clinical trial( Reference Gögebakan, Kohl and Osterhoff 2 ).
Diet is a cornerstone of any lifestyle intervention programme. The dietary plan that restricts energy and fat is the most common strategy, and based on it, several other dietary strategies have been proposed( Reference Ornish 3 – 5 ). The very-low-carbohydrate ketogenic diet (VLCKD) differs from these approaches. According to Accurso et al. ( Reference Accurso, Bernstein and Dahlqvist 6 ), in the early phases of this therapy, individuals must have approximately 50 g carbohydrates/d or 10 % of energy from a nominal 8400 kJ (approximately 2000 kcal) diet, unlike low-carbohydrate diets, which may have up to 130 g carbohydrates/d or 26 % of energy from a nominal diet. A major concern regarding the prescription of the VLCKD is the adherence of the individuals assigned to it, since it promotes important lifestyle changes( Reference Alhassan, Kim and Bersamin 7 ).
Given the importance of dietary counselling in weight loss, it is useful to investigate the effectiveness of different dietary therapies. A recent large randomised clinical trial, which assigned individuals to diets ranging from 35 to 65 % of dietary carbohydrate content, showed that, at this level of carbohydrate intake, there is no difference in weight loss between interventions( Reference Sacks, Bray and Carey 8 ). Nonetheless, evidence suggests that greater dietary carbohydrate restrictions lead to greater weight loss( Reference Krieger, Sitren and Daniels 9 ). Indeed, previous meta-analyses have shown that carbohydrate-restricted diets promote greater weight loss than conventional energy-restricted low-fat diets (LFD)( Reference Hession, Rolland and Kulkarni 10 , Reference Nordmann, Nordmann and Briel 11 ). However, these analyses did not exclusively focus on VLCKD studies( Reference Hession, Rolland and Kulkarni 10 ), or included mostly trials with 6 months of follow-up( Reference Nordmann, Nordmann and Briel 11 ); hence, these analyses do not guarantee the long-term effectiveness of the VLCKD.
A recent meta-analysis by Santos et al. ( Reference Santos, Esteves and da Costa Pereira 12 ) reported that low-carbohydrate diets lead to significantly favourable changes in body weight and major cardiovascular risk factors. Nevertheless, this analysis was based only on the individuals who had adopted a low-carbohydrate diet, comparing final values against baseline values. Although it was an important investigation, the question of whether an abrupt change to an individual's lifestyle, such as the adoption of a VLCKD, leads to relevant long-term clinical improvements remains unanswered.
Thus, the present meta-analysis evaluated randomised controlled trials to determine whether overweight and obese individuals assigned to a VLCKD achieve greater weight loss and manage cardiovascular risk factors more effectively than those assigned to a LFD over the long term (defined as 12 months or more post-intervention).
Methods
The present meta-analysis is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement( Reference Moher, Liberati and Tetzlaff 13 ). The protocol was previously published in the PROSPERO database (http://www.crd.york.ac.uk/PROSPERO), under registration no. CRD42012002408.
Search strategy
The following databases were searched until August 2012: MEDLINE, CENTRAL, ScienceDirect, Scopus, LILACS, SciELO and ClinicalTrials.gov. In addition, the following grey literature databases were searched: OpenGrey.eu, DissOnline.de, NYAM.org and ClinicalEvidence.com. There was no manual search of the included articles, and no specialists in the field were contacted to avoid the risk of citation bias( 14 ). The search strategy included terms related to the intervention (VLCKD), the primary outcome (weight loss) and the secondary outcomes (cardiovascular risk factors), as well as related terms designed to improve the sensitivity of a search for randomised controlled trials( Reference Robinson and Dickersin 15 ). The search was not restricted to any particular years of publication or languages. The complete search strategy is shown in the Supplementary material (available online).
Eligibility criteria
Only randomised controlled trials that met the following criteria were included: (1) the study participants were individuals older than 18 years old who were assigned to a LFD (i.e. a restricted-energy diet with less than 30 % of energy from fat) or to a VLCKD (i.e. a diet with no more than 50 g carbohydrates/d or 10 % of daily energy from carbohydrates); (2) the follow-up period was 12 months or more; (3) the participants had a mean BMI greater than 27·5 kg/m2. The third criterion allowed the inclusion of studies of populations who are already at high risk beyond this BMI threshold( 16 ).
The present analysis aimed to evaluate the differences in the outcomes of the prescribed diets, without addressing individual adherence to the diets. There were no restrictions based on sex, race or co-morbidities. At a minimum, the studies must have assessed weight loss as an outcome and must have reported mean values or the differences between the mean values. The exclusion criteria were as follows: (1) studies with a concomitant pharmacological intervention and (2) duplicate publications of the included trials.
Data extraction
The titles and abstracts of the retrieved articles were evaluated independently by two investigators who were not blinded to the authors or the journal titles. The full-text versions of potentially eligible articles were retrieved for further evaluation.
The primary outcome sought in the studies was the mean change between the baseline body weight and the final body weight (in kg), with the associated measure of dispersion. The secondary outcomes were the mean changes between the baseline and final values (with the associated measures of dispersion) for TAG (in mg/dl (to convert to mmol/l, multiply by 0·0113)), HDL-cholesterol (HDL-C) and LDL-cholesterol (LDL-C) (in mg/dl (to convert to mmol/l, multiply by 0·0259)), fasting blood glucose (in mg/dl (to convert to mmol/l, multiply by 0·0555)), insulin (in mU/ml (to convert to pmol/l, multiply by 6·945)), C-reactive protein (in mg/l (to convert to nmol/l, multiply by 9·524)), HbA1c (percentage), and systolic and diastolic blood pressure (SBP and DBP, respectively, in mmHg).
All the necessary information was extracted from the published articles, protocols and commentaries related to each study, and when necessary, the authors were contacted to obtain additional information. For the studies that had more than two experimental groups, the most suitable one was chosen. Any disagreements were resolved by consensus. A standard form for storing data was created based on the Cochrane Collaboration model( 17 ).
Assessment of risk of bias
Risk of bias was evaluated according to the Cochrane Handbook recommendations( 18 ), at the primary outcome level. The quality of the studies were assessed by two investigators independently in five categories: adequate sequence generation; allocation concealment; blinding of the outcome assessors; handling of missing data (intention-to-treat or per-protocol analysis); selective outcome reporting. The nature of the trials required an open intervention with no blinding of the trial participants or the investigators.
Data analysis
The absolute changes for each outcome, reported as the differences between the final and baseline mean values, were analysed. The treatment effects across the trials were pooled, and weighted mean differences (WMD) for the outcome measures were calculated. The study weights were assigned by using the inverse variance method( 19 ), and the calculations were performed using a random-effects model( Reference DerSimonian and Laird 20 ). An α value of 0·05 was considered to be statistically significant. When it was not possible to retrieve adequate data, imputations were performed( 21 ). These imputations are shown in the Supplementary material (available online).
Statistical heterogeneity among the studies was tested using the Cochran Q test, and inconsistency was tested using the I 2 test. A P value less than 0·10 was considered to be statistically significant. Whenever a result showed heterogeneity, it was explored in three different ways. First, each analysis was repeated, removing each study one at a time in order to assess whether a particular study explained the heterogeneity. Second, univariate meta-regressions were performed to analyse whether methodological covariates were influencing the results( Reference Harbord and Higgins 22 ). The covariates included the risk of bias in the study, adequate nutritional counselling of the individuals (studies that included individual or group meetings with a dietitian at least bimonthly until the end of the follow-up period were considered as adequate), the use of an intention-to-treat analysis, the study follow-up length in months and the presence of co-morbidities in the inclusion criteria for the participants in each study. Thereafter, it was planned to perform a multivariate meta-regression including all covariates that had a P value less than 0·10 in the univariate analysis. Finally, subgroup analyses were performed on studies that shared certain methodological features, including studies with a low risk of bias, studies using an intention-to-treat analysis and studies with 24 months of follow-up. Subgroup analyses were conducted regardless of heterogeneity.
Contour-enhanced funnel plots( Reference Peters, Sutton and Jones 23 ) were created and Egger's test( Reference Egger, Smith and Schneider 24 ) was performed to evaluate publication bias; P values less than 0·10 were considered to be statistically significant. All analyses were conducted using Stata software 9.0 (StataCorp). Graphs were plotted using RevMan 5.4 (Cochrane Collaboration).
Results
Included studies
From 3123 potentially relevant records identified by searching the databases, twenty-five full-text publications met the inclusion criteria and were retrieved for further assessment. From these, eleven were excluded after the full-text analysis, leaving fourteen full texts included in the qualitative and quantitative analysis (Table 1). The flow diagram illustrating the search and selection of studies is shown in Fig. 1. Reasons for exclusion are shown in the Supplementary material (available online).
Table 1 Characteristics of the included studies
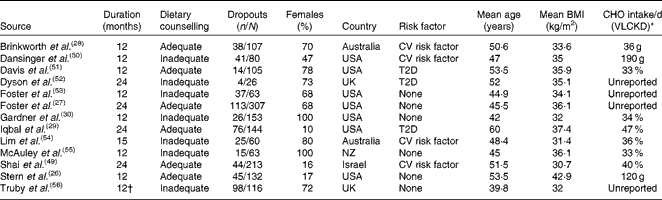
CHO, carbohydrate; VLCKD, very-low-carbohydrate ketogenic diet; CV, cardiovascular; T2D, type 2 diabetes mellitus; NZ, New Zealand.
* Mean carbohydrate intake in the VLCKD group at the end of the follow-up, measured by dietary assessment, shown as g/d or percentage of energy from carbohydrates per d.
† Truby et al. ( Reference Truby, Baic and deLooy 56 ) assessed only the body weight at 12 months.
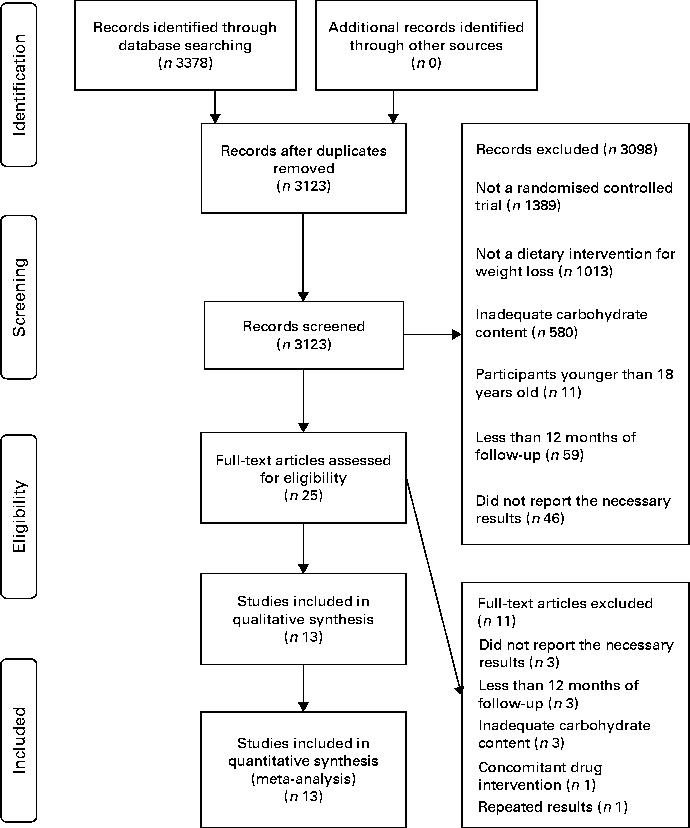
Fig. 1 Flow diagram of the study selection.
From the fourteen full-text articles included, the report by Vetter et al. ( Reference Vetter, Iqbal and Volger 25 ) had characteristics that were unexpected and not mentioned in the inclusion or exclusion criteria for the review. This report describes a body weight analysis of the individuals included in the study by Stern et al. ( Reference Stern, Iqbal and Seshadri 26 ), conducted 36 months after randomisation. Nevertheless, follow-up ceased after 12 months; thus, it was not possible to assess whether the individuals continued with the intervention in the period after follow-up, so the data from this full-text article were included in a sensitivity analysis.
In total, thirteen studies were included in the quantitative analysis, with a total of 1577 individuals randomised to a condition (787 to a LFD group and 790 to a VLCKD group). From these, six studies had more than two intervention groups, and it was determined by consensus which groups fit best in the analysis. Intervention groups of all studies are shown in the Supplementary material (available online).
Assessment of risk of bias
The risk of bias in the studies at the primary outcome level is shown in Table 2. In the final result, nine from the thirteen included studies were assessed as having a low risk of bias.
Table 2 Risk of bias of the included studies
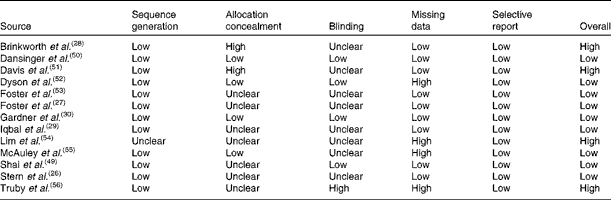
Of these nine studies, two did not report the sequence generation method used, while seven did not report using any measure to conceal the allocation. All the nine studies did not report blinding of the outcome assessors, but as all the outcomes are objective, it is unlikely that this domain affected the results of the trials. Regarding the handling of missing data, five studies were categorised as having a high risk of bias because they utilised a per-protocol analysis. There was no evidence of selective outcome reporting.
Data analysis
Body weight
All the thirteen included studies (1415 patients) were assessed (Fig. 2(a)). The individuals assigned to a VLCKD achieved a significantly greater weight loss compared with the individuals assigned to a LFD (WMD − 0·91 (95 % CI − 1·65, − 0·17) kg, P= 0·02; I 2= 0 %, P= 0·47). This result was consistent across all subgroup analyses, except for the subgroup of studies with 24 months of follow-up (data not shown). The substitution of the data from Stern et al. ( Reference Stern, Iqbal and Seshadri 26 ) for the data from Vetter et al. ( Reference Vetter, Iqbal and Volger 25 ) changed the results (WMD − 0·73 (95 % CI − 1·52, 0·06) kg, P= 0·07; I 2= 5 %, P= 0·39). There was no evidence of publication bias (P= 0·34). The contour-enhanced funnel plots for body weight and all other outcomes are shown in the Supplementary material (available online).
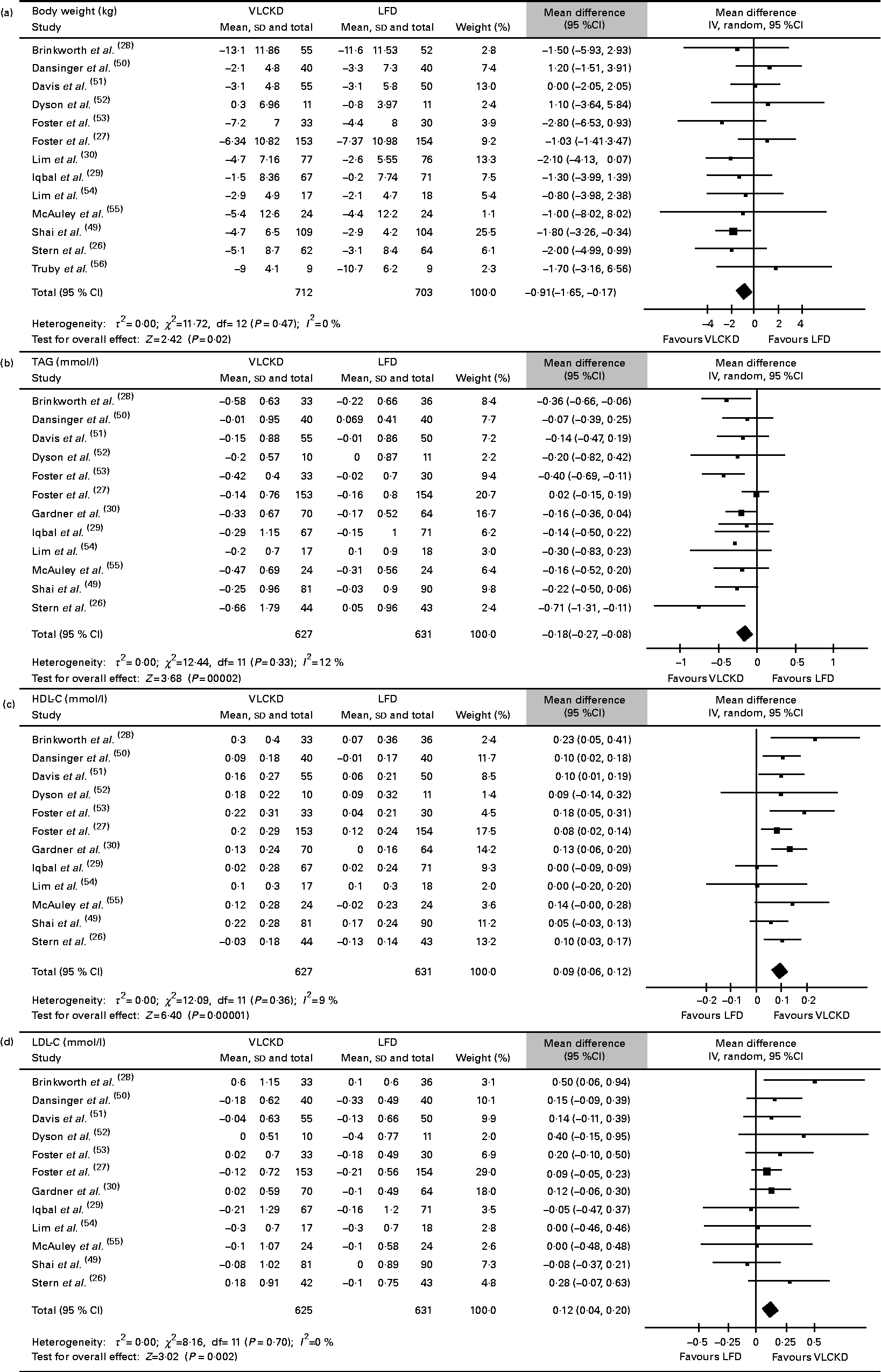
Fig. 2 Absolute changes in (a) body weight, (b) TAG, (c) HDL-cholesterol (HDL-C) and (d) LDL-cholesterol (LDL-C). VLCKD, very-low-carbohydrate ketogenic diet; LFD, energy-restricted low-fat diet.
TAG
In total, twelve studies (1258 patients) were assessed (Fig. 2(b)). The individuals assigned to a VLCKD showed a significantly greater reduction in TAG than the individuals assigned to a LFD (WMD − 0·18 (95 % CI − 0·27, − 0·08) mmol/l, P< 0·001; I 2= 12 %, P= 0·33). This result was consistent across all subgroup analyses, except for the subgroup of studies with 24 months of follow-up (data not shown). Heterogeneity was reversed when the study by Foster et al. ( Reference Foster, Wyatt and Hill 27 ) was excluded, and also when the study by Stern et al. ( Reference Stern, Iqbal and Seshadri 26 ) was excluded, but there were no statistically significant changes in the results. The evidence of publication bias (P= 0·04) was also reversed with the exclusion of both aforementioned studies. The meta-regression analysis showed that the covariate ‘study follow-up length’ affected the results significantly (r 2 87·19 %, P= 0·09; Table 3).
Table 3 Meta-regression analysis (Coefficients and 95 % confidence intervals)
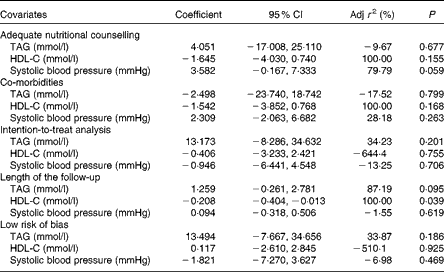
Adj r 2, adjusted r 2; HDL-C, HDL-cholesterol.
HDL-cholesterol
Overall, twelve studies (1257 patients) were assessed (Fig. 2(c)). The individuals assigned to a VLCKD achieved a significantly greater increase in their HDL-C levels compared with the individuals assigned to a LFD (WMD 0·09 (95 % CI 0·06, 0·12) mmol/l, P< 0·001; I 2= 9 %, P= 0·36). All the subgroups showed the same result (data not shown). The study by Brinkworth et al. ( Reference Brinkworth, Noakes and Buckley 28 ) and the study by Iqbal et al. ( Reference Iqbal, Vetter and Moore 29 ) were each individually responsible for the heterogeneity in the overall analysis, and the stepwise exclusion of both studies did not change the main result (data not shown). In the meta-regression analysis, only the covariate ‘study follow-up length’ significantly affected the results (r 2= 100 %, P= 0·03; Table 3). There was no evidence of publication bias (P= 0·53).
LDL-cholesterol
A total of twelve studies (1255 patients) were assessed (Fig. 2(d)). The individuals assigned to a VLCKD achieved a significantly greater increase in their LDL-C levels compared with the individuals assigned to a LFD (WMD 0·12 (95 % CI 0·04, 0·2) mmol/l, P= 0·002; I 2= 0 %, P= 0·7). The subgroup of studies with 24 months of follow-up was the only subgroup that showed different results (data not shown). There was no evidence of publication bias (P= 0·42).
Systolic and diastolic blood pressure
Overall, eleven studies (1298 patients) were included in the SBP (Fig. 3(A)) and DBP analyses (Fig. 3(B)). There were no differences in SBP between the groups (WMD in favour of the VLCKD − 1·47 (95 % CI − 3·44, 0·50) mmHg, P= 0·14; I 2= 33 %, P= 0·13), a result that held in the subgroup analyses. However, individuals assigned to a VLCKD had a significantly greater reduction in DBP than the individuals assigned to a LFD (WMD − 1·43 (95 % CI − 2·49, − 0·37) mmHg, P= 0·008; I 2= 3 %, P= 0·41).

Fig. 3 Absolute changes in (a) systolic blood pressure (SBP) and (b) diastolic blood pressure (DBP). LFD, energy-restricted low-fat diet; VLCKD, very-low-carbohydrate ketogenic diet.
The sensitivity analysis for SBP showed that the study by Gardner et al. ( Reference Gardner, Kiazand and Alhassan 30 ) was responsible for the heterogeneity, and its exclusion did not change the results (data not shown). The covariate ‘adequate nutritional counselling’ significantly affected the SBP results (r 2= 79·7 %, P= 0·05; Table 3). Due to the extremely low heterogeneity, neither a sensitivity analysis nor a meta-regression analysis was undertaken for DBP, and only the subgroup of studies with 24 months of follow-up showed different results (data not shown). There was no evidence of publication bias for SBP (P= 0·79), but the DBP analysis showed statistically significant publication bias (P= 0·04), which was not reversed by the exclusion of any study.
Fasting blood glucose, insulin, HbA1c and C-reactive protein
These analyses were performed in less than ten studies; thus, no sensitivity, subgroup, meta-regression and publication bias analyses were conducted. None of these analyses showed statistically significant results. The forest plots for these analyses are shown in the Supplementary material (available online). For the fasting blood glucose analysis, eight studies (770 patients) were assessed (WMD in favour of the VLCKD − 0·08 (95 % CI − 0·18, 0·02) mmol/l, P= 0·11; I 2= 0 %, P= 0·88). For the insulin analysis, six studies (584 patients) were assessed (WMD in favour of the VLCKD − 5·52 (95 % CI − 13·62, 2·57) pmol/l, P= 0·18; I 2= 26 %, P= 0·24). For the HbA1c analysis, four studies (319 patients) were assessed (WMD in favour of the VLCKD − 0·24 (95 % CI − 0·55, 0·06) %, P= 0·12; I 2= 0 %, P= 0·59). Finally, for the C-reactive protein analysis, four studies (355 patients) were also assessed (WMD in favour of the VLCKD − 1·85 (95 % CI − 6·66, 2·96) nmol/l, P= 0·45; I 2= 0 %, P= 0·55).
Discussion
The present meta-analysis showed that individuals assigned to a VLCKD achieve greater reductions in body weight, TAG and DBP, but they also demonstrate a greater increase in LDL-C and HDL-C levels over a treatment follow-up period of 12 months or more, compared with individuals assigned to a LFD. Only the change in HDL-C levels retained statistical significance in the subgroup analysis of studies with 24 months of follow-up; however, it is important to note that this analysis included only four studies. Low risk of bias was not unanimous, although this characteristic did not influence any of the results, since potential bias was explored by conducting subgroup and meta-regression analyses. Also, studies that included individuals with co-morbidities were not sources of heterogeneity. Furthermore, only the TAG and the DBP analyses revealed evidence of publication bias.
With regard to the primary outcome, the present findings are similar to the findings of previous meta-analyses( Reference Hession, Rolland and Kulkarni 10 , Reference Nordmann, Nordmann and Briel 11 ). The supposed beneficial effect of a VLCKD on body weight may be due to the modulation of resting energy expenditure. Under isoenergetic conditions, Ebbeling et al. ( Reference Ebbeling, Swain and Feldman 31 ) found that a carbohydrate-restricted diet is better than a LFD for retaining an individual's BMR. In addition, Westman et al. ( Reference Westman, Feinman and Mavropoulos 32 ) hypothesised that a VLCKD reduces insulin levels, which would explain the satietogenic effects of this diet. This hypoinsulinaemic effect of the VLCKD was not evidenced in this analysis.
TAG decreased significantly in individuals assigned to a VLCKD. The heterogeneity in the analysis and the evidence of publication bias were entirely attributable to the study by Foster et al. ( Reference Foster, Wyatt and Hill 27 ), which was the only study to present neutral results in this analysis. On the other hand, individuals assigned to a VLCKD showed significantly increased levels of both LDL-C and HDL-C levels. As discussed by Volek et al. ( Reference Volek, Sharman and Forsythe 33 ), the preservation of the circulating HDL-C and the hypotriacylglycerolaemic effect of a VLCKD might be explained by the reduction in the dieting individuals' postprandial lipaemia. Conversely, the increase in LDL-C concentration associated with the VLCKD is an expected finding that is attributable to the increase in saturated fat intake. However, this finding warrants further investigation. Krauss et al. ( Reference Krauss, Blanche and Rawlings 34 ) showed that high fat intake, combined with carbohydrate restriction, raises the levels of larger-sized LDL-C, which are known to be less atherogenic than the small, dense LDL-C( Reference Berneis and Krauss 35 ).
There was also evidence that individuals assigned to a VLCKD showed a significantly greater reduction in DBP. Hession et al. ( Reference Hession, Rolland and Kulkarni 10 ) analysed five studies and found that carbohydrate-restricted diets only influenced SBP. Usually, hypertension is attributable to obesity and Na intake, but Appel et al. ( Reference Appel, Sacks and Carey 36 ) showed that substituting carbohydrates for proteins and monounsaturated fats may decrease blood pressure beyond the decrease expected with Na restriction alone.
It is remarkable to note that although five outcomes demonstrated statistical significance, these findings must be carefully interpreted regarding its clinical significance( 37 ). For example, a typical 1·70 m-tall adult with a BMI of 30 kg/m2 weighs 87 kg; hence, a weight loss of 0·91 kg, as observed here, would represent only 1·04 % of the initial body weight. However, large randomised clinical trials with long-term dietary interventions aiming weight loss showed that individuals under intensive lifestyle interventions lose about 4·8 kg( Reference Gögebakan, Kohl and Osterhoff 2 , Reference Wadden, Volger and Sarwer 38 ). Hence, the further reduction of 0·9 kg in the individuals assigned to a VLCKD would represent almost 20 % of the awaited weight loss achieved with long-term dietary interventions. Additionally, if we assume the cut-off points of the metabolic syndrome( Reference Alberti, Zimmet and Shaw 39 ), similar percentages would be found regarding the other outcomes. The extra reduction of 1·43 mmHg in DBP achieved by individuals assigned to a VLCKD is similar to the reductions promoted by other dietary interventions, such as Mg supplementation( Reference Kass, Weekes and Carpenter 40 ) or consumption of flavonol-rich products( Reference Ried, Sullivan and Fakler 41 ).
Undoubtedly, the present findings demonstrate that a VLCKD has favourable effects on body weight and some cardiovascular risk factors, as stated by Santos et al. ( Reference Santos, Esteves and da Costa Pereira 12 ); however, in the long term and when compared with conventional therapy, the differences appear to be of little clinical significance, although statistically significant. Healthcare professionals should weigh the advantages and disadvantages of recommending a VLCKD and consider their patients' will power, since this therapy prominently alters an individual's daily habits.
The present meta-analysis has several limitations. First, it used aggregated data from the studies instead of individual patient data. Second, only blood risk factors were assessed, neglecting important pathological markers such as hepatic lipid infiltration( Reference Haufe, Engeli and Kast 42 ), endothelial function( Reference Phillips, Jurva and Syed 43 ), general cardiovascular events( Reference Lagiou, Sandin and Lof 44 ) and renal function( Reference Friedman, Ogden and Foster 45 ), which are important in assessing the safety of dietary therapies. Third, the adherence to the VLCKD in the included studies was low (Table 1). At the end of the follow-up period in most studies, carbohydrate intake was higher than the protocol allowed. However, in most cases, there was good adherence in the short term, which may explain why meta-analyses of 6-month studies show more impressive results than meta-analyses of longer-term studies, like the present analysis. Greenberg et al. ( Reference Greenberg, Stampfer and Schwarzfuchs 46 ) found that among dieters, the initial weight reduction in the first 6 months is the main predictor of both long-term retention and success in weight loss, which may explain the statistically significant differences observed here.
The Cochrane risk of bias tool was used in the present meta-analysis. Despite being the most recommended tool to assess the risk of bias in randomised controlled trials, it may face some limitations when assessing behavioural or lifestyle interventions, such as dietary ones( 47 ). These interventions are usually complex, i.e. have multiple components, which deem its fidelity (the extent to which the intervention has been delivered as planned) an important issue to be assessed( 48 ). Since the risk of bias tool does not directly address fidelity, it may be difficult to distinguish between an ineffective intervention and a failed implementation( 47 ).
Upcoming trials should focus on dietary adherence, implementing measures to ensure that individuals adhere to the protocol, as was done by some of the included studies( Reference Brinkworth, Noakes and Buckley 28 , Reference Shai, Schwarzfuchs and Henkin 49 ), permitting better investigation of the long-term effects of a VLCKD. Nevertheless, it is necessary to consider the feasibility of such measures, like those applied by Shai et al. ( Reference Shai, Schwarzfuchs and Henkin 49 ), where the investigators managed the lunches of all individuals, in a real-life scenario.
In conclusion, the present meta-analysis demonstrates that individuals assigned to a VLCKD achieve significantly greater long-term reductions in body weight, diastolic blood pressure and TAG, as well as greater LDL and HDL increases when compared with individuals assigned to a LFD; hence, the VLCKD may be an alternative tool against obesity. Investigations beyond that of blood cardiovascular risk factors merit further study.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114513000548
Acknowledgements
The present study was partially supported by Conselho Nacional de Pesquisa e Desenvolvimento Científico e Tecnológico (CNPq) grant 130639/2011-7, by means of a studentship to N. B. B. The sponsors of the study had no role in the study design, data collection, data analysis, data interpretation or writing of the report. N. B. B. and I. S. V. d. M. acquired, analysed and interpreted the data. All authors designed the study, drafted and critically reviewed the manuscript. The authors declare no conflicts of interest.








