Dementia is a major cause of disability worldwide( Reference Lefstad 1 ). In the absence of curative treatment, strategies for prevention of dementia are important. Since diet could be an important modifiable risk factor for dementia, nutritional interventions might help to decrease the incidence of dementia( Reference Feart, Samieri and Rondeau 2 ).
Citrus fruits are consumed worldwide. The edible parts of citrus, such as the segment epidermis tissue and juice vesicle tissue, are rich in citrus flavonoids( Reference Nogata, Sakamoto and Shiratsuchi 3 ). Previous in vivo and in vitro studies have reported that citrus flavonoids have antioxidant and anti-inflammatory bioactivities( Reference Parhiz, Roohbakhsh and Soltani 4 , Reference Alam, Subhan and Rahman 5 ), and can increase neuronal signalling( Reference Spencer 6 ), improve metabolic functions( Reference Orhan 7 , Reference Mulvihill and Huff 8 ) and cross the blood–brain barrier( Reference Youdim, Shukitt-Hale and Joseph 9 ). In addition, some flavonoids — such as naringin and hesperidin — are essentially unique to citrus( Reference Tripoli, Guardia and Giammanco 10 , Reference Rothwell, Perez-Jimenez and Neveu 11 ). Thus, it is expected that habitual intake of citrus would lower the risk of incident dementia through neuroprotective effects and promotion of brain function.
To date, only one epidemiological (cross-sectional) study has suggested that high intake of citrus( Reference Nurk, Refsum and Drevon 12 ) was positively associated with better cognitive function (including episodic memory, executive function, perceptual speed and executive function, visuospatial skills and global cognition). Thus, an assumption that a high intake of citrus might have a preventive effect against dementia could be reasonable. However, as cross-sectional studies may be generally affected by reverse causation, any association between citrus intake and onset of dementia would be better investigated by a cohort study. To our knowledge, however, no cohort study has yet examined this issue.
The purpose of the present cohort study was to examine the relationship between habitual citrus consumption and incident dementia.
Methods
Study cohort
The design of the Ohsaki Cohort 2006 Study has been described in detail elsewhere( Reference Kuriyama, Nakaya and Ohmori-Matsuda 13 ). In brief, the source population for the baseline survey comprised 31 694 men and women aged ≥65 years who were living in Ohsaki City, northeastern Japan, on 1 December 2006.
The baseline survey was conducted between 1 December and 15 December 2006, and follow-up of the participants started from 1 April 2007. A questionnaire was distributed by the heads of individual administrative districts to individual households and then collected by mail. In this analysis, 23 091 persons who provided valid responses formed the study cohort (Fig. 1). We excluded 6333 persons who did not provide written consent for review of their Long-term Care Insurance (LTCI) information, 2102 persons who had already been certified as having disability by the LTCI before follow-up (1 April 2007), sixty-two persons who had died or moved out of the district during the period of the baseline survey, 192 persons whose Doctor’s Opinion Paper and cognitive status in the paper were unavailable and 1029 persons whose citrus consumption data were missing. Thus, 13 373 responses were analysed for the purposes of this study. During the 5·7-year period, only 124 persons were lost to follow-up because of migration from the study area, without developing incident dementia, which provided a follow-up rate of 99·1 %. Among 66 338 person-years, incident dementia was determined for 1143 persons (8·5 %).

Fig. 1 Flow chart of the study participants: the Ohsaki Cohort 2006 Study. * Without developing incident disability.
Consumption of citrus and other foods
We asked about the consumption of citrus and other food items using a FFQ. The questionnaire included thirty-nine food items and several beverages. The term ‘total vegetables’ was defined as the sum of five food items (i.e. green vegetables, carrot and pumpkin, tomato, cabbage and lettuce and Chinese cabbage), and the term ‘other fruits’ referred to fruits (except citrus fruits) and fresh juice. For the majority of food items, five frequency categories were applied (almost never, 1–2 times/month, 1–2 times/week, 3–4 times/week, almost every day).
We conducted a validation study of the FFQ in which 113 respondents provided four 3-d food records within 1 year and subsequently responded to the questionnaire. The Spearman rank correlation coefficient between citrus consumption according to the questionnaire and that according to the food records was 0·56 for men and 0·51 for women; the correlation between the consumptions measured by the two questionnaires administered 1 year apart was 0·61 for men and 0·53 for women( Reference Ogawa, Tsubono and Nishino 14 ).
Covariates
BMI was calculated as the self-reported body weight (kg) divided by the square of the self-reported body height (m).
The K6 was used as an indicator of psychological distress( Reference Kessler, Andrews and Colpe 15 , Reference Kessler, Green and Gruber 16 ). Using six questions, respondents were asked about their mental status over the last month. Total point scores ranged from 0 to 24. As the optimal cut-off point for mental illness in the validation study, we classified individuals with scores of ≥13 as having psychological distress( Reference Kessler, Green and Gruber 16 ).
The Kihon Checklist was developed by the Ministry of Health, Labour, and Welfare of Japan to predict functional decline in community-dwelling elderly. With regard to the motor function score in the Kihon Checklist, respondents were asked about their current motor function status using five binary questions, yielding total point scores ranging from 0 to 5. As the optimal cut-off point for functional decline suggested in the validation study, we classified individuals with scores of <3 as having better motor function( Reference Tomata, Hozawa and Ohmori-Matsuda 17 ). With regard to the cognitive function score in the Kihon Checklist, respondents were asked about their current cognitive function status using three binary questions yielding total point scores ranging from 0 to 3. The validity of the cognitive function score in the Kihon Checklist had been confirmed in a previous study using the Clinical Dementia Rating as a gold standard( Reference Meguro and Team. 18 ).
Follow-up (incident dementia)
The primary outcome was incident dementia, defined as disabling dementia according to the criteria of the LTCI system used in Japan( Reference Ikeda, Yamagishi and Tanigawa 19 ).
The LTCI is a mandatory form of national social insurance to assist daily activity in the disabled elderly( Reference Ikegami 20 – Reference Imahashi, Kawagoe and Eto 22 ). Everyone aged ≥40 years pays premiums, and everyone aged ≥65 years is eligible for formal caregiving services under a uniform standard of disability certification. The procedure for disability certification comprises two parts: (1) assessment of the degree of functional disability using a questionnaire developed by the Ministry of Health, Labour, and Welfare, and (2) reference to the Doctor’s Opinion Paper prepared by the attending physician( Reference Moriyama, Tamiya and Kamimura 23 ).
Disabling dementia was defined as incident functional disability due to dementia according to the LTCI system, the dementia exceeding rank I (rank ≥II) on the Dementia Scale (Degree of Independence in Daily Living for Elderly with Dementia), as entered on the Doctor’s Opinion Paper. The Dementia Scale is classified into six ranks (0, I–IV, M. Rank M means that an individual has severe dementia-related behavioural disturbance that requires medical intervention), and a rank exceeding I is usually used as an outcome measure of incident dementia because individuals who have mild or moderate dementia are classified as rank II( Reference Ikeda, Yamagishi and Tanigawa 19 , Reference Yamamoto, Kondo and Hirai 24 – Reference Meguro, Tanaka and Kasai 26 ). A previous study has shown that the Dementia Scale is well correlated with the Mini Mental State Examination score (Spearman’s rank correlation coefficient=−0·736)( Reference Hisano 27 ).
We obtained a data set that included information on LTCI certification, death or emigration from Ohsaki City. All data were transferred from the Ohsaki City Government under an agreement related to Epidemiologic Research and Privacy Protection.
Ethical issues
We considered the return of completed questionnaires to imply consent to participate in the study involving the baseline survey data and subsequent follow-up of death and emigration. We also confirmed information regarding LTCI certification status after obtaining written consent along with the questionnaires returned from the subjects at the time of the baseline survey. The Ethics Committee of Tohoku University Graduate School of Medicine (Sendai, Japan) reviewed and approved the study protocol.
Statistical analysis
We counted the person-years of follow-up for each subject from 1 April 2007 until the date of incident dementia, date of emigration from Ohsaki City, date of death, incident functional disability without dementia or the end of the study period (30 November 2012), whichever occurred first.
In the present study, only 3·8 % of participants almost never consumed citrus fruits, and 11·6 % consumed them 1–2 times/month. Therefore, data for these two options were merged with the option ‘1–2 times/week’. Thus, citrus fruit consumption was categorised into three groups: ≤2, 3–4 times/week and almost every day.
The multiple adjusted Cox proportional hazards model was used to calculate the hazard ratios (HR) and 95 % CI for incident dementia according citrus consumption. Dummy variables were created for the citrus consumption groups, and respondents who consumed citrus ≤2 times/week (lowest) were defined as a reference category. Multivariate models were adjusted for the following variables. Model 1 was adjusted for age (65–69, 70–74, 75–79, 80–84 or ≥85 years) and sex. To examine whether the association between citrus consumption and dementia was attributable to a healthy physical status or other lifestyle factors, model 2 was further adjusted for BMI (<18·5, 18·5–25, ≥25 kg/m2 or missing), history of disease (stroke, hypertension, myocardial infarction or diabetes) (yes, no; for each term), education level (age at last school graduation: <16, 16–18, ≥19 years or missing), smoking (never, former, current or missing), alcohol drinking (never/former, current or missing), time spent walking (<1, ≥1 h/day or missing) and psychological distress score (<13, ≥13 or missing). In order to adjust for the influence of other dietary factors, model 3 added the consumption volume of total vegetables, and other fruits, intake of energy and protein (sex-specific tertile categories, or missing).
Considering possible reverse causality, we analysed whether the association would change if only individuals who had higher cognitive function at the baseline were selected. In this sensitivity analysis, ‘Cognitive function score in the Kihon Checklist=0 point’ was defined as higher cognitive function. Another sensitivity analysis was also conducted by excluding participants whose disability event occurred in the first 2 years of follow-up.
In addition, tests of interaction were performed to investigate whether there was any difference in the relationship between citrus consumption and incident dementia in terms of sex, age (<75 or ≥75 years), consumption volume of total vegetables and other fruits (≥median for both, or otherwise), and chronic conditions (stroke, hypertension, myocardial infarction or diabetes; having any one or more of these conditions, or having none of them).
All analyses above were performed by using SAS version 9.4 (SAS Inc.).
Finally, multiple imputations were conducted for missing values of all confounding factors in model 3. Based on age, sex and other observed values of confounding factors, missing values for each confounding factor were substituted with the most likely value by the multiple imputation procedure, and ten output data sets were created. Then, the Cox model was applied for the imputed data with confounding factors (without missing values) in model 3 to calculate the pooled HR and 95 % CI for incident dementia according to citrus consumption. Both the multiple data imputation and the Cox model were conducted using IBM SPSS Statistics 24.0 (IBM Software Group).
All statistical tests described here were two-sided, and differences at P<0·05 were accepted as significant.
Results
Baseline characteristics
The baseline characteristics of the 13 373 participants according to citrus consumption categories are shown in Table 1. Subjects with a higher citrus consumption frequency were less likely to be men, to have a history of stroke, to have been <16 years old upon completion of education, to be current smokers and alcohol drinkers, and to have psychological distress. Subjects with a lower citrus consumption frequency were less likely to consume vegetables and other fruits, and to intake protein and energy.
Table 1 Characteristics of participants by citrus consumption (n 13 373) (Mean values and standard deviations; percentages)

* Obtained by using χ 2 test for variables of proportion and one-factor ANOVA for continuous variables (missing value excluded).
† Age at last school graduation <16 years.
‡ Kessler six-item psychological distress scale score ≥13.
§ Motor function score of the Kihon Checklist<3.
Citrus consumption and incident dementia
The association between citrus consumption and incident dementia is shown in Table 2. In comparison with participants who consumed citrus ≤2 times/week, the incident dementia HR was 0·79 (95 % CI 0·69, 0·91) for participants who consumed citrus 3–4 times/week and 0·77 (95 % CI 0·67, 0·89) for those who consumed citrus almost every day (P trend<0·001 in the crude model). Even after adjustment for most confounding factors, the results for citrus consumption did not change substantially in model 2 (multivariate HR 1·00 (reference) for ≤2 times/week, 0·87 (95 % CI 0·76, 1·00) for 3–4 times/week and 0·79 (95 % CI 0·68, 0·91) for almost every day; P trend=0·001). However, the results in model 3 became marginally non-significant.
Table 2 Relationships between citrus consumption and incident dementia (n 13 373)Footnote * (Adjusted hazard ratios (HR) and 95 % confidence intervals)
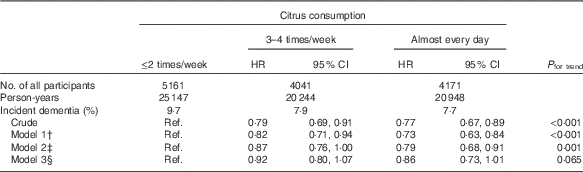
Ref., referent values.
* Analysis by Cox proportional hazards model.
† Model 1 was adjusted for age (65–69, 70–74, 75–79, 80–84 or ≥85 years) and sex.
‡ Model 2 was adjusted as for model 1 plus BMI (<18·5, 18·5–25, ≥25 kg/m2, or missing), history of disease (stroke, hypertension, myocardial infarction or diabetes (yes, no; for each term)), education level (age at last school graduation: <16, 16–18, ≥19 years or missing), smoking (never, former, current or missing), alcohol drinking (never/former, current or missing), time spent walking (<1, ≥1 h/d or missing), psychological distress score (<13, ≥13 or missing).
§ Model 3 was adjusted as for model 2 plus four groups of consumption volume of total vegetables, other fruits, intake of energy and protein (sex-specific tertile categories, or missing).
To consider the possibility that cognitive function at the baseline might affect the association between citrus consumption and incident dementia, we analysed the association after selecting 8283 participants who had better cognitive function (cognitive function score of the Kihon Checklist=0), as shown in Table 3. Although the point HR for higher citrus consumption in all models still lower than the reference group, results of models 3 and 4 were not significant.
Table 3 Relationships between citrus consumption and incident dementia (baseline lower cognitive function excluded) (n 8283)Footnote * (Adjusted hazard ratios (HR) and 95 % confidence intervals)
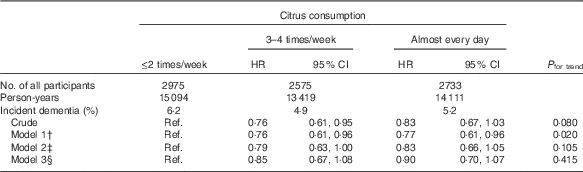
Ref., referent values.
* Analysis by Cox proportional hazards model.
† Model 1 was adjusted for age (65–69, 70–74, 75–79, 80–84 or ≥85 years) and sex.
‡ Model 2 was adjusted as for model 1 plus BMI (<18·5, 18·5–25, ≥25 kg/m2 or missing), history of disease (stroke, hypertension, myocardial infarction or diabetes (yes, no; for each term)), education level (age at last school graduation: <16, 16–18, ≥19 years or missing), smoking (never, former, current or missing), alcohol drinking (never/former, current or missing), time spent walking (<1, ≥1 h/d or missing), psychological distress score (<13, ≥13 or missing).
§ Model 3 was adjusted as for model 2 plus four groups of consumption volume of total vegetables, other fruits, intake of energy and protein (sex-specific tertile categories, or missing).
To examine possible reverse causality in that participants who had worse health status at the baseline might have consumed less citrus, we also analysed the association after excluding 321 participants who developed incident dementia in the first 2 years of follow-up (Table 4), but the results for citrus consumption did not change substantially; the multivariate HR was 1·00 (reference) for ≤2 times/week, 0·82 (95 % CI 0·69, 0·98) for 3–4 times/week and 0·77 (95 % CI 0·64, 0·93) for almost every day (P trend=0·006 in model 3).
Table 4 Relationships between citrus consumption and incident dementia (2-year dementia incidence excluded) (n 13 052)Footnote * (Adjusted hazard ratios (HR) and 95 % confidence intervals)
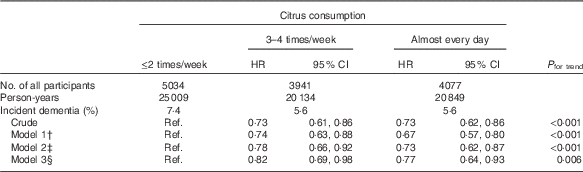
* Analysis by Cox proportional hazards model.
† Model 1 was adjusted for age (65–69, 70–74, 75–79, 80–84 or ≥85 years) and sex.
‡ Model 2 was adjusted as for model 1 plus BMI (<18·5, 18·5–25, ≥25 kg/m2 or missing), history of disease (stroke, hypertension, myocardial infarction or diabetes (yes, no; for each term)), education level (age at last school graduation: <16, 16–18, ≥19 years or missing), smoking (never, former, current or missing), alcohol drinking (never/former, current or missing), time spent walking (<1, ≥1 h/d or missing), psychological distress score (<13, ≥13 or missing).
§ Model 3 was adjusted as for model 2 plus four groups of consumption volume of total vegetables, other fruits, intake of energy and protein (sex-specific tertile categories, or missing).
Stratified analysis
We also conducted sensitivity analysis stratified for sex, age (<75 or ≥75 years), consumption volume of total vegetables and other fruits (≥median for both, or otherwise), and chronic conditions (stroke, hypertension, myocardial infarction or diabetes; having any one or more of these conditions, or none of them). After adjusting for multivariate factors, the inverse correlation between citrus consumption and incident dementia did not differ between subgroups (the P for interaction between citrus consumption and each of the confounders was: 0·45 for sex, 0·13 for age, 0·45 for consumption volume of total vegetables and other fruits and 0·08 for chronic conditions; data not shown).
Analyses of multiple imputed data
Another sensitivity analysis was also performed by using the multiple imputed data to assess the association between citrus fruits consumption and incident dementia without missing data for confounding factors. The multivariate-adjusted results remained consistent with the results of un-imputed data. The multivariate HR was 1·00 (reference) for ≤2 times/week, 0·92 (95 % CI 0·79, 1·06) for 3–4 times/week and 0·86 (95 % CI 0·73, 1·03) for almost every day (P trend=0·090 in model 3; data not shown).
Discussion
In this cohort study, we investigated the relationship between citrus consumption and incident dementia, and observed an inverse dose–response association between the two. Overall, our results are consistent with a previous cross-sectional study indicating potential benefits of citrus intake in terms of cognitive function( Reference Nurk, Refsum and Drevon 12 ). To our knowledge, this is the first cohort study to have investigated the association between citrus consumption and incident dementia in an elderly population.
Considering the possibility that individuals with weaker cognitive function might have less opportunity to consume citrus, we investigated the effects of reverse causality. However, even when we selected only individuals who had better cognitive function at the baseline, the point estimations (HR) for each of the categories were also almost the same. In addition, even after excluding individuals who developed incident dementia in the first 2 years of follow-up, the inverse association between citrus consumption and incident dementia persisted. These findings suggest that the present results are unlikely to have been attributable to reverse causality.
Even when we performed stratified analysis by sex, age, consumption volume of total vegetables and other fruits, and chronic conditions, the results for the relationship between citrus consumption and incident dementia did not change substantially. Therefore, the association between citrus consumption and incident dementia seems difficult to explain in terms of confounding factors.
In addition, in order to minimise potential bias attributed to missing values for confounding factors, analysis of multiple imputed data was performed. However, a similar inverse association was also observed after all confounding factors had been taken into consideration. Thus, the inverse association may not be ascribed to bias of analysis in which missing values were included.
In the area where the present study was conducted, mandarin oranges (such as Satsuma (Citrus unshiu)) are the most common citrus fruit consumed in daily life( Reference Shimizu 28 ). The segment epidermis tissue and juice vesicle tissue of these citrus fruits contain citrus flavonoids such as naringin, hesperidin, narirutin and neohesperidin( Reference Nogata, Sakamoto and Shiratsuchi 3 ). Some experimental biological studies have suggested that these citrus flavonoids have neuroprotective effects (antioxidant properties, anti-inflammation and signalling regulation)( Reference Williams, Spencer and Rice-Evans 29 – Reference Hwang, Lin and Shih 32 ). Therefore, the inverse relationship observed in the present study might have been attributable to these citrus flavonoids.
Our study had a number of strengths: (1) it was a large population-based cohort study involving 13 373 persons; (2) the follow-up rate was very high (99·1 %); (3) many confounding factors were taken into account; and (4) the study subjects lived in an area in which citrus fruits are widely consumed.
However, there were also several limitations. First, the causes of dementia were not evaluated, and therefore the mechanism responsible for reduction of incident dementia by citrus consumption remained unclarified. Second, information on citrus consumption using the FFQ was obtained only at the baseline, and the study subjects might have changed their citrus consumption during the course of follow-up. Third, potential confounding factors could not be disregarded. Particularly, it could not be ruled out that residual diet-related confounding factors were present, since our FFQ data could not fully consider diet quality. Lastly, the relatively short follow-up time of the participants might have compromised our findings. A cohort study with a longer follow-up might therefore be desirable.
In conclusion, the present study has shown that citrus consumption tended to be associated with a lower risk of incident dementia in elderly Japanese individuals. Our results suggest that habitual citrus consumption may have a preventive effect against the incident risk of dementia. Further prospective studies in other populations and settings will be necessary to confirm the association between citrus consumption and dementia.
Acknowledgements
The authors would like to thank Yoshiko Nakata, Yukiko Asano, Mao Suzuki and Mami Takahashi for their technical assistance.
This work was supported by Health Sciences Research grants (nos. H24-Choju-Ippan-005 and H26-Junkankitou (Seisaku)-Ippan-001) from the Ministry of Health, Labour and Welfare of Japan. Sponsors play no role in the design, methods, subject recruitment, data collections, analysis and preparation of paper.
S. Z. and I. T. designed research; Y. T. and K. S. conducted research; S. Z. and Y. T. analysed data; S. Z. wrote the paper; Y. T., K. S., Y. S. and I. T. gave the constructive suggestions; S. Z. had primary responsibility for final content. All authors read and approved the final manuscript.
None of the authors has any conflicts of interest to declare.








