Introduction
Exposure to childhood trauma is a prominent risk factor for the development of psychopathology (Cicchetti & Toth, Reference Cicchetti and Toth2005), and recent evidence suggests that the risk conferred by childhood trauma may even carry into future generations (Plant et al., Reference Plant, Pawlby, Pariante and Jones2018). At the same time, there are significant individual differences in people’s susceptibility to childhood or intergenerational trauma. Among youth exposed to childhood trauma or other adverse childhood experiences, less than 20% go on to develop posttraumatic stress disorder (PTSD; Alisic et al., Reference Alisic, Zalta, Van Wesel, Larsen, Hafstad, Hassanpour and Smid2014) and less than 30% go on to develop depression (Vibhakar et al., Reference Vibhakar, Allen, Gee and Meiser-Stedman2019). However, the biological factors that contribute to these differences in susceptibility remain unclear. To better understand potential risk versus protective mechanisms, the present study examines how epigenetic changes that reflect differences in biological aging may confer vulnerability or protection against the intergenerational impacts of maternal childhood trauma and adverse childhood experiences (ACEs).
Intergenerational impacts of childhood adversity
A broad body of literature has examined the detrimental impacts of childhood trauma – comprising experiences of maltreatment (i.e., emotional, physical, and sexual abuse) and neglect – as well as ACEs, which include non-traumatic stressors such as family dysfunction (e.g., parental substance abuse and parental incarceration). Trauma and adversity during childhood have prolonged consequences that not only increase risk for psychopathology in the individual but also in their offspring (Su et al., Reference Su, D’Arcy and Meng2022). Offspring of mothers who experienced childhood trauma demonstrate increased risk for psychopathology across multiple levels of analysis, ranging from emotional and behavioral symptomatology (Plant et al., Reference Plant, Pawlby, Pariante and Jones2018) to physiological alterations that characterize mood, trauma, and stress-related disorders (e.g., Buss et al., Reference Buss, Entringer, Moog, Toepfer, Fair, Simhan, Heim and Wadhwa2017, Daskalakis et al., Reference Daskalakis, Xu, Bader, Chatzinakos, Weber, Makotkine and Yehuda2021). Similarly, maternal ACEs have been significantly associated with both symptoms of psychopathology (e.g., negative emotionality, behavioral dysregulation, internalizing, and externalizing symptoms) and physiological correlates of psychopathology (e.g., cortisol, inflammatory cytokines, and HPA-axis functioning) in offspring (Cooke et al., Reference Cooke, Racine, Pador and Madigan2021; Zhang et al., Reference Zhang, Mersky, Gruber and Kim2022). As such, it has been proposed that this intergenerational risk may be transmitted through alterations to the mother’s physiological stress response, which in turn influences the fetal development of her offspring’s stress response to increase their sensitivity to environmental stressors and their risk for psychopathology (Babenko et al., Reference Babenko, Kovalchuk and Metz2015; Buss et al., Reference Buss, Entringer, Moog, Toepfer, Fair, Simhan, Heim and Wadhwa2017). Additionally, mothers with a history of childhood adversity have higher rates of perinatal psychopathology (e.g., depression and PTSD), which can influence mother-child attachment, parenting practices, and offspring exposure to childhood adversity, all of which likewise confer risk for the development of psychopathology in offspring (Plant et al., Reference Plant, Pawlby, Pariante and Jones2018).
Evidence from intra-generational studies suggests that the mechanisms underlying the links between childhood adversity and the development of psychopathology may differ according to the type of trauma exposure. For example, while childhood emotional, physical, and sexual abuse often co-occur and have generalized effects on the physiological mechanisms that confer risk for psychopathology (Cassier et al., Reference Cassiers, Sabbe, Schmaal, Veltman, Penninx and Van Den Eede2018; Finkelhor et al., Reference Finkelhor, Ormrod and Turner2007; Noll, 2021), there is also increasing evidence that trauma subtypes differentially impact physiological and behavioral outcomes. Neuroendocrine studies, for instance, have found that different forms of childhood maltreatment are associated with different patterns of HPA-axis activation. Findings from a recent study demonstrated that physical abuse was associated with greater HPA-axis activation following a stressor, while emotional abuse was associated with lower activation but a longer recovery to baseline (Kuhlman et al., Reference Kuhlman, Geiss, Vargas and Lopez-Duran2015). The authors posited that physical abuse may cause HPA-axis adaptations that make an individual more sensitive and reactive to threats in the environment, while emotional abuse may result in adaptations that lead to a more subtle but chronically activated stress response, which in turn may confer greater risk for depression and other psychopathology (Kuhlman et al., Reference Kuhlman, Geiss, Vargas and Lopez-Duran2015). In line with this interpretation, some studies have demonstrated that childhood emotional abuse is more strongly associated with depressive symptoms compared to physical or sexual abuse (Infurna et al., Reference Infurna, Reichl, Parzer, Schimmenti, Bifulco and Kaess2015; Mandelli et al., Reference Mandelli, Petrelli and Serretti2015; Paul & Eckenrode, Reference Paul and Eckenrode2015). However, the literature is mixed, as other studies have found that physically and sexually abused children are at a greater risk for depression (Cicchetti & Valentino, Reference Cicchetti and Valentino2006). Moreover, no studies to date have examined the differential impact of trauma subtypes on intergenerational outcomes. This is an important next step given the shared social and physiological mechanisms underlying intra- and intergenerational transmission (Babenko et al., Reference Babenko, Kovalchuk and Metz2015; Buss et al., Reference Buss, Entringer, Moog, Toepfer, Fair, Simhan, Heim and Wadhwa2017).
Individual differences in intergenerational transmission
Despite the increased risk conferred by maternal childhood trauma exposure, not all offspring of trauma-exposed mothers develop symptomatology. One factor that may contribute to these individual differences is offspring sex. Population studies show that many trauma-related outcomes – including depression, anxiety, and PTSD – are more prevalent in females than males (Kessler et al., Reference Kessler, Demler, Frank, Olfson, Pincus, Walters, Wang, Wells and Zaslavsky2005; Perkonigg et al., Reference Perkonigg, Kessler, Storz and Wittchen2000), and evidence suggests that this is particularly the case in the context of childhood trauma (Breslau et al., Reference Breslau, Davis, Andreski, Peterson and Schultz1997). However, a recent review found that male offspring may be more sensitive to maternal stress and/or HPA-axis dysregulation during fetal development compared to female offspring (Sutherland & Brunwasser, Reference Sutherland and Brunwasser2018). Although a dearth of studies has examined offspring sex differences in the context of maternal trauma exposure prior to pregnancy, it has been recognized as an important area for future research (Buss et al., Reference Buss, Entringer, Moog, Toepfer, Fair, Simhan, Heim and Wadhwa2017).
There is increasing evidence that epigenetic factors may also influence susceptibility to childhood trauma exposure. Epigenetic factors refer to environmentally sensitive molecular changes that alter the expression of genes without changing the genetic sequence itself (Goldberg et al., Reference Goldberg, Allis and Bernstein2007). These changes include histone modifications, changes to non-coding RNAs, and, most widely studied, DNA methylation at specific sites (referred to as CpG sites) that impact gene transcription. A variety of environmental factors have been shown to modify DNA methylation including nutrition, physical health, ecological exposures, and even psychological stressors such as trauma (Quach et al., Reference Quach, Levine, Tanaka, Lu, Chen, Ferrucci and Horvath2017; Zannas et al., Reference Zannas, Wiechmann, Gassen and Binder2016). While former studies have examined epigenetic mechanisms as a potential molecular pathway linking childhood trauma exposure to the development of psychopathology (Daskalakis et al., Reference Daskalakis, Xu, Bader, Chatzinakos, Weber, Makotkine and Yehuda2021; Nöthling et al., Reference Nöthling, Malan-Müller, Abrahams, Hemmings and Seedat2020; Yehuda & Lehrner, Reference Yehuda and Lehrner2018), more recent research has proposed that epigenetics may also moderate the link between maternal trauma and offspring psychopathology (Al Jowf et al., Reference Al Jowf, Snijders, Rutten, de Nijs and Eijssen2021; Conradt, Reference Conradt2017; Smeeth et al., Reference Smeeth, Beck, Karam and Pluess2021). In other words, differences in the epigenome may influence an offspring’s susceptibility to maternal childhood trauma, such that offspring with certain epigenetic signatures may be more resilient to the impacts of intergenerational trauma than others. The current study aims to better understand this potential buffering role.
Although no studies to date have examined whether epigenetic factors moderate the intergenerational association between maternal childhood adversity and offspring psychopathology, findings from studies of maternal prenatal stress provide preliminary support for the moderating role of epigenetic factors. For example, one study found that infants’ DNA methylation differences in the glucocorticoid receptor gene, NR3C1, moderated the relationship between maternal prenatal depression and infant self-regulation and health outcomes. Specifically, infants with high levels of methylation at a specific NR3C1 CpG site were susceptible to maternal prenatal depression, while infants with low levels of methylation were protected (Conradt et al., Reference Conradt, Lester, Appleton, Armstrong and Marsit2013). Given the potential role of NR3C1 in the development of psychopathology following maternal childhood trauma (Bowers & Yehuda, Reference Bowers and Yehuda2016), it is possible that similar effects may be demonstrated in the context of intergenerational trauma.
Epigenetic age acceleration
The above-noted findings highlight the importance of considering epigenetic factors when evaluating susceptibility to maternal childhood trauma. However, based on evidence from genome-wide association studies that psychopathology stems from variation across hundreds (if not thousands) of genes (Duncan et al., Reference Duncan, Ratanatharathorn, Aiello, Almli, Amstadter, Ashley-Koch, Baker, Beckham, Bierut, Bisson, Bradley, Chen, Dalvie, Farrer, Galea, Garrett, Gelernter, Guffanti, Hauser and Koenen2018), the significance of epigenetic variation within a single candidate gene is disputed. As such, researchers are increasingly turning to epigenome-wide measures to more robustly assess the interplay between environmental and epigenetic factors. One such measure is epigenetic age, also referred to as DNA methylation age (DNAm age), which uses DNA methylation patterns across hundreds of CpG sites to estimate biological aging. Interestingly, the discrepancy between an individual’s chronological age and their biological (i.e., epigenetic) age – often referred to as epigenetic age acceleration (EAA) – has been demonstrated as an informative biomarker of health and development (Horvath & Raj, Reference Horvath and Raj2018). While the majority of EAA studies have focused on predictors of adult health outcomes (e.g., GrimAge; Lu et al., Reference Lu, Quach, Wilson, Reiner, Aviv, Raj and Horvath2019), recent studies have determined that infant DNA methylation patterns associated with length of gestation (i.e., DNAm gestational age) are a useful predictor of infant health and early development. Contrary to adult studies, where epigenetic age acceleration has been associated with a variety of negative health outcomes (Oblak et al., Reference Oblak, van der Zaag, Higgins-Chen, Levine and Boks2021), studies of infants indicate that epigenetic age acceleration is often predictive of better outcomes such as higher birth weight and fewer socioemotional problems (Knight et al., Reference Knight, Craig, Theda, Baekvad-Hansen, Bybjerg-Grauholm, Hansen and Smith2016; Simpkin et al., Reference Simpkin, Howe, Tilling, Gaunt, Lyttleton, McArdle and Relton2017; Suarez et al., Reference Suarez, Lahti, Czamara, Lahti-Pulkkinen, Knight, Girchenko and Räikkönen2018), although it is important to note that some birth weight studies have found null or opposite findings (Girchenko et al., Reference Girchenko, Lahti, Czamara, Knight, Jones, Suarez and Räikkönen2017; Khouja et al., Reference Khouja, Simpkin, O’Keeffe, Wade, Houtepen, Relton, Suderman and Howe2018). As such, it is possible that infant epigenetic age acceleration may be protective against exposures that are known to contribute to developmental delays and adverse psychosocial outcomes, such as maternal stress. Together, these findings highlight the potential utility of using DNAm gestational age to more robustly assess how epigenetic factors may influence early developmental outcomes in the context of maternal childhood trauma.
Sociocultural considerations
Finally, epidemiological research indicates that Black Americans are disproportionately exposed to and impacted by childhood trauma and adversity (Mersky & Janczewski, Reference Mersky and Janczewski2018; Roberts et al., Reference Roberts, Gilman, Breslau, Breslau and Koenen2011). This can be attributed to a conglomeration of compounding factors stemming from systemic racism, spanning chronic daily and lifetime stressors as well as lower access to preventative factors and mental healthcare resources (Cooper et al., Reference Cooper, Hill and Powe2002; Williams, Reference Williams2018). Further, due to socioeconomic and racial inequities, Black American women are disproportionately impacted by factors that are negatively associated with infant epigenetic health, such as maternal prenatal stress and preterm birth (Purisch & Gyamfi-Bannerman, Reference Purisch and Gyamfi-Bannerman2017; Suarez et al., Reference Suarez, Lahti, Czamara, Lahti-Pulkkinen, Knight, Girchenko and Räikkönen2018; Szyf, Reference Szyf2021). As such, while we would not expect the mechanisms underlying the intergenerational impacts of maternal childhood adversity to differ for individuals of different racial/ethnic identities, we might expect these associations to differ according to moderators that vary for individuals of different backgrounds. In turn, focusing on the experiences of Black American women and offspring is important for better understanding the intergenerational impacts of maternal childhood trauma and how epigenetic factors may contribute to offspring resilience.
The present study
The present study used a prospective, longitudinal design leveraging data from three related projects that comprise a sample of Black American mother-child dyads followed from pregnancy through three years postpartum: 1) The Pregnancy Study (Corwin et al., Reference Corwin, Hogue, Pearce, Hill, Read, Mulle and Dunlop2017), which examines the impact of social and environmental exposures on maternal and infant birth outcomes, 2) the Maternal Stress and Infant Microbiome Study (Brennan et al., Reference Brennan, Dunlop, Smith, Kramer, Mulle and Corwin2019), which focuses on maternal stress and the infant gut-brain axis in the perinatal period; and 3) our local cohort of the Environmental Influences on Child Health Outcomes Study (ECHO; Gillman & Blaisdell, Reference Gillman and Blaisdell2018), which examines how biological, behavioral, and social factors relate to developmental outcomes in early childhood. We hypothesized that:
Hypothesis 1: Maternal childhood adversity – examined as childhood trauma and ACEs, separately – would be positively associated with offspring symptomatology (i.e., internalizing, externalizing, and posttraumatic stress symptoms).
Hypothesis 1.1 (exploratory): Subtypes of maternal childhood maltreatment (i.e., physical, sexual, and emotional abuse) would demonstrate differential impacts on offspring symptomatology.
Hypothesis 1.2 (exploratory): The association of maternal adversity and offspring symptomatology would be moderated by offspring sex.
Hypothesis 2: Offspring epigenetic aging would be negatively associated with offspring symptomatology. In other words, epigenetic age acceleration in infancy would predict lower symptomatology in early childhood.
Hypothesis 3: Significant associations between maternal adversity and offspring symptomatology would be moderated by offspring epigenetic aging, such that the impact of maternal childhood adversity on offspring symptomatology would be greater in offspring with decelerated (i.e., lower) epigenetic aging and attenuated in offspring with accelerated epigenetic aging.
Methods
Participants
Pregnant Black American women were initially recruited from prenatal clinics at public and private hospitals within a large metropolitan city in the southeastern United States. Mothers first enrolled in the Pregnancy Study, where data was collected at two prenatal visits (typically during 8–14- and 24–30-weeks’ gestation). After delivery, participants were invited to enroll in the Maternal Stress and Infant Microbiome Study, where an infant blood sample was collected shortly after birth or at roughly 41-weeks gestational age. When the child reached two years of age, participants were invited to enroll in the ECHO Study, which conducted annual follow-up visits from ages two to five years. Inclusion criteria for the three studies included: 1) Black/African American race (via self-report); 2) Maternal age of 18–40 years; 3) Singleton pregnancy (verified by clinical record); 4) Maternal comprehension of written and spoken English; and 5) Absence of infant congenital disorders. Additional inclusion criteria for the current study included: 6) Completion of at least one Pregnancy Study visit, 7) Completion of an ECHO Study visit at 2-, 3- or 4-years, and 8) Availability of infant methylation data. This resulted in a final sample of 80 mother-child dyads. Sample characteristics are shown in Table 1.
Table 1. Sample characteristics and descriptive statistics (n = 80)

Procedure
Study procedures were approved by Emory University’s Institutional Review Board and informed consent was obtained for each participant prior to enrollment in the Pregnancy Study, the Infant Microbiome Study, and the ECHO Study. Data collection was conducted by trained laboratory staff in participants’ homes or a clinical or laboratory setting. At the pregnancy visit, mothers self-reported on experiences of childhood adversity. At or shortly after birth, blood samples were collected from offspring, which were later assayed on a DNA methylation microarray. At the toddlerhood visits, mothers reported on offspring socioemotional and behavioral symptomatology. Covariates relevant to intergenerational adversity and child development (e.g., maternal socioeconomic status, child age, and child sex) were collected at all relevant time points.
Measures
Maternal exposure to adversity
Childhood Trauma. Maternal childhood trauma was measured using the short form of the Childhood Trauma Questionnaire (CTQ; Bernstein et al., Reference Bernstein, Stein, Newcomb, Walker, Pogge, Ahluvalia, Stokes, Handelsman, Medrano, Desmond and Zule2003). The CTQ has 28 items that are used to compute a total score and maltreatment subscale scores including emotional abuse, physical abuse, and sexual abuse. Responses are rated on a 5-point Likert scale ranging from “1 – Never True” to “5 – Very Often True.” Higher scores are associated with more severe neglect and abuse. The CTQ has been well-validated in non-clinical and Black American samples (Liebschutz et al., Reference Liebschutz, Buchanan-Howland, Chen, Frank, Richardson, Heeren and Rose-Jacobs2018). Internal consistency for the CTQ total score in the current sample was high (Cronbach’s α = 0.89), as were those for the CTQ subscale scores (Emotional Abuse: Cronbach’s α = 0.88; Physical Abuse: Cronbach’s α = 0.78; Sexual Abuse: Cronbach’s α = 0.91).
Adverse Childhood Experiences. Maternal experiences of childhood adversity were measured using a shortened form of the Adverse Childhood Experiences questionnaire (ACEs; Felitti et al., Reference Felitti, Anda, Nordenberg, Williamson, Spitz, Edwards, Koss and Marks1998), which eliminates items that overlap with the CTQ. The shortened form consists of ten items assessing adversities related to family dysfunction (e.g., mental illness, substance abuse, or suicidality within the household), parental loss (e.g., through divorce, imprisonment, death, or abandonment), and other childhood adversities (e.g., experiences of homelessness or foster care). Responses are rated in a yes/no format and items are coded as “0 –Absent” or “1 – Present.” The total score is calculated by summing the items, with higher scores indicating more adverse experiences. Internal consistency in the current sample was adequate (Cronbach’s α = 0.71).
Offspring Symptomatology. Internalizing, externalizing, and posttraumatic stress symptoms were measured using the Child Behavior Checklist for Ages 1.5–5 (CBCL/1.5–5), a standardized form in which mothers report their children’s behavioral and emotional symptoms (Achenbach & Ruffle, Reference Achenbach and Ruffle2000). The CBCL/1.5–5 contains 100 items in which the mother indicates the option that best describes her child now or within the past 2 months with one of the following: 0 = not true (as far as you know); 1 = somewhat or sometimes true; 2 = very true or often true. The internalizing symptoms score reflects the sum of 36 of these items, with possible scores ranging from 0 to 72, and the externalizing symptoms score reflects the sum of 24 items, with possible scores ranging from 0 to 48. The posttraumatic stress symptoms scale is based on a sum of 15 items (Dehon & Scheeringa, Reference Dehon and Scheeringa2006), with scores ranging from 0 to 30. The CBCL is a well-established measure of child emotional and behavioral concerns and demonstrates strong test-retest reliability in ethnically- and socioeconomically-diverse samples (e.g., Ivanova et al., Reference Ivanova, Achenbach, Rescorla, Harder, Ang, Bilenberg, Bjarnadottir, Capron, De Pauw, Dias, Dobrean, Doepfner, Duyme, Eapen, Erol, Esmaeili, Ezpeleta, Frigerio, Gonçalves and Verhulst2010).
Infant DNA Methylation and Epigenetic Age Acceleration (EAA). Infant blood samples were collected in one of two ways. For 53 offspring, blood spots were collected at birth according to standard procedures, described in detail by Schroeder et al. (Reference Schroeder, Smith, Brennan, Conneely, Kilaru, Knight, Newport, Cubells and Stowe2012). For 27 offspring, blood clots were collected from infants via heel stick at roughly 41-weeks-equivalent gestational age. For infants that were born full-term, blood clots were collected one week after birth; for infants that were born preterm, blood clots were collected one week after the expected due date. DNA was extracted, processed, and arrayed using the MethylationEPIC BeadChip (Illumina) at the Emory Integrated Genomics Core. Array chips were balanced according to offspring sex, gestational age at birth, and recruitment site (i.e, public vs. private hospital); preliminary analyses demonstrated no chip effects on epigenetic aging (t = −1.00, p = 0.32). Initial quality control was performed using R package minfi (Aryee et al., Reference Aryee, Jaffe, Corrada-Bravo, Ladd-Acosta, Feinberg, Hansen and Irizarry2014), which removed probes and samples with low signal and missing data. Cross-reactive probes identified by McCartney and colleagues were removed (McCartney et al., Reference McCartney, Walker, Morris, McIntosh, Porteous and Evans2016). Following quality control, the Noob normalization method implemented in minfi was used for dye bias equalization.
Epigenetic gestational age was calculated based on a weighted average of 148 CpG sites, according to procedures developed by Knight et al. (Reference Knight, Craig, Theda, Baekvad-Hansen, Bybjerg-Grauholm, Hansen and Smith2016). The Knight epigenetic clock was selected for several reasons. First, it was developed and normed on a sample that focused on the developmental age of the current sample and is shown to accurately predict gestational age at birth for neonates born between 24- and 44-weeks’ gestation, a range that is broader than and encompasses that of the current sample (34- to 41-weeks’ gestation). Moreover, the training and test samples for the Knight epigenetic clock represented a wide range of ancestries, and the measure did not demonstrate differences in predictive accuracy for infants of African ancestry. In line with expectations, we found that epigenetic gestational age using the Knight measure was positively correlated with clinically estimated gestational age (Pearson correlation r = 0.45, p < 0.001). The difference between epigenetic gestational age and clinically estimated gestational age (i.e., epigenetic age acceleration) was defined by taking the residual of a linear regression of epigenetic gestational age on clinically estimated gestational age.
To account for the potential effects of cell type heterogeneity in infant blood, cell type proportions were estimated for CD4+ T cells, natural killer cells, B cells, neutrophils, and monocytes.
Statistical analysis
Analyses were performed in R version 4.1.3. Complete data were available for all measures apart from the CTQ, which had missing data for five participants. This data was imputed using predictive mean matching via the MICE package (Van Buuren & Groothuis-Oudshoorn, Reference Van Buuren and Groothuis-Oudshoorn2011). All analyses adjusted for covariates that have been previously associated with intergenerational trauma and/or socioemotional development: offspring age at the time of CBCL collection, pre- or post-COVID-19 onset at the time of CBCL collection, offspring sex, and maternal socioeconomic status (SES; operationalized as a factor score comprised of maternal education, insurance status, marital status, and income). Analyses involving epigenetic age acceleration additionally adjusted for cell type proportions and maternal prenatal tobacco use. Biosample type (i.e., blood spot vs. blood clot) was not significantly associated with offspring epigenetic aging or symptomatology after adjusting for cell type proportions, so biosample type was not adjusted for in subsequent analyses. Multiple regressions were used to test our hypotheses that maternal childhood adversity would be positively associated with offspring symptoms (Hypothesis 1 and exploratory Hypothesis 1.1), that offspring epigenetic aging would be negatively associated with offspring symptoms (Hypothesis 2), and that offspring epigenetic aging (Hypothesis 3) and offspring sex (exploratory Hypothesis 1.2) would moderate the significant associations between maternal childhood adversity and offspring symptoms. Power analyses indicated that the analyses were adequately powered to detect medium and large effects but potentially underpowered to detect small effects (f 2 > 0.078, power = 0.8, p = 0.05). The Benjamini-Hochberg procedure was used to adjust for multiple comparisons (Thissen et al., Reference Thissen, Steinberg and Kuang2002).
Results
Descriptive statistics and bivariate correlations for the primary variables are displayed in Tables 1 and 2, respectively. Bivariate correlations indicated that maternal childhood trauma was moderately correlated with maternal ACEs (r = .43, p < 0.001), and childhood maltreatment subtypes were highly correlated with each other (rs = .67 to .83, ps < 0.001). Offspring internalizing, externalizing, and posttraumatic stress symptoms were also highly intercorrelated (rs = .79 to .83, ps < 0.001). Maternal childhood adversity measures were not associated with moderator variables (i.e., offspring sex and epigenetic aging), other than a small correlation between maternal childhood emotional abuse and offspring sex (r = −0.22, p = 0.047) such that male offspring had mothers with higher scores.
Table 2. Bivariate correlations

Note. CTQ = Childhood Trauma Questionnaire; EA = Emotional Abuse; PA = Physical Abuse; SA = Sexual Abuse; EAA = Epigenetic Age Acceleration.
With respect to main effects, maternal childhood trauma was significantly correlated with offspring internalizing, externalizing, and posttraumatic stress symptoms (rs = .22 to .39, ps = 0.001 to 0.049), as were maternal sexual and emotional abuse (rs = .27 to .39, ps = 0.001 to 0.01). Maternal physical abuse was only significantly correlated with offspring internalizing symptoms (r = .27, p = 0.01). Offspring sex was significantly correlated with offspring externalizing symptoms, such that males demonstrated higher symptoms (r = −.22, p = 0.048); offspring sex was not correlated with internalizing or posttraumatic stress symptoms. Offspring epigenetic aging was significantly correlated with offspring externalizing (r = −.26, p = 0.02) and posttraumatic stress (r = −.24, p = 0.03) symptoms, and demonstrated a trend with internalizing symptoms (r = .21, p = 0.06).
Finally, with respect to potential confounders, maternal SES was significantly and negatively correlated with maternal childhood trauma, maternal childhood emotional abuse, and offspring internalizing symptoms. Pre/post-COVID, a binary variable that represented whether the CBCL was administered prior to or after March 2020, was significantly associated with offspring internalizing (r = −.35, p = 0.002), externalizing (r = −.23, p = 0.04), and posttraumatic stress (r = −.27, p = 0.01) symptoms such that offspring assessed after the onset of the pandemic (n = 59) were reported as demonstrating higher symptoms. As such, these variables were included as covariates in all appropriate regression analyses.
Intergenerational association of maternal adversity and offspring symptomatology
First, we examined whether maternal experiences of childhood adversity were intergenerationally associated with offspring symptomatology, after adjusting for maternal SES, pre/post-COVID, offspring age, and offspring sex. Results indicated that maternal ACEs were not significantly associated with any offspring outcomes and maternal childhood trauma was not significantly associated with offspring externalizing or posttraumatic stress symptoms (Table 3). However, maternal childhood trauma was significantly positively associated with offspring internalizing symptoms (β = 0.24, p = 0.03). To further examine the impact of maternal childhood trauma, we then examined the individual contributions of maltreatment subtypes (i.e., emotional, physical, and sexual abuse). Results indicated that maternal experiences of childhood sexual abuse were associated with offspring internalizing (β = 0.29, p = 0.003) and externalizing (β = 0.22, p = 0.02) symptoms, with a trend for posttraumatic stress symptoms (β = 0.18, p = 0.09). Maternal childhood experiences of physical and emotional abuse were not significantly associated with offspring outcomes, although maternal emotional abuse demonstrated a trend (ps < 0.1). All regression results are presented in Table 3.
Table 3. Main effect of maternal childhood adversity on offspring symptomatology
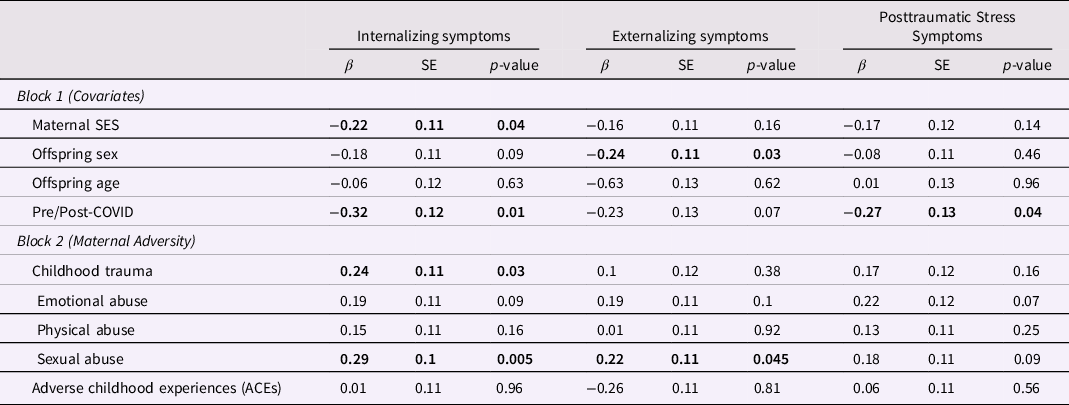
Note. Separate regressions were conducted for each form of maternal adversity. Results are presented together for consolidation purposes. Bold indicates statistical significance.
Association of offspring sex and early childhood symptomatology
Results indicated a significant sex difference for externalizing symptoms (β = −0.24, p = 0.03), such that males exhibited greater externalizing symptoms than females. No sex differences were demonstrated for internalizing symptoms (Table 3).
Does offspring sex moderate associations between maternal adversity and offspring symptomatology?
We then examined whether the significant intergenerational associations between maternal adversity and offspring symptoms differed across male and female offspring. Results demonstrated a significant interaction between maternal childhood sexual abuse and offspring sex to predict offspring externalizing symptoms (β = −0.72, p = 0.03; Table 4). The interaction survived correction for multiple testing using the Benjamini-Hochberg procedure (i = 3, m = 6, Q = 0.05, p < 0.035). Upon further probing, results indicated that the significant association between maternal childhood sexual abuse and offspring externalizing symptoms was driven by male offspring, and there was no association for female offspring (Figure 1). No other moderating sex effects were found (Table 4).
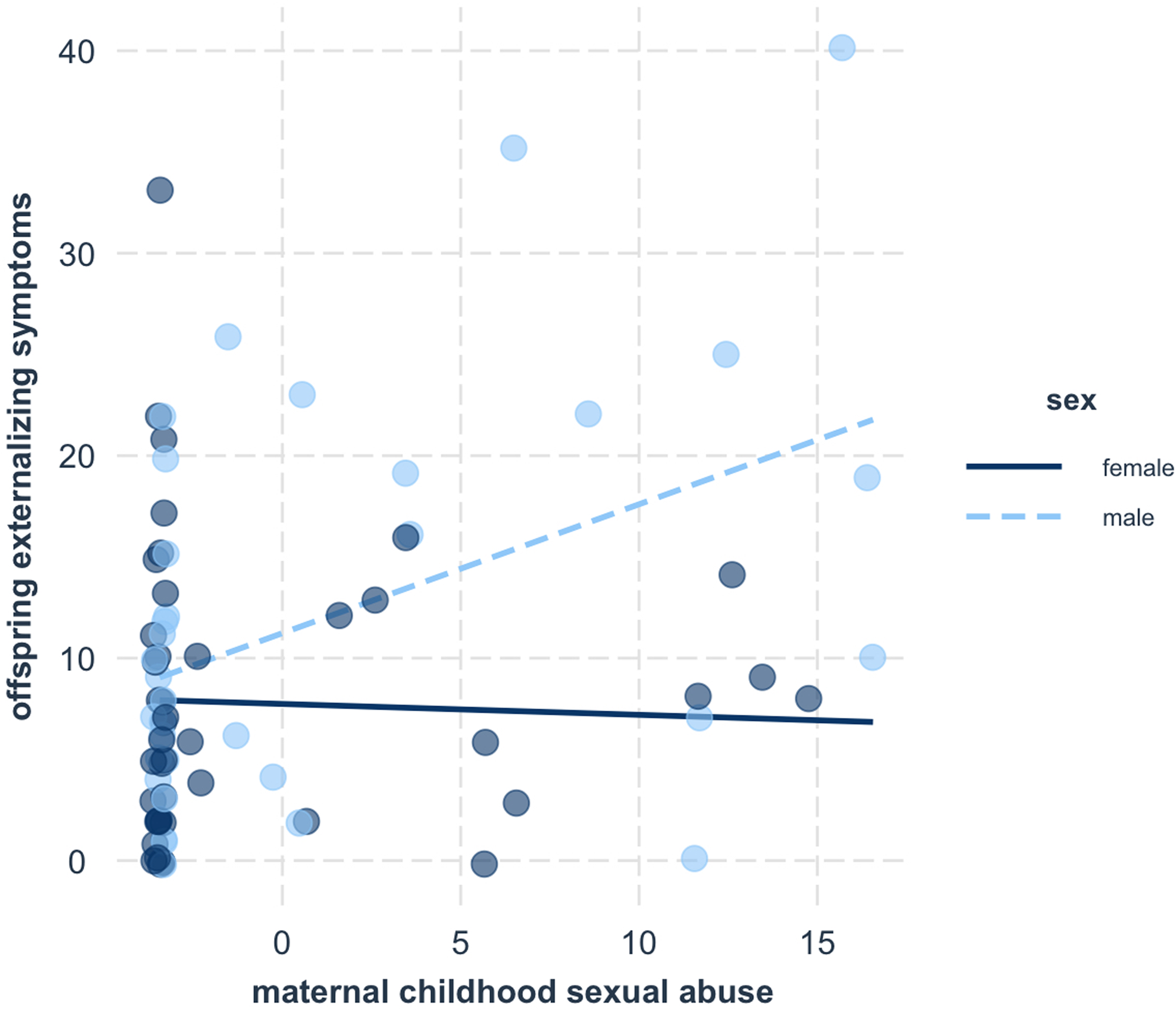
Figure 1. Male offspring demonstrated elevated externalizing symptoms in the context of maternal childhood sexual abuse, while female offspring did not.
Table 4. Interaction of maternal childhood trauma (top) and maternal childhood sexual abuse (bottom) with offspring sex to predict offspring symptomatology
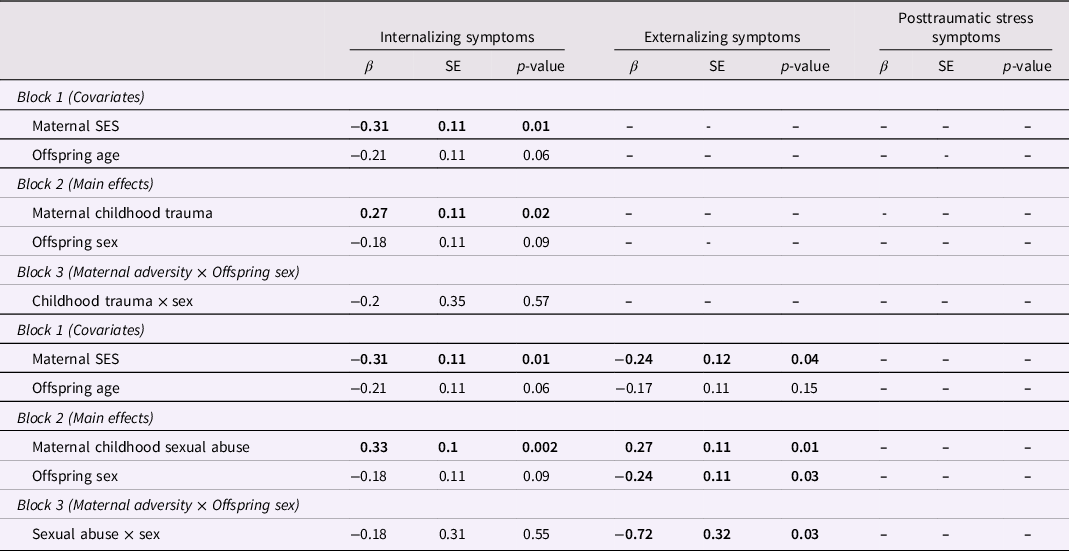
Note. Bold indicates statistical significance.
Association of infant epigenetic aging and early childhood symptomatology
Next, we examined the main effects of offspring epigenetic aging on symptomatology. Although offspring epigenetic aging was initially correlated with offspring symptoms, regression results indicated that epigenetic aging was no longer significantly associated with internalizing, externalizing, or posttraumatic stress symptoms after adjusting for cell type proportions, maternal prenatal tobacco use, and other relevant covariates (Table 5).
Table 5. Main effect of offspring epigenetic aging on offspring symptomatology
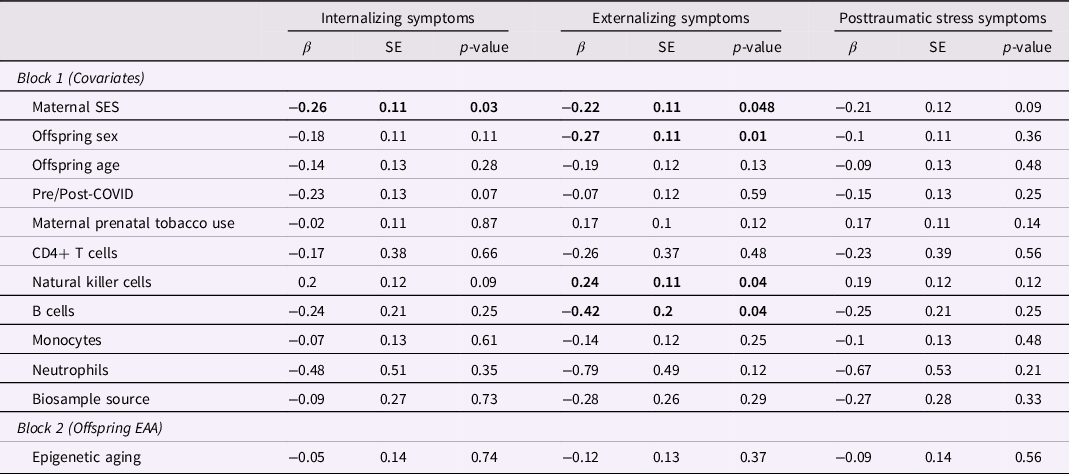
Note. Bold indicates statistical significance.
Does infant epigenetic aging moderate associations between maternal adversity and offspring symptomatology?
Finally, we examined whether infant epigenetic aging moderated the significant associations between maternal adversity and offspring symptomatology (Table 6). Results indicated that epigenetic age acceleration attenuated the impact of maternal childhood sexual abuse on both offspring internalizing (β = −0.31, p = 0.004; Figure 2a) and externalizing (β = −0.28, p = 0.01; Figure 2b) symptoms. Both interactions survived correction for multiple testing using the Benjamini-Hochberg procedure (i = 1, m = 6, Q = 0.05, p < 0.008 for internalizing; i = 2, m = 6, Q = 0.05, p < 0.02 for externalizing). Post-hoc regions of significance tests indicated that at high levels of maternal childhood sexual abuse (i.e., scores greater than 5.85 for internalizing symptoms and 5.93 for externalizing symptoms), offspring symptoms were significantly attenuated in offspring with epigenetic age acceleration. No other moderating effects of infant epigenetic aging were found.
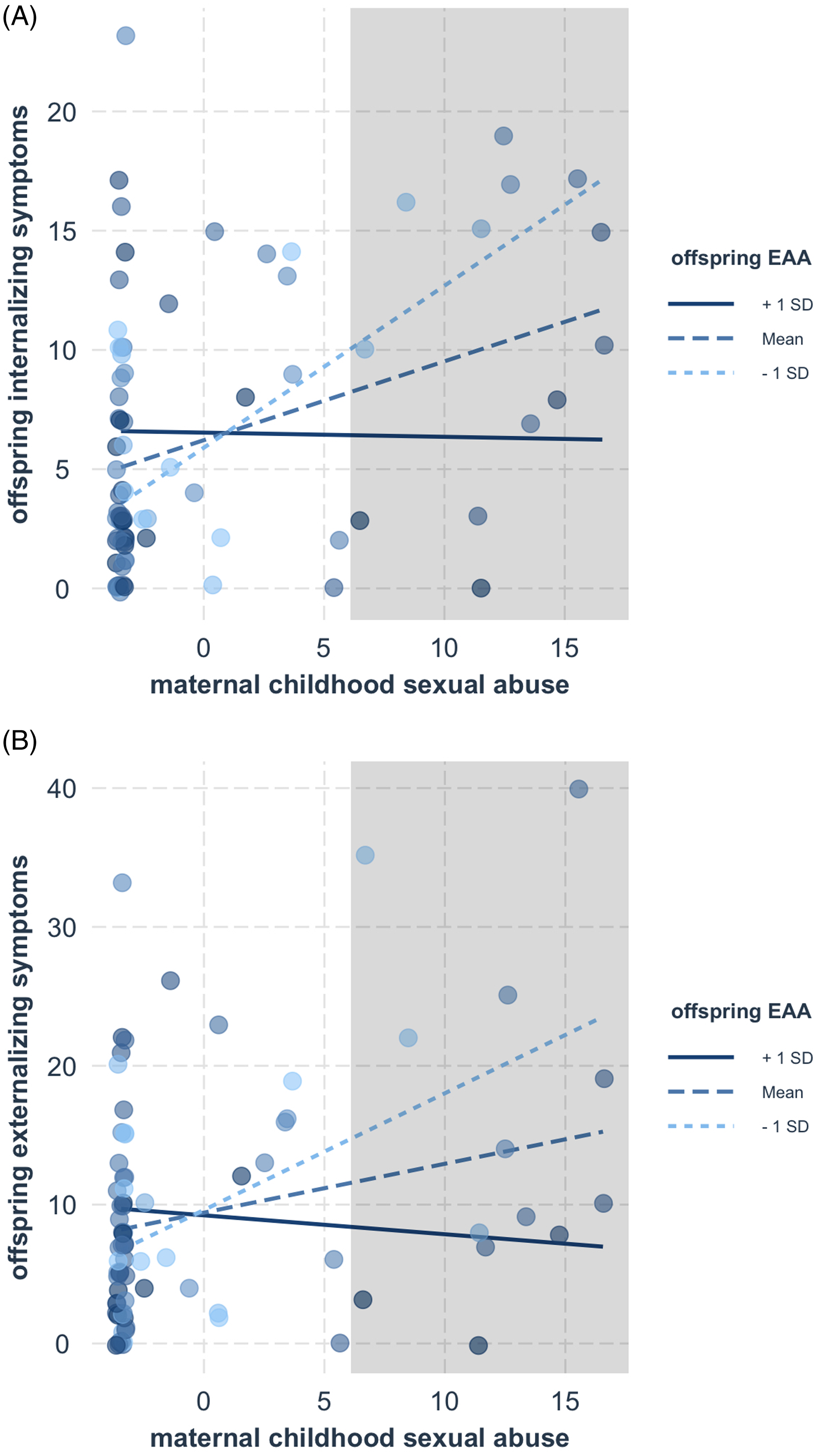
Figure 2. The associations between maternal childhood sexual abuse and offspring internalizing (A) and externalizing (B) symptoms were attenuated in offspring with accelerated epigenetic aging. The shaded regions indicate where the associations between maternal abuse and offspring symptoms significantly differed according to offspring epigenetic aging.
Table 6. Interaction of maternal childhood trauma (top) and maternal childhood sexual abuse (bottom) with offspring epigenetic aging to predict offspring symptomatology

Discussion
In the present study, we explored the intergenerational associations of different types of maternal childhood adversity with offspring behavioral outcomes and whether infant epigenetic aging might buffer these associations. We found that maternal experiences of childhood trauma were significantly associated with symptoms of psychopathology in her offspring, with particularly pronounced effects of sexual abuse on offspring symptoms. However, the intergenerational associations between maternal sexual abuse and offspring outcomes were attenuated in offspring with accelerated epigenetic aging at birth. These findings extend prior research demonstrating the differential impacts of childhood maltreatment subtypes not only on an individual’s risk for developing psychopathology but also extending into future generations. Further, it provides novel preliminary evidence that epigenetic mechanisms may play an important role in moderating the association between intergenerational stress and early developmental outcomes.
Across both internalizing and externalizing symptoms, the associations between maternal sexual abuse and offspring symptomatology were significant for offspring with lower epigenetic aging at birth but were attenuated in offspring with accelerated epigenetic aging. While this was the first study to explore the moderating effect of epigenetic aging in the context of intergenerational trauma, these findings are consistent with theoretical and empirical evidence from intra-generational studies that epigenetic differences associated with psychopathology-related mechanisms (e.g., neuroendocrine reactivity) moderate the relationship between early life stress and psychopathology (Al Jowf et al., Reference Al Jowf, Snijders, Rutten, de Nijs and Eijssen2021; Conradt et al., Reference Conradt, Lester, Appleton, Armstrong and Marsit2013; Conradt, Reference Conradt2017; Smearman et al., Reference Smearman, Almli, Conneely, Brody, Sales, Bradley and Smith2016; Smeeth et al., Reference Smeeth, Beck, Karam and Pluess2021). Prior studies suggest that epigenetic aging may reflect differences in neuroendocrine and inflammatory processes that in turn confer greater susceptibility to environmental stressors (Lu et al., Reference Lu, Quach, Wilson, Reiner, Aviv, Raj and Horvath2019; Wolf et al., Reference Wolf, Zhao, Hawn, Morrison, Zhou, Fein-Schaffer, Huber, Miller and Logue2021). For example, epigenetic aging has been associated with methylation differences in the stress-regulatory gene FKBP5 (Beach et al., Reference Beach, Ong, Lei, Carter, Simons, Gibbons and Philibert2022) as well as differential expression of inflammation genes (Wolf et al., Reference Wolf, Zhao, Hawn, Morrison, Zhou, Fein-Schaffer, Huber, Miller and Logue2021). Given that neuroendocrine and inflammatory pathways have been shown to mediate the intra-generational relationship between childhood maltreatment (including childhood sexual abuse; Nunes et al., Reference Nunes, Watanabe, Morimoto, Moriya and Reiche2010) and the development of depression and other psychopathology (McCrory et al., Reference McCrory, De Brito and Viding2012), it follows that differential expression of genes implicated in these pathways might influence an individual’s susceptibility to maltreatment.
Importantly, similar mechanisms have been posited to mediate the intergenerational link between maternal maltreatment and offspring psychopathology. Evidence stemming from the fetal programing hypothesis has demonstrated that maternal experiences of adversity can cause alterations to her physiological stress response that then influence the development of her offspring’s stress response during fetal development (Babenko et al., Reference Babenko, Kovalchuk and Metz2015; Buss et al., Reference Buss, Entringer, Moog, Toepfer, Fair, Simhan, Heim and Wadhwa2017). In turn, offspring of women who experienced maltreatment tend to demonstrate differences in fear and neuroendocrine systems that influence stress sensitivity and risk for psychopathology (Brand et al., Reference Brand, Brennan, Newport, Smith, Weiss and Stowe2010; Hendrix et al., Reference Hendrix, Dilks, McKenna, Dunlop, Corwin and Brennan2020). Given these effects, it is possible that methylation differences that are reflected by infant epigenetic age acceleration may also be acting on neuroendocrine and inflammation mechanisms to buffer the impact of maternal maltreatment on the development of offspring psychopathology. However, these mechanisms are still poorly understood and future research is needed to better understand the physiological processes that relate to infant epigenetic aging and how those processes play a role in the intergenerational transmission of maltreatment.
Our findings corroborate prior evidence that maternal childhood trauma is associated with greater offspring symptomatology (Bush et al., Reference Bush, Noroña-Zhou, Coccia, Rudd, Ahmad, Loftus and LeWinn2023) and additionally suggest that different maltreatment types confer differential risk for offspring psychopathology. The present study is the first to our knowledge that examines subtype differences in the association between maternal childhood maltreatment and offspring psychopathology, and it adds to the growing evidence that examining subtypes of childhood maltreatment is important for understanding long-term outcomes. Specifically, we found maternal experiences of childhood sexual abuse were associated with offspring internalizing and externalizing symptoms. Experiences of emotional abuse demonstrated a similar (albeit, nonsignificant) trend, while experiences of physical abuse were not associated with any offspring outcomes. These results are consistent with evidence from intra-generational studies, which have found that sexual abuse may account for unique variation in adverse psychological and behavioral outcomes. For example, a systematic review of neuroimaging studies found that while some structural and connectivity differences are shared across all types of childhood maltreatment, sexual maltreatment confers unique risk for structural changes to reward circuitry (Cassier et al., Reference Cassiers, Sabbe, Schmaal, Veltman, Penninx and Van Den Eede2018). These findings are interesting given the well-established role of reward circuits in the development of depression and other trauma-related psychopathology (Nestler & Carlezon, Reference Nestler and Carlezon2006). Similarly, Cassier et al. (Reference Cassiers, Sabbe, Schmaal, Veltman, Penninx and Van Den Eede2018) found that emotional abuse was uniquely associated with structural changes to fronto-limbic socioemotional networks, which have also been associated with the development of depression and other psychopathology (Du et al., Reference Du, Zeng, Liu, Tang, Meng, Li and Fu2017). As such, it is possible that similar mechanisms could underlie the positive trend between maternal emotional abuse and offspring symptoms, particularly considering recent evidence that maternal childhood emotional (but not physical) neglect is associated with fronto-limbic connectivity in newborn offspring (Hendrix et al., Reference Hendrix, Dilks, McKenna, Dunlop, Corwin and Brennan2020). While it is important not to overinterpret these novel findings, together this evidence suggests that future research may benefit from examining the effects of maternal maltreatment subtypes on offspring neural circuitry.
It is also possible that experiences of maternal sexual (and potentially emotional) abuse confer offspring risk for psychopathology through the same mechanisms as other forms of maternal stress, but demonstrate a stronger association because they elicit a more chronic or severe stress response. For example, evidence from a recent review indicated that childhood sexual abuse confers the most unique risk (i.e., over and above the influence of other childhood adversities) for disorders characterized by HPA-axis dysregulation (Noll, 2022). As such, it is possible that mothers who experience sexual abuse during childhood develop more significant alterations to their HPA-axis that then disproportionately influence the fetal programing of their offspring’s stress response. Additionally, studies have shown that emotional abuse is associated with more chronic activation of the stress response compared to physical abuse (Kuhlman et al., Reference Kuhlman, Geiss, Vargas and Lopez-Duran2015). In turn, it is possible that mothers who experience emotional abuse during childhood demonstrate more sustained changes to their HPA-axis that are then transferred to their offspring during pregnancy (Babenko et al., Reference Babenko, Kovalchuk and Metz2015; Buss et al., Reference Buss, Entringer, Moog, Toepfer, Fair, Simhan, Heim and Wadhwa2017). While these interpretations are speculative, our findings highlight the importance of better understanding how different forms of childhood maltreatment may also differentially influence the physiological (e.g., neuroendocrine) systems that contribute to intergenerational risk.
Finally, we found a moderating effect of sex such that male – but not female – offspring demonstrated elevated externalizing symptoms following maternal experiences of sexual abuse. It is possible that these sex differences are related to the higher prevalence of externalizing disorders among males (Leadbeater et al., Reference Leadbeater, Kuperminc, Blatt and Hertzog1999), as we found a significant association between offspring sex and offspring externalizing symptoms such that males demonstrated greater symptoms. However, given that no other sex moderation effects were identified and a recent study in a larger sample (n = 1,948) found no sex differences in the intergenerational relationship between maternal childhood trauma and offspring behavioral outcomes (Bush et al., Reference Bush, Noroña-Zhou, Coccia, Rudd, Ahmad, Loftus and LeWinn2023), this finding may have been a statistical artifact and requires replication.
Strengths and limitations
A notable strength of the current study is our focus on Black American women and children, which is important for several reasons. First, given that Black Americans are disproportionately exposed to and impacted by childhood trauma and adversity (Mersky & Janczewski, Reference Mersky and Janczewski2018; Roberts et al., Reference Roberts, Gilman, Breslau, Breslau and Koenen2011), concentrating on Black women’s and children’s experiences increases the relative impact and practical utility of our findings. Second, given that Black Americans experience unique factors that influence risk and resilience to trauma, it is important to specifically examine intergenerational trauma among Black Americans rather than assuming that prior findings from predominantly White samples generalize to all individuals. Indeed, a recent multi-cohort study examining the impact of maternal childhood adversity on offspring symptoms of psychopathology found that intergenerational associations were similar across individuals of different racial, ethnic, socioeconomic, and regional backgrounds (Bush et al., Reference Bush, Noroña-Zhou, Coccia, Rudd, Ahmad, Loftus and LeWinn2023), but the effect sizes were smaller for the cohort that was primarily comprised of Black women and children from the southeastern United States. The authors posited that these differences may be due to the presence of other adversities (e.g., racial stressors and neighborhood crime) that outweigh the impact of the measured stressors (Bush et al., Reference Bush, Noroña-Zhou, Coccia, Rudd, Ahmad, Loftus and LeWinn2023). In the context of our study, this may explain why ACEs were not significantly associated with offspring outcomes despite evidence from predominantly White samples that ACEs demonstrate an intergenerational impact (Cooke et al., Reference Cooke, Racine, Pador and Madigan2021; Zhang et al., Reference Zhang, Mersky, Gruber and Kim2022). Alternatively, these lower intergenerational effects may be due to unmeasured protective factors within the Black community such as family and community support (Bush et al., Reference Bush, Noroña-Zhou, Coccia, Rudd, Ahmad, Loftus and LeWinn2023). While our study provides evidence that early epigenetic processes may serve as a protective factor, many other biological and psychosocial factors also contribute to resilience. Together, this evidence emphasizes the importance of continuing to increase representation of Black Americans in the realms of developmental, biological, and psychological research to better understand individual differences in risk and resilience.
Our findings must also be considered in light of several limitations. Most importantly, although power analyses indicated a sufficient sample size to detect medium effects, our sample size was limited, and we were underpowered to detect small effects. Given that we would expect small effect sizes for tests of moderation, it is possible that we did not capture significant relationships of smaller magnitudes. Moreover, the significant interaction effects that we did identify should be considered preliminary and require replication. In addition, while a strength of our study was its prospective design which provides some directionality for the relationship between maternal childhood trauma, infant epigenetic aging, and offspring outcomes, we cannot establish causality from the present study. Our study was also limited to using peripheral tissue (i.e., blood) to assess DNA methylation. Although there is evidence that methylation in peripheral tissue is reflective of systemic changes to inflammation and may be comparable to methylation of brain tissue (Braun et al., Reference Braun, Han, Hing, Nagahama, Gaul, Heinzman and Shinozaki2019; Smeeth et al., Reference Smeeth, Beck, Karam and Pluess2021; Smith et al., Reference Smith, Kilaru, Kocak, Almli, Mercer, Ressler and Conneely2014), it may not accurately reflect DNA methylation within the central nervous system. Finally, we were not able to assess how the timing or severity of maternal childhood traumatic experiences may influence offspring psychopathology, and we relied solely on maternal report to assess childhood adversity and offspring symptomatology. Although the CTQ and CBCL are well-validated measures that have been shown to align with corroborating reports and behavioral observations, some studies have suggested that mothers’ mental health can bias maternal reports (e.g., Kohen et al., Reference Kohen, Brooks-Gunn, McCormick and Graber1997), albeit to a minimal degree (Olino et al., Reference Olino, Michelini, Mennies, Kotov and Klein2021). Given prior evidence that maternal psychopathology is one mechanism by which maternal childhood maltreatment confers risk for offspring outcomes, controlling for maternal mental health posed the risk of masking the effects we aimed to examine. As such, future research would benefit from including additional measures of offspring outcomes such as secondary reports, behavioral observations, or physiological measures (e.g., cortisol reactivity) to corroborate these findings. Moreover, it would be interesting for future longitudinal studies to examine whether and how infant epigenetic aging may moderate a more proximal relationship between maternal psychopathology and offspring symptomatology, particularly considering the bidirectional relationship between these factors (Bagner et al., Reference Bagner, Pettit, Lewinsohn, Seeley and Jaccard2013).
Conclusion
Experiences of childhood trauma confer intergenerational risk for psychopathology, and it is important to consider the type of maltreatment when assessing level of risk. Moreover, biological factors such as accelerated epigenetic aging may reduce a child’s susceptibility to intergenerational trauma in early life. Better understanding the physiological processes that contribute to “epigenetic resilience” against the prolonged outcomes associated with childhood trauma has the potential to deepen our understanding of developmental psychopathology and develop biologically informed intervention and prevention efforts.
Acknowledgments
First and foremost, we would like to thank the families who participated in this research for their trust and commitment to science. We also thank Julie Carroll for her management of this project and all members of the BUILD Lab for their assistance with data collection. This work was supported by the National Institute on Minority Health and Health Disparities [R01MD009746 to PAB and EJC; R01MD009064 to AKS and ALD], National Institute of Nursing Research [R01NR014800 to EJC and ALD], National Institute of Environmental Health Sciences [R24ES029490 to ALD], National Institute of Social Sciences [dissertation grant to BGM], and the American Psychological Foundation [Elizabeth Munsterberg Koppitz Child Psychology Graduate Fellowship to BGM]. Author BGM was supported by the NSF Graduate Research Fellowship Program (DGE-1444932).
Competing interests
The authors declare none.










