The gut is not only a digestive tract, but also the key borderline against enteropathogens, whose assaults usually result in host intestinal inflammation, injury and dysfunction(Reference Blikslager, Moeser and Gookin1). In particular, as a result of enterotoxigenic Escherichia coli K88 infection, post-weaning diarrhoea (PWD) is the main cause of piglet death that brings out a great economic loss in the pig industry around the world(Reference Fairbrother, Nadeau and Gyles2). E. coli K88 colonies in the small intestine releases specific enterotoxins and stimulates secretion of pro-inflammatory cytokines such as IL-1β, IL-6 and TNF-α (Reference Che, Xu and Wu3, Reference Liu, Piao and Thacker4), and subsequently impairs intestinal barrier functions by depressing the expression of tight junction proteins, then leads to diarrhoea and even death(Reference Yang, Jiang and Zheng5, Reference Xu, Wang and Sun6). Over the past few decades, various antibiotics and heavy metal compounds such as zinc oxide and copper sulfate had been widely added in piglet diets in order to control PWD and promote growth performance of piglets. However, growing public concern has been drawn over the emergence and prevalence of bacterial resistance and heavy metal pollution(Reference Erik and Knudsen7). Thus, great efforts have been made to seek safe alternatives to control PWD.
Herbal extracts exhibit a promising role in protecting intestine against enterotoxigenic E. coli infection and keeping intestinal health in piglets. Scutellaria baicalensis is a widely used herb in Chinese traditional medicine prescriptions for its substantial anti-inflammatory and antibacterial effects against E. coli in the treatment of diarrhoea, dysentery, inflammation and respiratory infections in rats and humans(Reference Qing, Xiao-Ya and Cathie8, Reference Shan, Cai and Brooks9). Flavonoids are major functional components of S. baicalensis extracts (SBE), among which baicalin (C21H18O11; 5,6,7-trihydroxyflavone 7-O-β-d-glucuronid or baicalein 7-O-β-d-glucuronic acid or 7-d-glucuronic acid-5,6- dihydroxyflavone) had been demonstrated to compose major functional roles of SBE(Reference Qing, Xiao-Ya and Cathie8, Reference Sowndhararajan, Deepa and Kim10). Flavonoids derived from S. baicalensis can alleviate the inflammatory responses in animal models and clinical trials(Reference Wang, Zhang and Bai11–Reference Dinda, Dinda and Dassharma13), and mitigate the over-release of pro-inflammatory cytokines(Reference Pérez-Cano and Castell14). Recently, it had been demonstrated that flavonoids could be of great application in conditions of acute or chronic intestinal inflammation in vivo and in vitro models(Reference Vezza, Rodríguez-Nogales and Algieri15), such as dextran sulfate sodium-induced colitis(Reference Yang, Chen and Yang16) and intestinal allergy in mice(Reference Sun, Lee and Choi17) as well as TNF-α-induced injury in IEC-6 cell line(Reference Wang, Zhang and Chen18).
However, there are few studies focused on the influence of SBE supplementation on piglet intestinal health. Therefore, we investigated the effect of dietary supplementation of SBE on intestinal morphology and barrier function together with small intestinal immune and inflammatory responses in E. coli K88-challenged piglets.
Materials and methods
Ethics approval
All experimental protocols for the present study were approved by the Animal Care and Use Committee of China Agricultural University and carried out in accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals.
Preparation of Scutellaria baicalensis extracts premix
In the present study, SBE product (purity ≥ 85 % baicalin), the brown-yellow powder with size of 120–180 µm, was purchased from Xi′an Tianrui Biotech Co., Ltd. As per the company product specification sheet, the quality control of the product is performed according to Chinese Veterinary Pharmacopoeia (2015 Edition) by monitoring baicalin content by HPLC. Briefly, about 10 mg SBE was dissolved in 25 ml methanol, filtered through a 0·22 μm membrane filter (Millipore), and then 10 μl of the standard (baicalin, C21H18O11; Y0001273; Sigma-Aldrich) or samples were injected into the HPLC system. The sample was analysed on an Agilent SB-C18 analytical column (4·6 mm × 250 mm, 5 μm). The oven temperature was maintained at 25°C. The mobile phase comprised methanol and 0·2 % phosphoric acid (47:53, v/v). The flow rate was 0·8 ml/min. Chromatograms were acquired at 280 nm by UV detection. In the HPLC chromatogram, baicalin (retention time = 18·646 min) was found in the extract sample (online Supplementary Fig. S1(b)). The SBE premix (purity ≥ 12 % baicalin) was prepared with maize starch, and then mixed in the formulation of the experimental diet.
Experimental design
A total of 72 Duroc × Landrace × Large White crossbred castrated male piglets (7·44 ± 0·80 kg, weaned at 24 ± 2 d) were randomly assigned to one of two dietary treatments to receive either the maize–soya bean meal basal diet (as control; CTRL) or the basal diet supplemented with 1000 mg SBE/kg diet with six replicates (pens) per treatment and six piglets per pen. The maize–soya bean meal basal diet was formulated according to the National Research Council (2012) for 7–15 kg piglets without any antibiotic additives (Table 1). Piglets were housed in a nursery room equipped with air condition and ventilation, and all piglets had free access to feed and water.
Table 1. Ingredients and nutrition levels of the basal diet (as-fed basis)
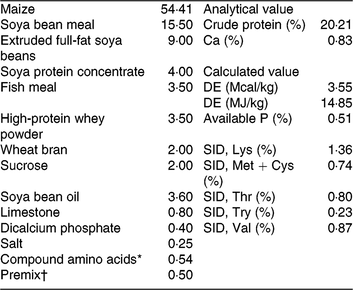
DE, digestible energy; SID, standardised ileal digestibility.
* Compound amino acids – L-lysine HCl, L-threonine, DL-methione, L-tryptophan and valine.
† Premix (antibiotic-free) provided per kg of the complete diet for 7–15 kg piglets. 2000 IU vitamin A, 2500 IU vitamin D3, 30 IU vitamin E, 12 μg of vitamin B12, 3 mg vitamin K3, 15 mg D-pantothenic acid, 40 mg nicotinic acid, 400 mg choline, 30 mg Mn as MnSO4, 80 mg Zn as ZnSO4, 90 mg Fe as FeSO4, 10 mg Cu as CuSO4, 0·35 mg iodine as ethylenediamine dihydroiodide and 0·3 mg Se as Na2SeO3.
During the feeding trial of 14 d, the diarrhoea incidence of piglets was recorded. At the end of the feed trial, piglets were weighed to calculate the average daily gain, and diet disappearance was checked to calculate the average daily feed intake and feed conversion ratio.
Piglets challenged with Escherichia coli K88 and sample collection
After feeding trial, twelve healthy piglets from each treatment were selected, and among them six piglets from each treatment received an oral ingestion of E. coli K88 (serotype O149, China Institute of Veterinary Drug Control) approximately 1 × 109 colony-forming units/ml in 10 ml PBS, and other six piglets received equivalent amount vehicle (PBS), respectively. The piglets that were challenged with E. coli K88 were housed in separate pens while unchallenged piglets were housed in other pens at different areas of the piggery. It is noteworthy that pens were equipped with exclusive cleaning tools and were cleaned twice per d individually with specially assigned workers, avoiding the cross-contamination during the cleaning and feeding between pens.
After 48 h of E.coli K88 challenge, blood from the piglets was sampled from the anterior vena cava into 10 ml heparinised vacuum tubes (Becton Dickinson Vacutainer System) after overnight starvation for 16 h. The plasma was harvested and immediately stored at −20°C for further analysis of inflammatory cytokines, diamine oxidase (DAO) activity and IgA, IgG as well as IgM.
Following blood collection, the same piglets were humanely killed for sampling intestine tissue. The small intestine was dissected free of its mesentery and immediately placed on ice, and the 3 and 10 cm segments were removed from the mid-jejunal and mid-ileal segments, respectively. The 3 cm intestinal segments were gently flushed with PBS solution and stored in 4 % neutral-buffered paraformaldehyde for analysis of intestinal morphology. The 10 cm intestinal samples were opened longitudinally and flushed gently to remove luminal chime. The mucosa sample was obtained via scraping with glass slides, then rapidly frozen in liquid nitrogen and stored at −80°C for further analysis.
Histological examination of the intestinal morphology
Histological examination of the intestinal morphology was conducted (described earlier)(Reference Xu, Chen and Wang19). Briefly, after a 24 h fixing, the intestinal segments were dehydrated in ethanol concentration gradient and cleared up with xylene, embedded in paraffin, cut in cross sections with a thickness of approximately 5 μm with a microtome and then stained with haematoxylin and eosin. Villous height and crypt depth of two intestinal segments were measured and villous height/crypt depth (VCR) was calculated.
Measurement of plasma inflammatory cytokines
The plasma levels of IL-1β, IL-6, IL-10 and TNF-α were measured by double antibody sandwich ELISA method using commercial swine ELISA kits (eBioscience) according to the manufacturer’s instructions and the absorbance measured at 450 nm. The minimal detection limit was 0·3 pg/ml for IL-1β, 0·92 pg/ml for IL-6, 1 pg/ml for IL-10 and 5 pg/ml for TNF-α, respectively.
The plasma levels of IgA, IgG and IgM were detected with turbidimetric inhibition immunoassay and DAO activity were measured with the ELISA method using corresponding commercial kits on the basis of product manuals (Nanjing Jiancheng Bioengineering Institute). The absorbance was measured at 340 nm for IgA and IgM, 405 nm for IgG and 460 nm for DAO and the minimal detection was 0·7 g/l for IgA, 0·3 g/l for IgM, 1 g/l for IgG and 1·28 ng/ml for DAO, respectively. The intra-assay was <5 % and inter-assay CV was <6 % for each assay.
Quantitative real-time PCR assay
Total RNA was extracted from intestinal mucosa using the Trizol reagent (TaKaRa Biotechnology) according to the manufacturer’s instructions. The quality and integrity of RNA samples and purity of the PCR products were assessed by electrophoresis on 1 % agarose gel and nucleic acid analyser (NanoDrop 1000; Thermo Fisher), respectively. The RNA was used to synthesise cDNA using reagent kit (PrimeScript™ RT reagent Kit with gDNA Eraser), the reaction system is 42°C for 15 min, followed by RT inactivation at 85°C for 5 s. The product of reverse transcription was diluted by 1:10 with diethylpyrocarbonate (DEPC)-treated water. The quantitative real-time PCR were conducted by ABI 7500 Real-Time PCR system (Applied Biosystems). The primers for toll-like receptor 4 (TLR4), myeloid differentiation factor 88 (MyD88), NF-κB, IL-1β, IL-6, TNF-α and β-actin used in the study are listed in Table 2. The reaction mixture (10 μl) contained 5 μl of fresh TB Green™ Premix Ex Taq™ (Tli RNaseH Plus) and 0·5 μl of the primers, 4 μl of RT products and 0·5 μl of DEPC-treated water. The following PCR protocol was used: one cycle at 94°C for 5 min, 40 cycles at 94°C for 30 s, annealing temperature for 30 s and 72°C for 30 s. Each gene was measured in triplicate and β-actin was used as the reference gene. The relative mRNA expression of the target genes was analysed by the 2−ΔΔCT method(Reference Pfaffl, Horgan and Dempfle20).
Table 2. Information of the primers used for the detection of mRNA specific for toll-like receptor 4 (TLR4), myeloid differentiation factor 88 (MyD88), NF-κB, IL-1β, TNF-α and β-actin
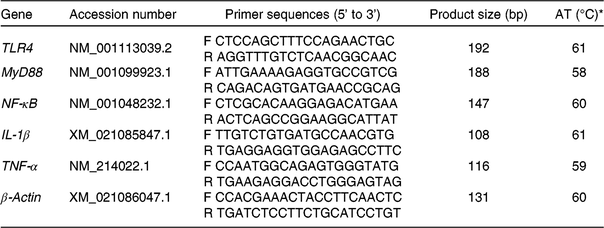
AT, annealing temperature; F, forward primer; R, reverse primer.
* The reaction mixture (10 μl) contained 5 μl of fresh TB Green™ Premix Ex Taq™ and 0·5 μl of the primers, 4 μl of reverse transcription products and 0·5 μl of diethylpyrocarbonate-treated water. The following PCR protocol was used: one cycle at 94°C for 5 min, forty cycles at 94°C for 30 s, annealing temperature for 30 s and 72°C for 30 s. Each gene was measured in triplicate and β-actin was used as the reference gene.
Western blot analysis
Protein expression levels of TLR4, MyD88, NF-κB, TNF-α, IL-1β, occludin, zonula occludens-2 (ZO-2) and P38 in both jejunal and ileal mucosa were measured by Western blot(Reference Qiu, Qin and Luo21) and normalised by the protein expression of β-actin. Briefly, about 100 mg of frozen jejunal or ileal mucosa were powdered in liquid nitrogen and lysed in RIPA buffer containing 50 mM Tris-HCl (pH 7·4), 150 mM NaCl, 1 % NP-40 and 0·1 % SDS, plus a Halt protease inhibitor cocktail (Thermo Fisher Scientific). The homogenate was centrifuged at 14 000 g for 15 min at 4°C and the supernatant was used for Western blot analysis. The protein concentration was measured by the bicinchoninic acid method (described earlier)(Reference Smith, Krohn and Hermanson22).
Statistical analysis
Data on growth performance and diarrhoea rate obtained in feeding trial were analysed using Student’s t test and the pen was used as the experimental unit. Differences between means on intestinal morphology, barrier integrity and immune response as well as mRNA protein expression levels were valued by one-way ANOVA followed by the Student–Newman–Keuls test for E. coli K88-challenged piglet experiments, with each animal as an experimental unit. Statistical analysis was performed by using the SAS software (version 9.2, SAS Institute). Values are presented as means and pooled standard errors and P < 0·05 was considered statistical significant. Graphpad Prism 6 was used to create the artwork.
Results
Growth performance and diarrhoea frequency of weaning piglets
As shown in Table 3, dietary supplementation of SBE significantly decreased average daily feed intake (P = 0·04), improved the feed conversion ratio (P = 0·04) and reduced diarrhoea frequency (P = 0·03), but did not alter average daily gain compared with the CTRL group during the 14 d of the feeding trial.
Table 3. Effects of Scutellaria baicalensis extracts (SBE) on growth performance and diarrhoea rate in weaned pigs (n 6 per group)
(Mean values with pooled standard errors)
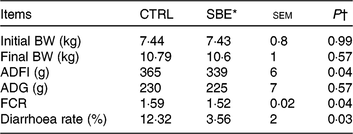
CTRL, control (antibiotic-free basal diet); BW, body weight; ADFI, average daily feed intake; ADG, average daily gain; FCR, feed conversion ratio.
* 1000 mg SBE/kg basal diet.
† Differences were considered as significant when P < 0·05.
Intestinal morphology and tight junction protein expression
Compared with the CTRL group, jejunal and ileal villous height and VCR were decreased by E. coli K88 challenge (P < 0·05) while crypt depth was not altered (P > 0·10) in relative to the CTRL piglets offered the basal diet (Fig. 1). Meanwhile, dietary supplementation of SBE to piglets challenged by E. coli K88 significantly improved jejunal and ileal villous height as well as ileal VCR (P < 0·05), but did not alter jejunal and ileal crypt depth.
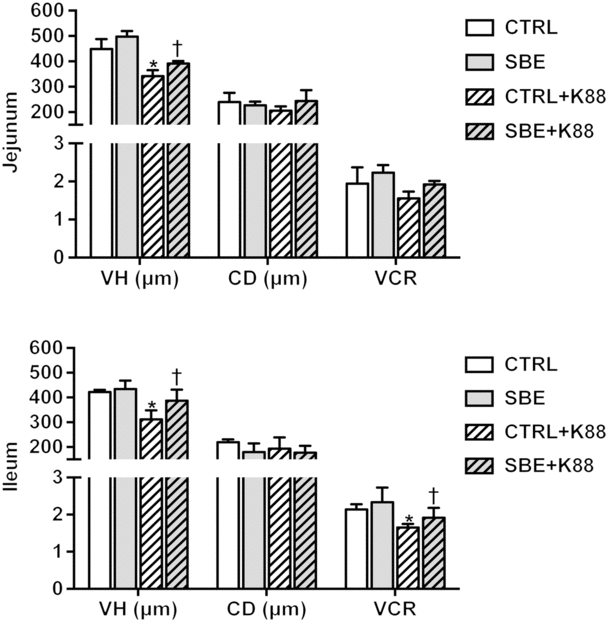
Fig. 1. Effects of Scutellaria baicalensis extracts (SBE) supplementation on small intestinal morphology in Escherichia coli K88-challenged piglets. VH, villous height; CD, crypt depth; VCR, villous height/crypt depth; CTRL, piglets were offered the antibiotic-free basal diet; SBE, piglets were offered SBE-containing diet; CTRL + K88, piglets fed the basal diet were challenged with E. coli K88; SBE + K88, piglets offered SBE-containing diet were challenged with E. coli K88, n 6. *P < 0·05 as compared with the CTRL group. † P < 0·05 as compared with the CTRL + K88 group.
With regard to intestinal integrity, E. coli K88 challenge significantly increased plasma DAO activity (P < 0·05) (Fig. 2) and decreased the protein expression of occludin and ZO-2 both in jejunal and ileal mucosa of piglets (P < 0·05) (Fig. 3). Notably, SBE supplementation to piglets challenged by E. coli K88 decreased plasma DAO activity and effectively alleviated the negative influence on intestinal integrity to a certain extent by increased protein expression of occludin and ZO-2 in jejunal mucosa and ZO-2 expression in ileal mucosa (P < 0·05). As for the piglets free of E. coli K88 challenge, SBE supplementation did not alter the protein expression levels of occludin and ZO-2 in jejunal and ileal mucosa (P > 0·05).
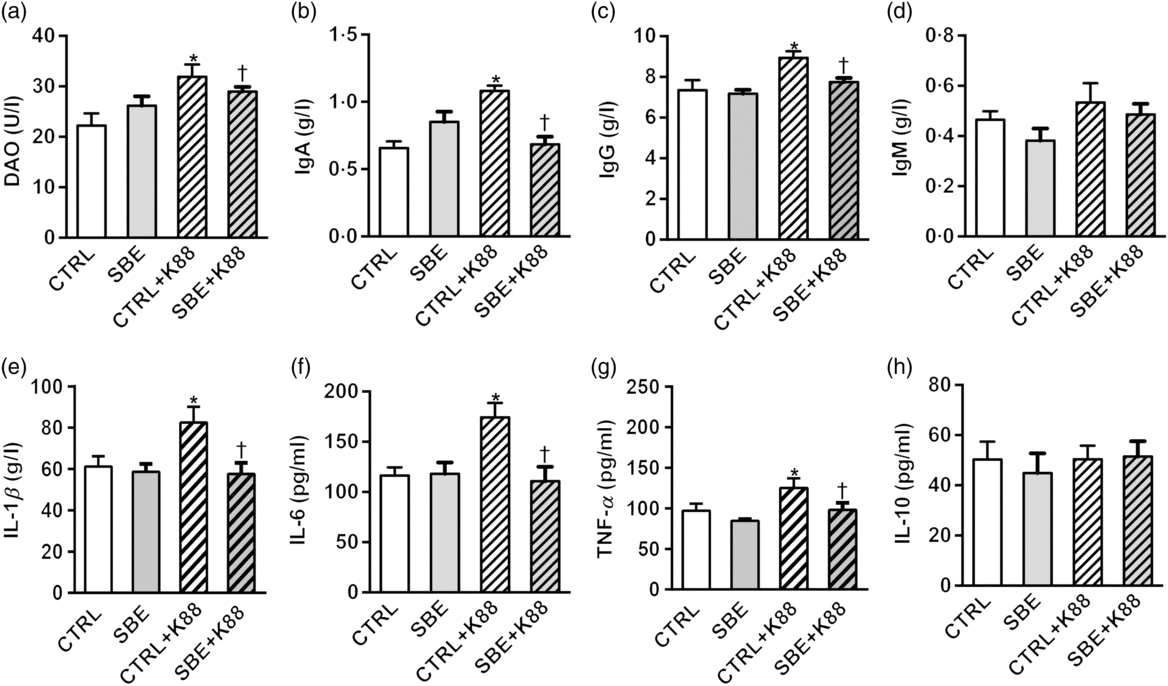
Fig. 2. Effects of Scutellaria baicalensis extracts (SBE) supplementation on plasma indices in Escherichia coli K88-challenged piglets. DAO, diamine oxidase; CTRL, piglets were offered the antibiotic-free basal diet; SBE, piglets were offered SBE-containing diet; CTRL + K88, piglets fed the basal diet were challenged with E. coli K88; SBE + K88, piglets offered SBE-containing diet were challenged with E. coli K88, n 6. *P < 0·05 as compared with the CTRL group. † P < 0·05 as compared with the CTRL + K88 group.

Fig. 3. Effects of Scutellaria baicalensis extracts (SBE) supplementation on protein abundance of occludin and zonula occludens-2 (ZO-2) in jejunal and ileal mucosa in Escherichia coli K88-challenged piglets. The densitometric values of these proteins were normalised to β-actin. CTRL, piglets were offered the antibiotic-free basal diet; SBE, piglets were offered SBE-containing diet; CTRL + K88, piglets fed with the basal diet were challenged with E. coli K88; SBE + K88, piglets offered SBE-containing diet were challenged with E. coli K88, n 6. *P < 0·05 as compared with the CTRL group. † P < 0·05 as compared with the CTRL + K88 group.
Plasma immune indices in piglets
Compared with the CTRL group, plasma levels of IL-1β, IL-6 and TNF-α as well as IgA and IgG were increased by E. coli K88 challenge (P < 0·05), while no alteration was observed in plasma IL-10 and IgM (P > 0·05) (Fig. 2). Dietary supplementation of SBE to piglets with exposure to E. coli K88 challenge significantly decreased plasma levels of IL-1β, IL-6 and TNF-α as well as IgA and IgG (P < 0·05).
mRNA and protein expression of toll-like receptor 4 and downstream inflammatory factors in the small intestine
To investigate the role of SBE supplementation in protecting the intestine health against immune stimulation induced by E. coli K88 challenge, we evaluated the expression of TLR4, MyD88, NF-κB, IL-1β and TNF-α in jejunal and ileal mucosa.
In jejunal mucosa, the mRNA expression of TLR4, MyD88, NF-κB, IL-1β and TNF-α was significantly stimulated by E. coli K88 challenge compared with the CTRL group (P < 0·05) (Fig. 4(a)–(f); jejunum). Meanwhile, SBE supplementation to piglets with or without exposure to E. coli K88 challenge decreased the mRNA expression of TLR4, MyD88, NF-κB, IL-1β and TNF-α (P < 0·05) in the jejunal mucosa relative to the corresponding CTRL animals.

Fig. 4. Effects of Scutellaria baicalensis extracts (SBE) supplementation on mRNA expression of NF-κB signalling pathway in jejunum and ileum in Escherichia coli K88-challenged piglets. All data were obtained by using real-time PCR and normalised to β-actin. TLR4, toll-like receptor 4; MyD88, myeloid differentiation factor 88; CTRL, piglets were offered the antibiotic-free basal diet; SBE, piglets were offered SBE-containing diet; CTRL + K88, piglets fed with the basal diet were challenged with E. coli K88; SBE + K88, piglets offered SBE-containing diet were challenged with E. coli K88, n 6. *P < 0·05 as compared with the CTRL group. † P < 0·05 as compared with the CTRL + K88 group.
In ileal mucosa, E. coli K88 challenge also stimulated the mRNA expression of TLR4, NF-κB, IL-1β and TNF-α (P < 0·05) except MyD88 compared with the CTRL piglets. Consistently, SBE supplementation to piglets with exposure to E. coli K88 challenge also decreased mRNA expression of TLR4, NF-κB and IL-1β (P < 0·05), except MyD88 and TNF-α in ileal mucosa. As for piglets without exposure to E. coli K88 challenge, SBE supplementation also decreased mRNA expression of MyD88 (P < 0·05) in ileal mucosa compared with piglets offered the control diet (Fig. 4(a)–(f); ileum).
Furthermore, E. coli K88 significantly stimulated protein expression of TLR4, MyD88, NF-κB, P38, IL-1β and TNF-α both in jejunal and ileal mucosa of piglets compared with the CTRL animals (P < 0·05). Additionally, we found that dietary supplementation of SBE to piglets challenged by E. coli K88 remarkably reversed the inflammatory stimulation to a certain extent by the alleviated level of TLR4, MyD88, NF-κB, P38, IL-1β and TNF-α (Figs. 5 and 6).
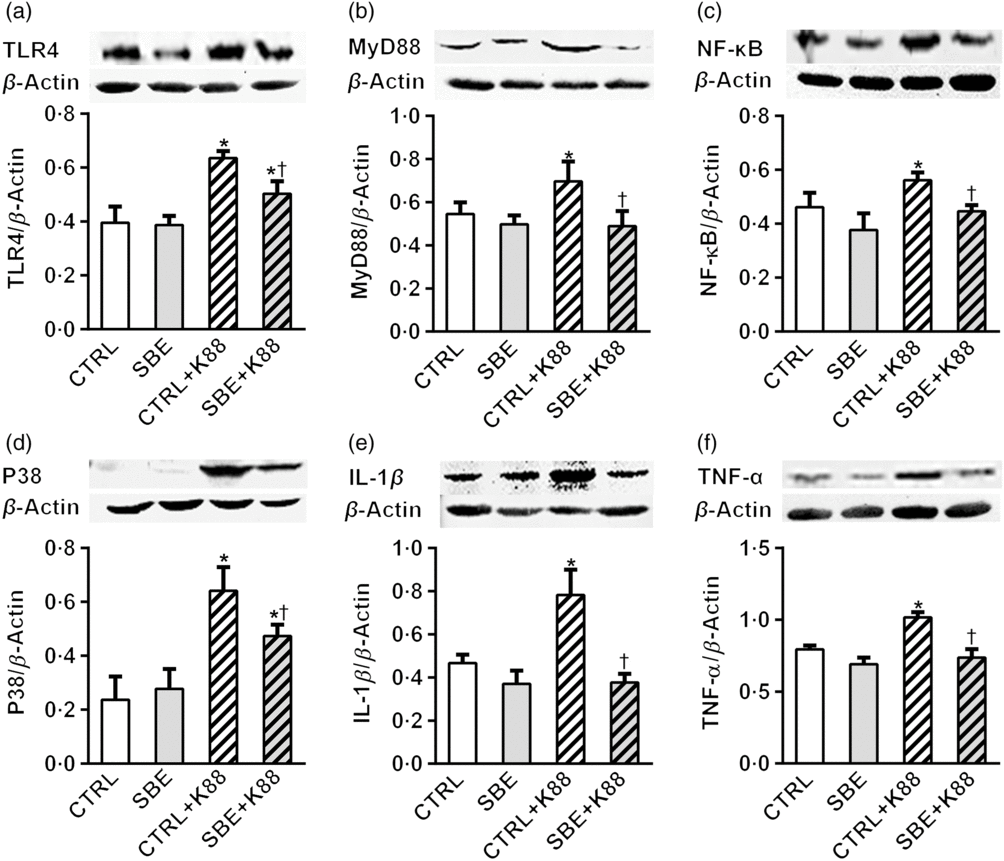
Fig. 5. Effects of Scutellaria baicalensis extracts (SBE) supplementation on jejunal protein abundance of NF-κB and P38 signalling pathway in Escherichia coli K88-challenged piglets. The figure shows a Western blot of immunoprecipitated toll-like receptor 4 (TLR4), myeloid differentiation factor 88 (MyD88), NF-κB, P38, IL-1β, TNF-α and the densitometric values of these proteins normalised to β-actin. CTRL, piglets were offered the antibiotic-free basal diet; SBE, piglets were offered SBE-containing diet; CTRL + K88, piglets fed with the basal diet were challenged with E. coli K88; SBE + K88, piglets offered SBE-containing diet were challenged with E. coli K88, n 6. *P < 0·05 as compared with the CTRL group. † P < 0·05 as compared with the CTRL + K88 group.
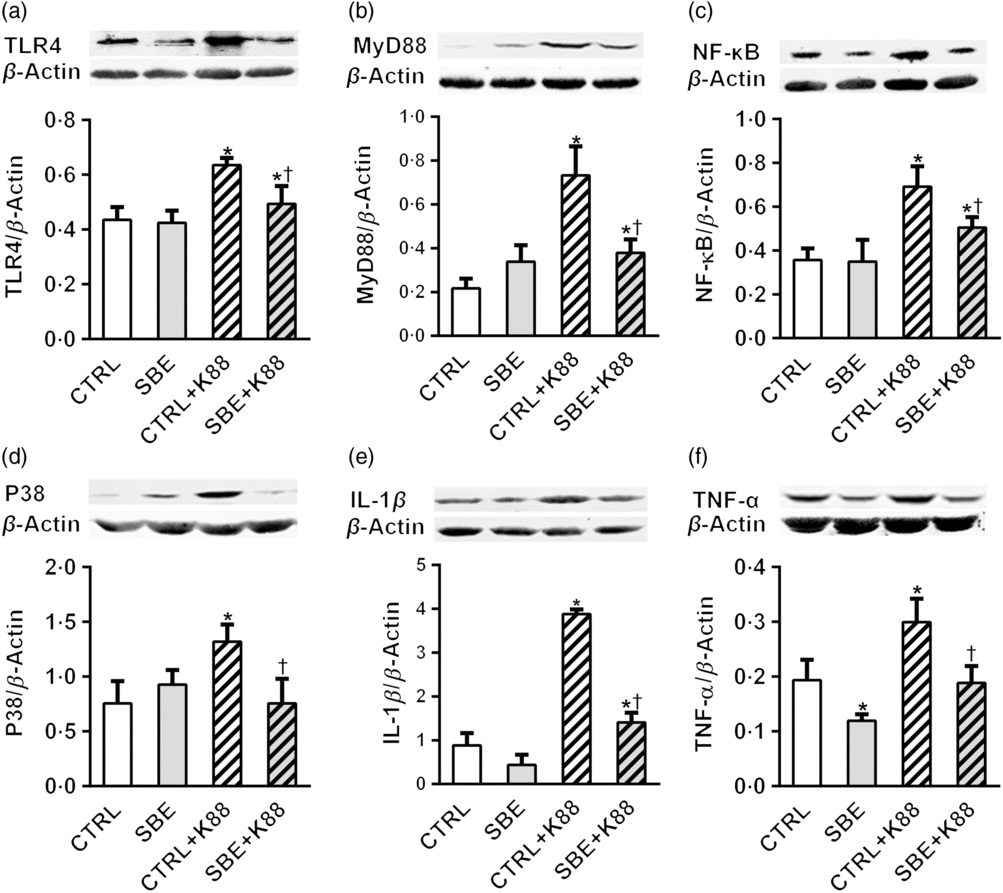
Fig. 6. Effects of Scutellaria baicalensis extracts (SBE) supplementation on ileal protein abundance of NF-κB and P38 signalling pathway in Escherichia coli K88-challenged piglets. The figure shows a Western blot of immunoprecipitated toll-like receptor 4 (TLR4), myeloid differentiation factor 88 (MyD88), NF-κB, P38, IL-1β, TNF-α and the densitometric values of these proteins normalised to β-actin. CTRL, piglets were offered the antibiotic-free basal diet; SBE, piglets were offered SBE-containing diet; CTRL + K88, piglets fed with the basal diet were challenged with E. coli K88; SBE + K88, piglets offered SBE-containing diet were challenged with E. coli K88, n 6. *P < 0·05 as compared with the CTRL group. † P < 0·05 as compared with the CTRL + K88 group.
Discussion
The attacks from pathogenic E. coli often lead to intestinal injury and resultant diarrhoea in baby animals and even in children in developing countries. SBE had been shown to occupy multi-characteristics such as antimicrobial, anti-inflammatory, antioxidant and immune-regulative function(Reference Shan, Cai and Brooks9, Reference Gaire, Moon and Kim23, Reference Shang, He and Jia24), attracting great interests in farm animal feeding. However, the role of SBE in protecting farm animal intestinal health against attacks from enteropathogenic bacteria remains unclear.
In the present study, we tested whether SBE supplementation could protect intestinal health against infection by pathogenic E. coli in a model of weaned piglets. During the 14 d feeding trial, ingestion of the diet containing SBE did not alter the average daily gain of piglets, but significantly decreased diarrhoea rate and improved feed efficiency compared with CTRL. Feed intake was decreased, which may be due to the bitter tasting SBE. It demonstrated that dietary supplementation of SBE could effectively improve intestinal health of weaned piglets. What is more, the sample size of six replicates (n 6) in the present study is proper for detecting the effect of SBE on intestinal barrier function and immune response on E. coli K88 challenge; however, as for the effect on growth performance, it warrants continual study with a greater population of piglets.
Intestinal health in terms of intestinal integrity can be assessed by intestinal morphology and intestinal barrier function. Intestinal morphology can be directly reflected by villous height, crypt depth and VCR(Reference Adelman, Murray and Wu25). Intestinal barrier function can be assessed by circulative DAO activity(Reference Fukudome, Kobayashi and Dabanaka26, Reference Wang, Liu and Shi27) and tight junction protein expressions(Reference Fasano28, Reference Suzuki and Hara29). In the present study, we observed that E. coli K88 challenge not only induced fever, anorexia, inactivity, shivering within 6 h and appearing diarrhoea within 1 d in piglets, but also damaged intestinal morphology and increased plasma DAO activity. The villous height in the intestine of piglets was shrunk by E. coli K88 challenge, which was consistent with previous studies(Reference Che, Xu and Wu3, Reference Heim, Sweeney and O’Shea30, Reference Owusu-Asiedu, Nyachoti and Marquardt31). The DAO is abundant in enterocytes at the tip of small intestinal villous and regulates epithelial cell proliferation through degradation of polyamine as an indispensable substance for mitosis and meiosis(Reference Wolvekamp and de Bruin32). The DAO is a key marker for monitoring intestinal barrier permeability(Reference Thompson, Vaughan and Forst33). When the intestinal mucosal barrier integrity is injured, intestinal DAO will release into the circulation and plasma DAO activity increased(Reference Zhao, Luo and Jia34). In the present study, plasma DAO activity was significantly increased after piglets being challenged by E. coli K88, indicating that intestinal barrier function was attainted, which was consistent with previous study(Reference Pan, Zhao and Ma35).
It had been shown that occludin and ZO-2 play crucial roles in maintaining the tight junction structure and permeability of the intestinal epithelium(Reference Vivian and Gumbiner36–Reference Tsukita, Katsuno and Yamazaki38). Through altering the protein expression of the tight junction, enteric pathogen and endotoxin translocations result in increased intestinal paracellular permeability(Reference Groschwitz and Hogan39). In the present study, the protein expressions of occluding and ZO-2 in jejunal and ileal mucosa was decreased by E. coli K88 challenge in piglets compared with the CTRL piglets offered the CTRL diet, which demonstrated that intestinal barrier integrity was damaged by E. coli K88 challenge in piglets. Consequently, we successfully established acute intestinal injury model in piglets with the oral ingestion of E. coli K88.
Stimulation of serum IgG and IgA is a prerequisite for induction of an immune response in piglets with E. coli K88 challenge(Reference Van, Cox and Goddeeris40). Moreover, pro-inflammatory cytokines such as TNF-α and IL-1β have been shown to exert negative roles in maintaining the tight junction and cytoskeleton structure and function(Reference Nusrat, Turner and Madara41). In the present study, plasma pro-inflammatory cytokines TNF-α, IL-1β, IL-6 and immunoglobulins IgA and IgG were stimulated by E. coli K88 challenged in piglets offered the CTRL diet, which was consistent with previous studies(Reference Ewaschuk, Murdoch and Johnson42–Reference Gao, Han and Huang44).
We observed that dietary SBE supplementation to E. coli K88-challenged piglets effectively restored villous height both in jejunum and ileum, which was supported by previous studies in which herbal extracts could alleviate the intestinal morphology injuries in pigs or rodents(Reference Nofrarías, Manzanilla and Pujols45–Reference Franki, Volj and Salobir47). Therefore, we hypothesised that SBE supplementation improved intestinal health through inhibiting intestinal inflammatory response. In the present study, plasma level of TNF-α, IL-1β, IL-6, IgA and IgG were stimulated by E. coli K88 challenge in piglets offered the CTRL diet. However, the stimulated plasma levels of these indices were rescued in piglets offered the SBE diet. Similarly, the mRNA expression of NF-κB and IL-1β in the jejunum and ileum and TNF-α in the jejunum was stimulated by E. coli K88 challenge in piglets offered the CTRL diet while being rescued by SBE supplementation. Furthermore, after we validated it on the protein expression level, we found that protein abundance of TNF-α and IL-1β was stimulated in both jejunum and ileum after E. coli K88 challenge in piglets offered the CTRL diet, but SBE supplementation could effectively rescue the protein expression in jejunum and ileum. From this, we reasonably deduced that SBE supplementation could effectively repress immune stimulation induced by E. coli K88 challenge and consequently improve intestinal health in piglets.
Pro-inflammatory cytokines are often associated with the activation of inflammatory signalling pathways, in which TLR4, as a member of transmembrane TLR, distributing on all types of intestinal cells, plays a crucial role in recognising pathogen-associated molecular patterns and modulating inflammatory responses with the receptor complex of TRL4/MD-2/CD14 and transduces a signal sensed by MyD88, which recruits IL-1 receptor-associated kinase 1 (IRAK1) to activate TNF receptor-associated factor 6 (TRAF6) and the IKK complex. The activated IKK phosphorylates the IκB family, and then leads to the activation of NF-κB pathway as well as mitogen-activated protein kinase (MAPK) pathway, including the extracellular signal-regulated kinase 1/2 (ERK1/2), the Jun N-terminal kinase (JNK) and p38, and eventually stimulates synthesis of the pro-inflammatory cytokines such as TNF-α, IL-1β, IL-6 and so on(Reference Baldwin48–Reference Ghosh, May and Kopp50). To further detect the pathway through which SBE supplementation to piglets suffered E. coli K88 challenge alleviated the activated immune response, we detected whether the key elements involved in TLR4-mediated inflammatory reaction were altered. We found that, even though mRNA expression of MyD88 and TNF-α in the ileum remained unchanged, mRNA abundance of jejunal and ileal TLR4, NF-κB and IL- 1β as well as jejunal MyD88 and TNF-α was stimulated by E. coli K88 challenge, while SBE supplementation effectively recovered the stimulated immune status, which was also strongly supported by the fact that SBE supplementation to piglets suffered E. coli K88 challenge reduced the protein expression of TLR4, MyD88, NF-κB, and P38, together with IL-1β and TNF-α.
Therefore, we evidenced that TLR4, MyD88, NF-κB as well as P38 were involved in dietary supplementation of SBE ameliorating immune stimulation induced by E. coli K88 challenge, which was indicated by the alteration both of mRNA abundance and protein expression of inflammatory cytokines in response to dietary supplementation of SBE to E. coli K88-challenged piglets.
In conclusion, combining with results of plasma IL-1β, IL-6 and TNF-α as well as IgA and IgG, it could be reasonable to deduce that E. coli K88 challenge injured the intestinal health in terms of intestinal morphology and barrier function through stimulating inflammation, and dietary supplementation of SBE could effectively rescue the intestinal injury via restraining NF-κB/P38 pathways.
Acknowledgements
The present study was funded by the National Key R&D Program (grant no. 2016YFD0700201, 2018YFD0500402), and 111 Project (B16044).
The authors’ contributions are: J. Y. was the principal investigator contributing to getting financial support, the study design and interpretation of the findings, and wrote the manuscript. C. H., Y. W. and X. H. carried out the animal feeding trial and sample collection. C. H. contributed to sample analysis, statistical analysis and preparation of the first draft; X. P. contributed to the study design and arrangement; N. J., X. Z., K. Q. contributed to the sample collection and data analyses. All authors read and approved the final version of the manuscript.
The authors have no financial or personal conflicts of interest to declare.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0007114519000928












