INTRODUCTION
Infection by Campylobacter bacteria is currently responsible for the largest fraction of bacterial gastroenteritis in developed countries and represents a heavy burden on public health resources [Reference Butzler1–Reference Tam4]. In humans, the majority of Campylobacter infections are believed to result from the ingestion of contaminated foods of animal origin although other sources of infection may include contact with domestic and farm animals as well as surface waters [Reference Park5–Reference Kapperud8]. Most cases of human campylobacteriosis are sporadic [Reference Altekruse9] and the epidemiology of Campylobacter has a distinct seasonality with incidence peaking in the late spring or early summer in temperate climates [Reference Nylen10–Reference Tam12]. Despite its wide distribution and pervasiveness in the environment and throughout the food chain, the ecology of campylobacteriosis remains complex, making its prevention and control problematic [Reference Frost13, Reference Skelly and Weinstein14].
A key factor in targeting interventions and in developing prevention and control measures for Campylobacter infection is determining where the illness was acquired. This information not only gives clues to investigate potential sources, reservoirs, and determinants, but also assists in assessing the geographical distribution of the infection for surveillance activities. It is often impossible to establish the true location of disease transmission and because of this limitation, residential addresses of cases [Reference Michaud, Ménard and Arbeit15–Reference Rajda and Middleton18] are frequently used as a proxy measure. However, the use of such a proxy location may cloud our understanding of the geographical distribution of the disease and may distort the estimation of ecological associations between the disease and putative local environmental factors.
The public health authorities in Iceland routinely collect information on confirmed cases of campylobacteriosis. Between 2000 and 2006, detailed epidemiological data were also collected from all reported cases as part of larger research initiative [Reference Stern19], for which more in-depth questions regarding risk factors, including regional travel, were gathered. The population of Iceland in 2003 was 290 570 with the majority of the population living in the Southwest area of the island, which includes the capital city Reykjavík (39% of the island's population) [20]. The second largest city Akureyri, located in the northeast area of the island, has a much smaller population than Reykjavík (6% of the population in 2003). A large portion of the interior of Iceland is covered by permanent ice or lava and therefore human settlement is mostly limited to the rim of the island [Reference Wilcox and Latif21].
The objective of this study was to describe the potential public health impact of failing to incorporate domestic travel information when conducting surveillance on cases of campylobacteriosis. This objective was carried out by calculating various measures of campylobacteriosis incidence in Iceland from 2001 to 2005, based upon the cases' region of residence, adjusting location of domestic travel cases to their travel region, as well as separate estimations for travellers and non-travellers.
METHODS
Surveillance for campylobacteriosis in Iceland is based upon laboratory reports of all culture-confirmed cases and reports from the attending physicians. Two hospital-based laboratories, Landspitali University Hospital and Akureyri District Hospital, process all the specimens and all positive cultures are confirmed at the reference laboratory at Landspitali University Hospital. A clinical microbiologist at Landspitali University Hospital notifies the attending physician and obtains information regarding likely sources, symptoms, and travel information. If the information received from the physician was insufficient, permission is then given for the clinical microbiologist to contact directly the patient or guardian of the patient. The incubation period was defined as the 10 days prior to symptom onset date [Reference Mentzing22, Reference Heymann23]. Regions were designated according to the Icelandic Census [20], which are not administrative regions but utilized for statistical purposes. Domestic travel was defined as any case having travelled outside their region of residence but within Iceland at any time during the incubation period. Cases were excluded if accurate information regarding the region or city destination of their domestic travel was not obtained. Additionally, cases that travelled internationally within their incubation period were also excluded from the analysis as they may have become infected outside Iceland, which would not represent domestic acquisition of the infection. Descriptive statistics were calculated for the cases and stratified by domestic travel status. Continuous variables were compared by Student's t test; dichotomous variables were analysed by Pearson's χ 2 test, and a significance level of 0·05 was applied to all tests.
Estimates of the proportion of cases from each region and cumulative incidence rates for each region were calculated using two different approaches. The first method determined the estimate in each region based upon the case's region of residence, termed the ‘crude’ proportion or incidence rate estimate. The second approach, the ‘adjusted’ proportion or rate, involved geographically reassigning the cases that travelled during their incubation period to another region of Iceland. If a case travelled to more than one region during their incubation period (which occurred for 15% of those who travelled), time in the travel regions was divided equally as information collected on time spent within each region was not collected. Additionally, the cumulative incidence rates were estimated for non-travellers only, in order to serve as a comparator for the crude and adjusted rates. Since 2003 is the midpoint of the study period, (2001–2005), in estimating rates, at-risk person-time for each region was determined using the 2003 Icelandic Census.
To estimate the probability of a case arising from a region, we used Bayesian methods to estimate multinomial probabilities assuming a Dirichlet prior. In order to determine whether a significant difference existed in the distribution of cases in Iceland's eight regions after accounting for domestic travel during the incubation period, we first determined whether there was a significant overall change in the eight regional proportions using a marginal homogeneity test [Reference Agresti24]. For this test, we only included travellers that had visited no more than one destination during their incubation period. Next, we examined the ratio of adjusted to crude rate estimates for each region. For this, we applied a simple Bayesian hierarchical model to estimate the crude regional rates, the adjusted regional rates and the ratio of the two rates. Bayesian hierarchical models were used to increase the precision of the estimates as data scarcity, particularly in the low-populated areas, caused the risk measures to be unstable. We used a lognormal model for global smoothing rather than one with both local and global smoothing as we had only a few large areas [Reference Bernardinelli25]. Global smoothing shrinks unstable risk estimates to the overall risk of the study region with greater shrinkage occurring in areas with more unstable and unusual risk estimates. To determine if accounting for domestic travel significantly changed the rate of campylobacteriosis for a given region, we calculated the 95% credible interval (95% CI) of the ratio comparing the adjusted to the crude proportions and rate estimates for each region.
In implementing the Bayesian models, we produced three Markov Chain Monte Carlo sequences of risk estimates for every region, each chain starting from a different initial value and then assessed convergence via the Gelman–Rubin statistic [Reference Gelman26]. Convergence was achieved at 50 000 iterations but we produced a total of 100 000 iterations for each chain and discarded the first 80 000, leaving the last 20 000 from each chain for parameter estimation. In addition, sensitivity to the choice of hyper-parameters was assessed but only small changes in the risk parameter estimates were observed.
A population-based map of Iceland, divided into the eight regions was created to aid in the visualization of our analysis, using ArcGIS v. 9.1 software (ESRI Inc., USA). The map illustrates the percent change between the crude and adjusted rate estimates as well as the flow of domestic travellers into and out of each region.
A limitation of analysing rates of infection in those travelling to or residing in a region, is the difficulty in estimating at-risk person time. To accurately determine the at-risk person time for an area requires accounting for all individuals in the area at all times of the study period and this would include residents travelling out of the area as well as those travelling to the area. Data describing this complex flow of travel is not available but an analysis of rates of infection given regional volume of overnight trips could be informative and possibly validate our other findings. We used data on the total number of trips per region taken by Icelandic residents from May 2007 to April 2008 [27] to determine the rates of infection for domestic travellers. The regional distribution of overnight trips was compared to the geographic distribution of cases for travellers using Bayesian hierarchical (global smoothing) models. The relative risk of infection for each region was estimated by the ratio of the rate of infection from overnight travels to the region to the rate of infection from all overnight trips in Iceland. Similar analyses giving relative risks and identifying high-risk areas were conducted on the ‘crude’ data (i.e. regional counts of cases by region of residence) and on the ‘adjusted’ data (i.e. regional counts of cases by residence while adjusting for travel). High-risk regions were established by using a cut-off probability of 0·8 for the posterior proportion and a reference threshold R 0 of 1·2 [Reference Richardson28].
All analyses were conducted using WinBUGS v. 1.4 software (MRC Biostatistics Unit/Imperial College School of Medicine, UK) and R v. 8.1.2 software (R Foundation for Statistical Computing, Austria).
RESULTS
A total of 400 cases of human campylobacteriosis were reported from April 2001 to December 2005 in non-foreign travel cases. Twenty-four cases were excluded due to missing information on domestic travel. The final dataset included 376 cases, 37% of whom travelled domestically within their estimated incubation period (Table 1). Non-travellers and travellers had significantly different seasons of onset for campylobacteriosis, with travellers having a significantly higher proportion of Campylobacter infections in the summer (83% vs. 48%) than non-travellers.
Table 1. Demographic information for all cases (n=376), non-travellers (n=236) and travellers (n=140)
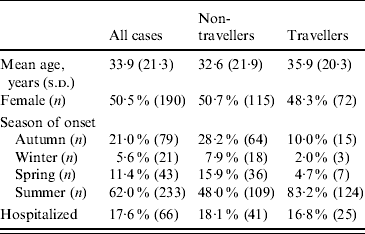
s.d., Standard deviation.
The Capital region bore the largest burden of all Campylobacter cases in Iceland (63%) which decreased to 40% when domestic travellers from the Capital region were reassigned to their travel region (Table 2). The proportion of total cases significantly increased in the West, East, and South regions and significantly decreased in the Capital and South Peninsula regions. The test of marginal homogeneity indicated an overall difference in the geographic distribution of cases after accounting for domestic travel during the incubation period (P value <0·0001).
Table 2. Number of cases and proportion of campylobacteriosis in Iceland from 2001 to 2005
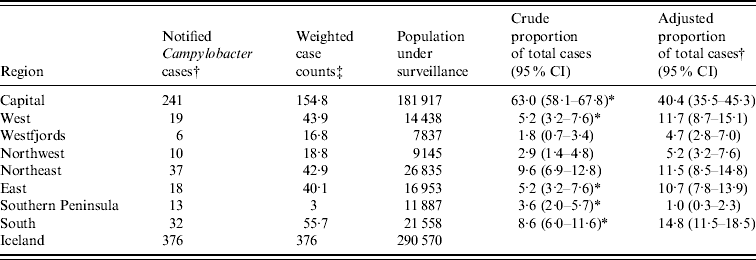
CI, Credible interval.
† Based upon region of residence.
‡ Based upon travel region for travellers and region of residence for non-travellers.
* Significantly different adjusted proportion relative to crude proportion.
The national cumulative incidence rate of domestic campylobacteriosis in Iceland from April 2001 to December 2005 was 129·4/500 000 (95% CI 122·2–136·8) (Table 3). The regional crude incidence rate estimates were similar in magnitude to one another as well as to the national rate. The adjusted estimates ranged from a low of 53·6/500 000 (95% CI 18·6–102·6) in the Southern Peninsula to a high of 291·3/500 000 (95% CI 211·1–383·9) in the West region. As with the adjusted proportion estimations, the adjusted cumulative incidence rate estimates also increased considerably in the West, East, and South regions while greatly decreasing in the Capital and Southern Peninsula regions relative to the crude rate estimates. The cumulative national incidence rates for non-travellers was 78·1/500 000 (95% CI 72·9–83·5) and the regional non-traveller rate estimates did not significantly differ from the national rate calculated for non-travellers.
Table 3. Cumulative regional incidence rate estimates of campylobacteriosis/500 000 in Iceland
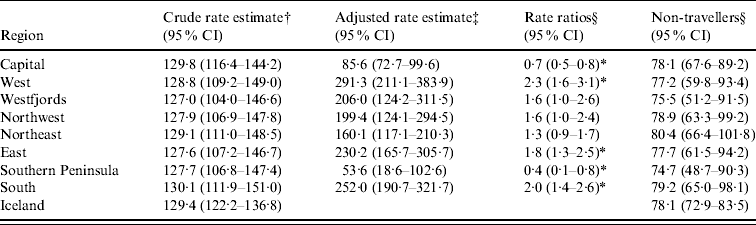
CI, Credible interval.
† Based upon region of residence.
‡ Based upon travel region for travellers and region of residence for non-travellers.
§ Rate ratios of crude rate relative to adjusted rate.
* Significantly different adjusted rate estimate relative to crude rate estimate.
The largest change in incidence rate estimates, when comparing the adjusted to the crude, occurred in the West region with a 126% increase and the smallest percentage change occurring in the Northeast with a 24% increase (Fig. 1). The figure also shows the flow of travellers into and out of each region. For example, 71% of the domestic travellers lived in the Capital region (and left the Capital region during their incubation period) and 10% of the domestic travellers entered the Capital region during their incubation period. This results in a 61% net loss of Capital region's travellers and a total of 56% of Capital's cases.
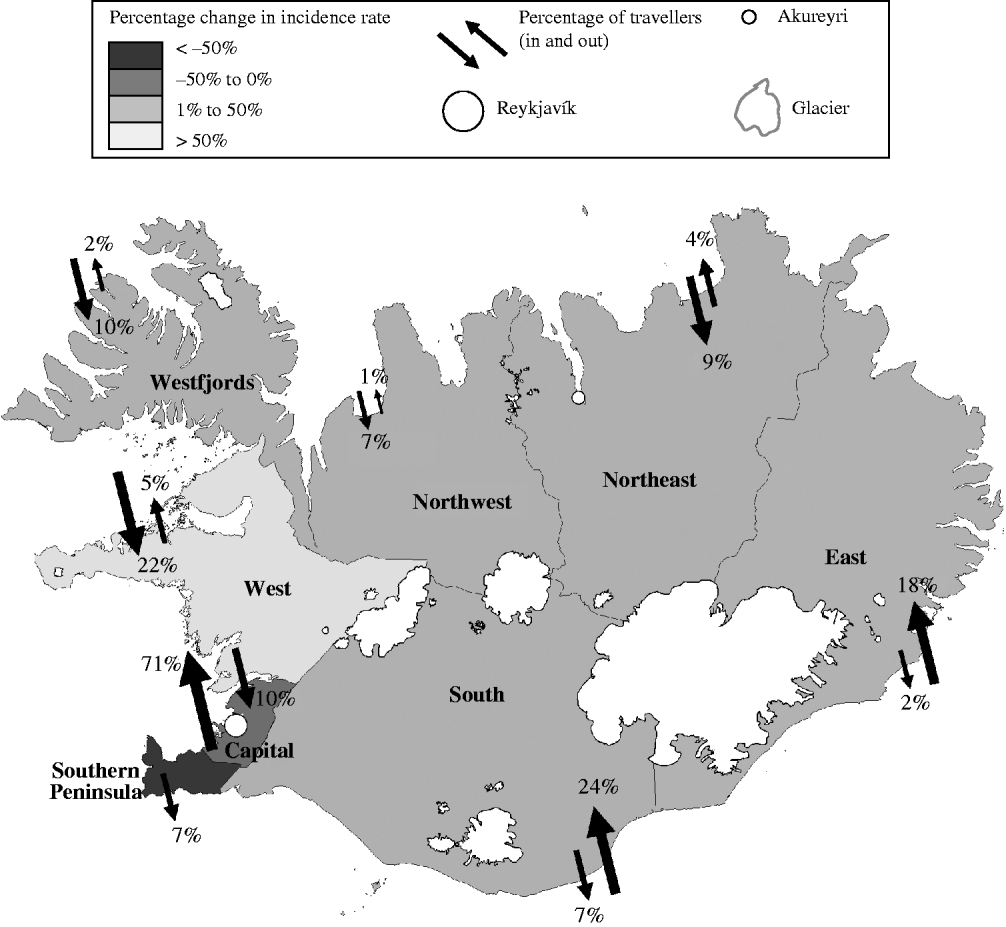
Fig. 1. Map of Iceland showing regional percentage change in campylobacteriosis incidence rates and flow of travellers from 2001 to 2005.
When comparing the risk ratios from the crude and adjusted assignment and that of travellers (Table 4), the results varied considerably. The risk ratios resulting from the crude assignment were relatively uniform throughout the nation. We found greater correspondence between the adjusted risk ratios and those of the travellers. However, the between-region variation in risk ratios was not as pronounced for the travellers. For example, we found that the risk of travellers to the East region was significantly higher than the overall risk of travellers; a finding that was not reflected in the results using residential address as proxy but was found in the ‘adjusted’ analysis that accounted for travel. There were no high-risk regions identified when using residence as proxy yet five regions were identified for the adjusted assignment, and one region was identified as high-risk from the spatial analysis of travellers.
Table 4. Regional risk ratios (RR) and posterior proportions (PP) for crude, adjusted, and travellers

* Based upon region of residence.
† Based upon travel region for travellers and region of residence for non-travellers.
‡ Based upon travellers and their travel region using total domestic trips as the denominator.
§ Rate ratio of regional rate relative to national rate.
|| High-risk region.
DISCUSSION
In Iceland, about 37% of all reported domestic cases of human campylobacteriosis were thought to be outside their residential area at a time when exposure probably occurred. This effect demonstrates the importance of collecting and using domestic travel information when calculating Campylobacter rates by geographical regions. The regional rate estimates changed substantially when corrected for domestic travel and ignoring travel information could lead to inappropriate interpretations of the data, misguiding prevention and control measures that are based upon the identification of high-risk areas and the investigation of associated regional environmental sources [Reference Michaud, Ménard and Arbeit15, Reference Green, Krause and Wylie17]. For example, prior to correcting for domestic travel, all regional rate estimates were fairly similar and after the adjustment, five of the eight regions differed considerably from their respective crude estimates. Ignoring domestic travel is likely to provide a false representation of the local burden of Campylobacter for each region and fail to highlight the regional differences. Furthermore, it is common practice in case-control studies to match a control to a Campylobacter case based upon a postal code [Reference Kapperud7, Reference Green, Krause and Wylie17] or a municipality [Reference Schönberg-Norio6, Reference Kapperud8, Reference Michaud, Ménard and Arbeit15], although if performed with the intention of matching on exposure location, bias could result due to the inappropriate matching between cases and controls.
As shown in the analysis, non-travellers had a significantly lower proportion of Campylobacter infections occurring in the summer compared to travellers. These findings are not surprising as 56% of all domestic trips in Iceland occur between May to August [27] and reflects a preference in Icelanders to travel domestically during the summer. The increased campylobacteriosis in travellers in the summer coincides with other studies that have found a distinct seasonal pattern of campylobacteriosis in temperate regions [Reference Louis2, Reference Nylen10–Reference Tam12, Reference Eberhart-Phillips29, Reference Pearson and Healing30]. Known risk factors for Campylobacter infection that are summer-related include eating barbeque-prepared meals [Reference Kapperud8, Reference Meldrum31, Reference Patrick32], drinking untreated water from streams and other natural sources [Reference Kapperud8, Reference Hearnden33], aquatic recreation [Reference Neimann34], and increased contact with farm animals if travelling into the countryside [Reference Green, Krause and Wylie17, Reference Allerberger35, Reference Godoy36]. It has also been cited that eating food prepared at a restaurant and in particular chicken, poses a greatly increased risk of campylobacteriosis [Reference Friedman37] and it is probable that people eat at restaurants more frequently when travelling. Studies in Iceland have implicated domestic animals and pets [Reference Steingrimsson38], unpasteurized milk, as well as surface water [Reference Steingrimsson and Alfredsson39, Reference Thorsteinsson40] as local sources of campylobacteriosis. Additionally, the consumption of fresh chicken is frequently cited as a source of infection for human cases of Campylobacter in Iceland [Reference Reiersen41]. These potential sources indicate that certain regions may pose different risks and that there is an underlying divergence in characteristics between travellers and non-travellers; travellers may constitute a different risk group for campylobacteriosis than non-travellers.
The main limitations of this study are a result of the assumptions that were made when geographically reassigning domestic travellers. It was assumed that if a case travelled outside their region of residence, that they spent their entire 10-day incubation period in that particular region. Therefore, the 24% of travellers that visited the South region during their incubation period did not necessarily acquire their campylobacteriosis in that particular region because of this assumption. If a case travelled to more than one region during their incubation period, time spent in the travel regions was divided equally as a time dimension was not collected in the travel component of the case follow-up questionnaire.
It is also likely that there was underreporting of campylobacteriosis during this time as it has been estimated that for every laboratory-based report of gastrointestinal illness, there are 313 cases occurring in the community, although this estimate was based upon Canadian data [Reference Majowicz42]. While it is not possible to infer this estimation directly on the Icelandic population, the higher proportion of hospitalizations in the cases included in the study (18%) is greater than that typically expected for Campylobacter cases (5–10%) [Reference Shirrow, Blaser, Nachamkin and Blaser43], which supports the hypothesis of underreporting, as more severe cases are more likely to seek medical attention.
When attempting to adjust for travel (the ‘adjusted’ rate estimates), we were not able to accurately adjust our denominators to reflect the true population at-risk due to data limitations concerning domestic travel in Iceland. However, as a form of validation, using data on the number of overnight trips per region, we estimated the regional rates of infection given the regional volume of overnight trips (Table 4). This analysis validated our approach to risk estimation, despite its limitations, as significant differences were found between the flow of all domestic travellers and the geographic distribution of cases of domestic travellers. This analysis also demonstrated that there would have been no regions identified as high-risk using the region of residence as the basis for surveillance. One high-risk region was identified using only travellers while five high-risk regions were identified using the adjusted approach.
Due to the assumptions made when geographically reassigning domestic travellers, it is probable that a significant overcorrection for domestic travel occurred. Pragmatically, and in consideration of the possible ambiguity relating to the incubation period, it may be difficult to capture sufficient detail to accurately place each case with respect time and place of exposure when conducting routine surveillance and investigations. In this context, the exclusion of domestic travellers from the analysis, or conducting a stratified analysis based upon travel status would provide an interesting alternative to understanding the regional-level occurrence of campylobacteriosis, although more work is needed in this area to determine the best analytical approach.
Despite the limitations of this study, we feel that this analysis demonstrates the importance of considering domestic travel information when calculating incidence rates of campylobacteriosis based upon geographical location. It provides evidence for collecting additional information from confirmed cases of campylobacteriosis on domestic travel and duration. In order to reduce the numbers of Campylobacter infections, accurate local surveillance is required, followed by appropriate preventive measures [Reference Adak44]. For example, public health could choose to target messages to residents and those travelling to regions whose incidence rates increased substantially with the incorporation of domestic travel information. By locating cases more accurately and understanding the local burden of Campylobacter, the determination of associated aetiological sources can then be achieved which would inform control and prevention measures. These findings have the potential for a broader application than exclusively to campylobacteriosis and should be explored for other priority enteric diseases as well as for other countries.
ACKNOWLEDGEMENTS
Special thanks are due to the clinical microbiologists at Landspitali University Hospital: Guđrun S. Hauksdóttir, Hördur Harđarson, Ingibjörg Hilmarsdóttir, and Ólafur Steingrímsson.
APPENDIX. ‘Campy-on-Ice’ Consortium
Iceland: Haraldur Briem, Vala Friđriksdóttir, Eggert Gunnarsson, Franklín Georgsson, Hjördís Harđardóttir, Karl Kristinsson, Guđrún Sigmundsdóttir, Jarle Reiersen. Sweden: Eva Berndtson. Canada: Jean-Robert Bisaillon, Pascal Michel, Greg Paoli, Aamir Fazil, Ruff Lowman. USA: Norman Stern, Kelli Hiett, Ken Callicott.
DECLARATION OF INTEREST
None.







