INTRODUCTION
Streptococcus pneumoniae is a leading cause of invasive infections such as lobar pneumonia, septicaemia, and meningitis, which are major contributors to morbidity and mortality in children and adults. Since the discovery of pneumococcal strains resistant to penicillin G (PEN) [Reference Hansman and Bullen1], these strains have spread rapidly worldwide [Reference Klugman2, Reference Appelbaum3] and have been the subject of several epidemiological surveillance studies of capsule serotype distribution and antibiotic susceptibility in many countries [Reference Phongsamart4–8].
In Japan, the prevalence of PEN-resistant S. pneumoniae (PRSP) and PEN-intermediate S. pneumoniae (PISP) in clinical isolates has increased rapidly since the late 1990s, especially in younger children [Reference Ubukata9, Reference Ubukata10]. Characteristically, PRSP strains show simultaneous resistance to cephalosporin antibiotics used in ambulatory practice [Reference Ubukata9]. In PRSP and PISP, genotypic abnormalities in three penicillin-binding protein (PBP) genes, pbp1a, pbp2x, and pbp2b, which encode the PBP1A, PBP2X, and PBP2B enzymes, respectively, have been identified by polymerase chain reaction (PCR) using primers to detect mutations in these genes [Reference Ubukata9, Reference Ubukata11]. The prevalence of PRSP possessing the three abnormal pbp genes currently exceeds 50% in Japan [Reference Chiba12].
Given this background, therapeutic choices for patients with invasive pneumococcal disease (IPD) in Japan have been gradually eroded. A carbapenem antibiotic such as panipenem (PAM), which has been used only in Japan, Korea, and China, was administered in preference to intravenous third-generation cephalosporins such as cefotaxime (CTX) and ceftriaxone (CRO). Additionally, rapid increases in numbers of adults and elderly persons with various underlying diseases, is thought to increase the threat of IPD.
A heptavalent pneumococcal conjugate vaccine (PCV7) for children has been introduced in many countries [Reference Reinert13], beginning with the USA [14]. This vaccine has been reported to contribute to a decrease in IPD when causative strains are covered [15–Reference Black18]. In contrast, IPD caused by non-PCV7 serotypes of S. pneumoniae, such as 19A, continues to increase [Reference Pelton19–21]. As a result, a second-generation pneumococcal conjugate vaccine such as PCV13 is now being developed to cover a wider range of serotypes.
We therefore focused on understanding the serotype distribution and antibiotic susceptibility of isolates from IPD in children and adults throughout Japan, where clinical trials of PCV7 for children have been concluded and approval is expected. Here we describe the serotype distribution and antibiotic susceptibility of the isolates according to their pbp genotype by PCR. We also extrapolate from the data the expected PCV7 and PCV13 coverage rates for children and those of PPV23 and PCV13 for adults.
MATERIALS AND METHODS
We examined 496 S. pneumoniae isolates from patients with IPD [Reference Ubukata22]. Isolates were cultured from clinical samples processed in the laboratories of 186 medical institutions from August 2006 to July 2007 throughout Japan and then sent to our laboratory with an anonymous application form written by the reporting doctor. All isolates were from normally sterile samples such as cerebrospinal fluid (CSF), blood, or pleural or joint fluid.
Haematological tests in IPD patients
To statistically determine risk factors in adults, we requested an anonymous report including patient's age, disease presentation, underlying disease, white blood cell count (WBC), C-reactive protein (CRP), and platelet count (PLT); and outcome, including presence or absence of neurological sequelae.
Serotype and antimicrobial susceptibility
Serotypes of all S. pneumoniae isolates were determined by the capsule swelling reaction using antiserum purchased from the Statens Serum Institute (Denmark) [Reference Ubukata23]. Minimal inhibitory concentrations (MICs) of penicillin (PEN), ampicillin (AMP), cefotaxime (CTX), meropenem (MEM) and vancomycin (VAN) were determined by agar dilution methods using Muller–Hinton II agar (MH; Becton Dickinson, USA) supplemented with 5% defibrinated sheep blood [24]. S. pneumoniae ATCC49619 was used as a quality control strain.
Genotypic identification of resistance by PCR
To confirm that isolates were S. pneumoniae, the lytA gene encoding the autolysin enzyme specific to S. pneumoniae [Reference Garcia25] was amplified simultaneously with the three PBP genes. Each primer set used for detection of the three PBP genes was designed to amplify a part of the normal pbp1a, pbp2x, and pbp2b genes detected only in susceptible strains [Reference Ubukata9]. Portions of each gene corresponding to the primers were positioned in blocks of highly divergent sequences within or near conserved amino-acid motifs. Each reaction tube for PCR contained two primer sets, for detecting lytA and pbp1a in tube A; pbp2x and pbp2b in tube B; and mef(A) and erm(B) in tube C. These tubes contained 30 μl reaction mixture as previously described [Reference Ubukata9, Reference Ubukata22, Reference Nagai26].
One colony was chosen from sheep blood agar and suspended in 30 μl lysis solution [Reference Ubukata11]. The tube then was placed in a thermal cycler (Gene Amp PCR System 9600R; PerkinElmer Cetus, USA) and heat-treated for 10 min at 60°C and for 5 min at 94°C to obtain template DNA. Next, 2 μl template DNA was added to each of the three tubes marked A, B, and C containing 30 μl reaction mixture. PCR cycling conditions consisted of 30 cycles at 94°C for 15 s, 53°C for 15 s, and 72°C for 15 s and amplified using a Takara PCR Thermal Cycler (Model TP600; Takara Bio, Japan). Amplified DNA fragments were analysed by electrophoresis on a 3% agarose gel. In the presence of all three DNA fragments corresponding to pbp1a, pbp2x, and pbp2b, the PBP genes were regarded as having essentially the same sequences as the sensitive R6 strain (PEN-susceptible S. pneumoniae, PSSP). We regarded the absence of DNA fragments as indicative of sequences other than those in PSSP. Genotypic determination is indicated by adding ‘g’ to designations as follows: gPSSP, gPISP (pbp2x), gPISP (pbp2b), gPISP (pbp1a+2x), gPISP (pbp2x+2b), and gPRSP (pbp1a+2x+2b).
Pulsed-field gel electrophoresis (PFGE)
PFGE was performed using a modification of a method described previously [Reference Chiba12]. For digestion, DNA plugs were incubated in 1 ml restriction enzyme buffer with 100 U of ApaI at 37°C for 16 h. Electrophoresis was performed with a CHEF Mapper (Bio-Rad Laboratories, USA) at 5·7 V/cm at 14°C for 18 h.
RESULTS
IPD
IPD was classified into five groups as follows: septicaemia and bacteraemia (including two cases of bacterial endocarditis); pneumonia, where S. pneumoniae was isolated from blood cultures; meningitis diagnosed by clinical findings, where S. pneumoniae was isolated from CSF or blood; suppurative arthritis or osteomyelitis; and others. In 193 children aged ⩽17 years, septicaemia was predominant with 114 (59·1%) cases, followed by pneumonia with 44 (22·8%) cases, and meningitis with 30 (15·5%) cases; other diseases were rare. Almost 92% of IPD cases in children were aged ⩽4 years. In the 303 adults, septicaemia and pneumonia predominated with 115 (38·0%) cases and 112 (37·0%) cases, respectively, followed by meningitis with 57(18·8%) cases. The median age of adults with septicaemia and meningitis was 66 years, but was somewhat higher in patients with pneumonia (73 years).
Outcomes and underlying diseases
Table 1 shows outcomes and underlying diseases in 138 children (71·5% of those studied), and 195 adults (64·4%), according to reports returned by collaborating institutions. In children, 20 (14·5%) had underlying diseases, mostly congenital abnormalities. Adverse outcomes for children included death in two (1·4%) cases and neurological sequelae in four (2·9%) cases.
Table 1. Outcome based on presence or absence of underlying diseaseFootnote *
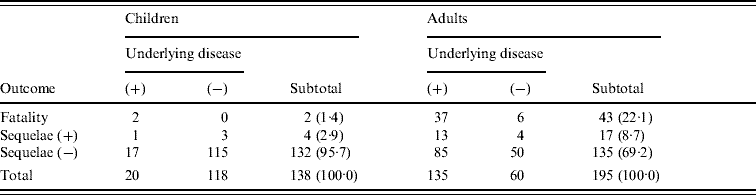
* Patients with unknown status concerning underlying disease and outcome were excluded from analysis.
In adults, 135 (69·2%) had underlying diseases, the most common being cancer surgery (38), diabetes (30), cardiovascular disease (18), hepatic disease (16), kidney disease (9), immunological deficiency (3), and splenectomy (2). Deaths were numerous [43 (22·1%)], but 37 of those patients had underlying diseases, and the cause of death was not considered in detail. The median hospital stay in adults who did not survive was 2 days. Seventeen patients, including 13 with underlying disease, had severe neurological sequelae. When outcomes in cases with underlying diseases and those without underlying diseases were compared separately for children and adults, the mortality and sequelae rates were statistically higher in both children and adults having underlying diseases (Fisher's test: children, P=0·0395; adults, P=0·0043).
Haematological findings and outcomes in adults
We compared WBC, CRP, and PLT at time of admission between the non-surviving and surviving adults. Analysis was carried out using a non-parametric Kruskal–Wallis test and the results are shown in Table 2. The median WBC in non-survivors and survivors was 5·1×109 and 13·2×109 cells/l, respectively; the odds ratio between patients with WBC below and above 5·0×109 cells/l was calculated as 7·64. A clear difference in the PLT was also noted between the two groups; and the odds ratio for mortality between patients with PLT below and above 130×109 cells/l was 4·15. No significant difference in CRP was evident between non-survivors and survivors. In addition, no significant difference in resistance type of gPSSP, gPISP, and gPRSP or in serotype (PPV23) was found between the non-survivors and survivors (P=0·1200, P=0·9891, respectively).
Table 2. Clinical laboratory findings associated with fatal outcome in adults with invasive pneumococcal disease
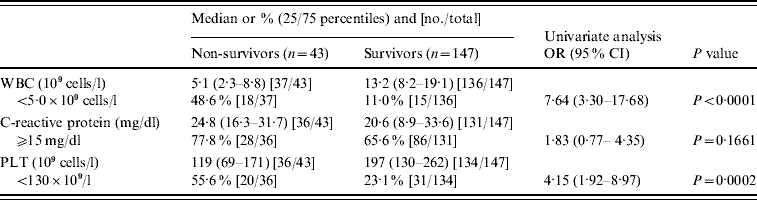
OR, Odds ratio; CI, confidence interval; WBC, white blood cell count; PLT, platelet count.
PBP gene alterations and β-lactam susceptibility
Table 3 shows results of MIC90 of PEN, AMP, CTX, MEM, and VAN. Genotype was based on PCR results for the pbp1a, pbp2x, and pbp2b genes. PEN susceptibility declined according to addition of altered pbp genes, from a MIC90 of 0·063 μg/ml for gPISP (pbp2x) to 2 μg/ml for gPRSP (pbp1a+2x+2b). In particular, susceptibility to CTX was affected by alterations of pbp2x, a pattern markedly different from that of susceptibility to PEN. In contrast, although susceptibility to MEM was affected by the gene alterations, the effect was much less. The MIC90 of VAN for all S. pneumoniae strains was 0·5 μg/ml.
Table 3. MIC90 and resistance genes identified by PCR in S. pneumoniae
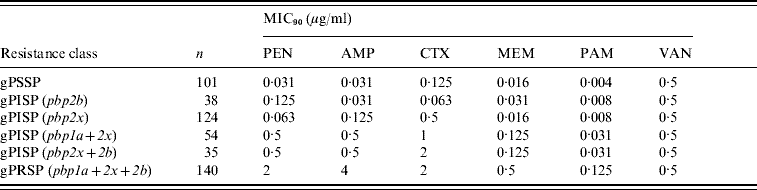
Each pbp gene alteration detected by PCR appears within parentheses.
MICs were determined for the following antibiotics: PEN, penicillin; AMP, ampicillin; CTX, cefotaxime; MEM, meropenem; PAM, panipenem; VAN, vancomycin.
Strains tested MICs: 492 isolates grown on sheep blood agar plate from stock at −80°C.
Relationship between serotype and resistance genotype for β-lactams
The serotypes of S. pneumoniae isolates from children, classified as either PCV7 or non-PCV7 types, in decreasing order of prevalence are shown in Figure 1 and the percentage rate of resistance genotypes for β-lactams is also given for each serotype. Serotype 6B predominated in the PCV7 types, followed in order by 19F, 14, and 23F. Coverage by PCV7, to which types 9V, 4, and 18C were added, was calculated as 75·4%. PCV7 covered types 6B, 19F, 14 and 23F, all of which showed high rates of gPRSP. In addition, coverage by PCV13 was calculated as 93·7%. The resistance rate of gPRSP (pbp1a+2x+2b) was highest, at 46·1%, followed by gPISP (pbp2x) at 19·9%, gPISP (pbp1a+2x) at 12·6%, gPISP (pbp2x+2b) at 6·3%, and gPISP (pbp2b) at 1·0%. The rate of gPSSP was only 14·1%.
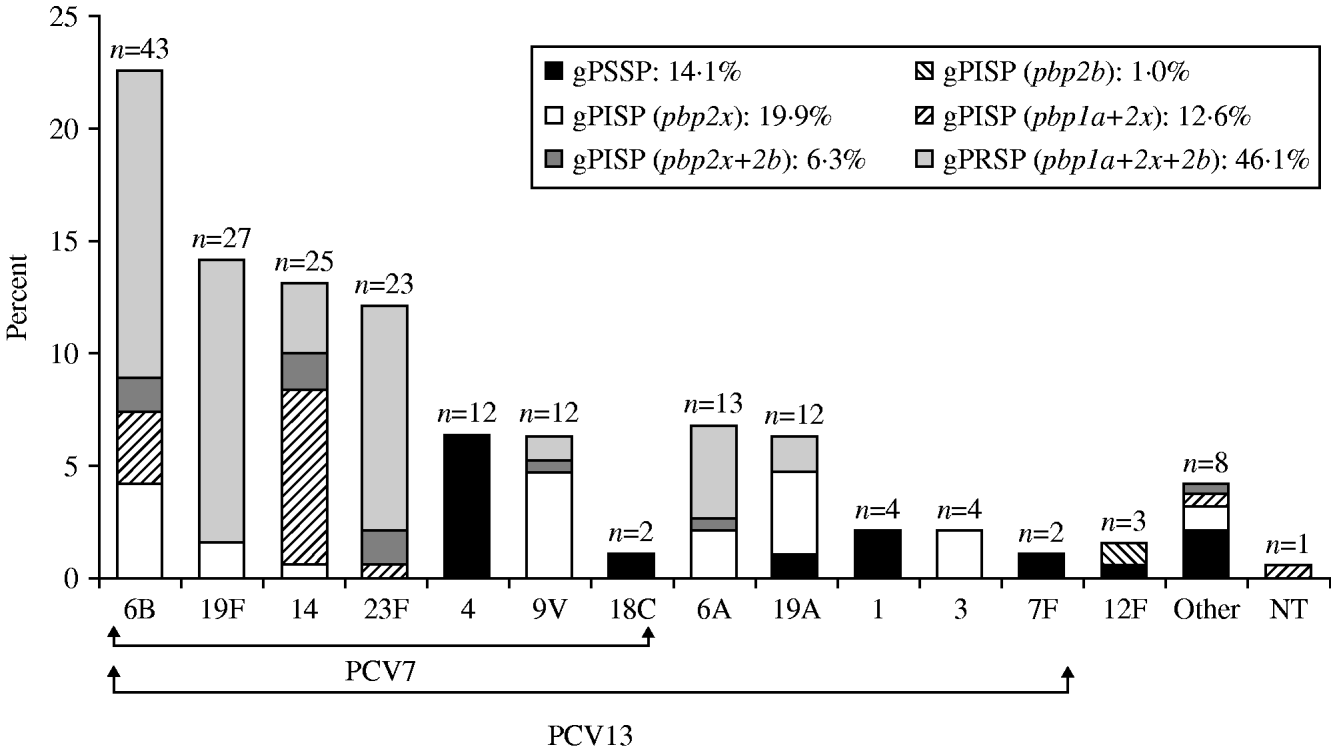
Fig. 1. Serotype distribution and resistance genes identified by PCR in S. pneumoniae isolated from children. ‘Other’ category includes serotypes 15B, 23A, 8, 24, 34, 35, and 38.
The serotypes of S. pneumoniae isolates from adults that were covered by PPV23 are shown in Figure 2, in decreasing order of prevalence. These results differed markedly from those for children. The most prevalent type, 12F, accounted for 14·3% of the total; interestingly, almost all had gPISP (pbp2b). Serotype 3 (11·3%), with a high incidence of gPISP (pbp2x), was second only to 12F. Other common serotypes were, type 6B (10·3%), with a high frequency of gPRSP (pbp1a+2x+2b), while type 14 (7·6%) showed a high frequency of gPISP (pbp1a+2x). PPV23 and PCV13 provided coverage in 85·4% and 61·5%, respectively. Non-survivors and patients with sequelae had developed IPD involving strains of various serotypes. The predominant resistance genotype in adults was gPISP (pbp2x) at 28·6%, followed by gPSSP at 24·6%, gPRSP (pbp1a+2x+2b) at 17·3%, gPISP (pbp2b) at 12·0%, gPISP (pbp1a+2x) at 10·0%, and gPISP (pbp2x+2b) at 7·6%. The serotype and the resistance genotype of strains differed significantly between children and adults (both P<0·0001).
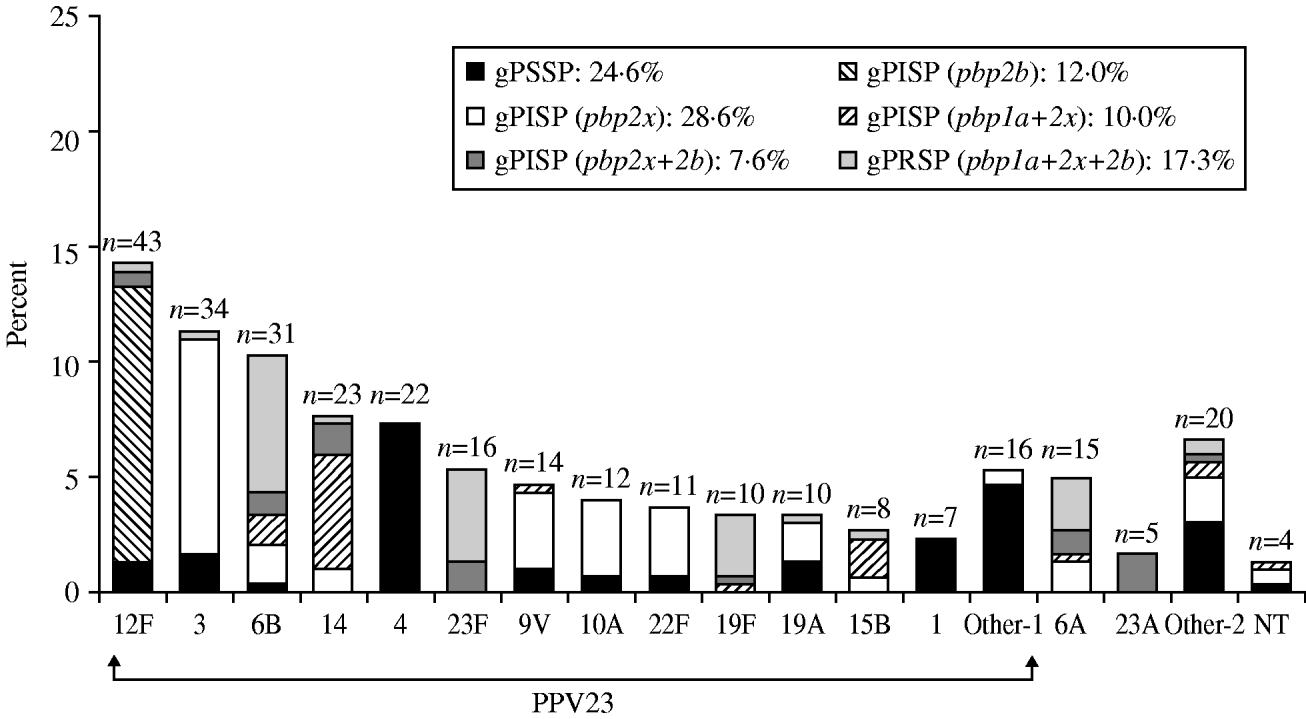
Fig. 2. Serotype distribution and resistance genes identified by PCR in S. pneumoniae isolated from adults. ‘Other-1’ category includes serotypes 9N, 11A, 33, 18C, 20, 2, 7F, 8. ‘Other-2’ category includes serotypes 35, 7C, 15A, 38, 15C, 31, 16, and 36.
PFGE pattern of strains serotyped 12F
Figure 3 shows PFGE patterns of ApaI DNA digests of serotype 12F strains. The 27 strains pictured, namely five gPSSP, two gPISP (pbp2x+2b), one gPRSP, and 19 gPISP (pbp2b), were selected randomly from 38 strains which were isolated from patients throughout Japan. DNA restriction patterns of strains with the same resistance genotype were homogeneous, suggesting that S. pneumoniae strains possessing the same pbp alterations had spread widely. There has been a rapid increase in the prevalence of serotype 12 in Japan and this serotype is present in 18% of cases with a poor prognosis in adults. This increase is therefore considered to be of clinical significance.

Fig. 3. PFGE patterns of ApaI digests of chromosomal DNA from serotype 12F isolates. A, gPISP (pbp2b) (lanes 1–19); B, gPSSP (lanes 20–24); C, gPISP (pbp2x+2b) (lanes 25, 26); D, gPRSP (pbp1a+2x+2b) (lane 27).
DISCUSSION
S. pneumoniae is a major causative agent of diseases such as pneumonia, meningitis, and acute otitis media (AOM), as well as various other serious invasive infections. In the USA, the PCV7 vaccine was developed for children and approved in 2000, and has been incorporated into the paediatric vaccination schedule [14]. Immunization programmes using PCV7 have spread widely, and are presently conducted in almost 70 countries worldwide [Reference Center27]. The incidence of IPD involving vaccine-type S. pneumoniae has been reported to have decreased significantly [15, Reference Poehling17, Reference Black18], and a related decrease in IPD in adults has been noted [Reference Lexau16]. However, the incidence of IPD caused by non-vaccine-type S. pneumoniae has increased; particularly type 19A [Reference Pelton19–21]. In order to provide increased coverage, a new vaccine, PCV13, is being developed, which will include types 19A, 6A, and 3 [Reference Scott28].
Much clinical attention has been drawn to a rapid increase in PRSP in S. pneumoniae isolates. These strains have been causative agents of paediatric AOM [Reference Yamanaka, Hotomi and Billal29] and meningitis [Reference Ubukata22] in Japan since 1990 and this increase is strongly related to a shift from prescribing oral penicillins for outpatients to using oral cephalosporins. The increase may also be related to use of macrolides, considering that most PRSP are multidrug-resistant S. pneumoniae (MDRSP) also resistant to macrolides [Reference Ubukata, Iwata and Sunakawa30]. In addition, Japan's high population density tends to accelerate increases in resistant organisms.
We previously compared pbp gene alterations in S. pneumoniae strains that had been isolated in the same time period from the USA and Japan [Reference Ubukata10]. In the USA, where use of penicillins predominated, increases were evident in resistant strains with the pbp2b gene alteration whereas in Japan, where cephalosporins predominated, many strains characteristically had the pbp2x gene alteration. As shown in this study, the latter pattern still persists in Japan.
According to USA guidelines [Reference Tunkel31], the use of third-generation cephalosporins – CTX, CRO, or either of these in combination with VAN – is recommended for meningitis caused by PRSP. In Japan, however, carbapenems such as PAM and MEM are recommended as first-choice antibiotics in this situation. A major reason for this practice is that 60% of Japanese paediatric meningitis cases are caused by Haemophilus influenzae type b (Hib), of which about 36·2% show resistance to AMP and CTX, reflecting β-lactamase non-producing and AMP-resistant H. influenzae as the causative pathogens [Reference Hasegawa32]. Therefore, in Japan, the preferred paediatric treatment increasingly involves concomitant use of a carbapenem, with its superior bactericidal effect against S. pneumoniae, plus CTX or CRO, with superior activity against H. influenzae; treatment now is basically the same for adults.
As for vaccines against S. pneumoniae, PPV23 has been introduced in Japan, where it is used mainly on a voluntary basis for elderly people as well as adults and children with underlying diseases. The PCV7 vaccine is currently under review by the Japanese Ministry of Health, Labour and Welfare, and approval is expected soon. Nevertheless, one needs to know the extent to which PCV7 covers IPD. According to our epidemiological surveillance in the current study, PCV7 covers 75·4% of strains isolated from children with IPD. However, the incidence of types 6A and 19A, which are non-vaccine types, is significant, so the introduction of PCV13 will be beneficial.
In Japan, a recent rapid increase in IPD in adults may reflect the rapid ageing of society and an increase of lifestyle-related diseases in the adult population. The current situation whereby PPV23 vaccination is voluntary, limits its effectiveness against this increase. Development of disease caused by S. pneumoniae in adults with underlying disease often triggers disseminated intravascular coagulation (DIC), leading to death or serious sequelae for which the prognosis is extremely poor. Also of concern is the poor prognosis for adults who develop IPD caused by S. pneumoniae with intermediate PEN resistance. In addition, serotype 12F was very rare in 2000, but in the current study accounted for 12·0% of IPD cases and strains show essentially the same PFGE pattern as gPISP (pbp2b). The reason why this type of S. pneumoniae has increased so rapidly in adults is unknown, and requires further investigation. Finally, but importantly, the impact of the forthcoming introduction of PCV7 will need to be assessed by continued epidemiological surveillance of IPD throughout Japan.
ACKNOWLEDGEMENTS
We deeply thank all the collaborators for their cooperation. This work was supported by grants for a ‘Research Project for Emerging and Re-emerging Infectious Diseases’ (H-19-002 and H-20-002) from the Japanese Ministry of Health, Labour and Welfare.
DECLARATION OF INTEREST
None.








