The production of enteric methane (CH4) is considered an undesired (but partially unavoidable) side-effect of the fermentation of plant material by micro-organisms present in the gastrointestinal tract (GIT) of herbivores. It represents a loss of energy to the animal (2–12 %)( Reference Johnson and Johnson 1 ), and contributes substantially to the greenhouse effect( 2 ). In ruminants, the production of CH4 is the last step of the fermentation process and is carried out by methanogenic Archaea (methanogens). These methanogens utilise H2 as an energy source to reduce carbon dioxide to CH4 ( Reference Moss, Jouany and Newbold 3 ). This is the predominant way of H2 utilisation, despite the presence of alternative, more energy-efficient H2 sinks in the ruminant forestomach, such as acetogens( Reference Joblin 4 ) or propionic acid production( Reference Russell 5 ).
Previous studies have suggested that a reduced residence time of ingested plant material in the digestive tract (measured as mean retention time (MRT)) is associated with lower CH4 emission (CH4exhal) in ruminants( Reference Pinares-Patiño, Ulyatt and Lassey 6 – Reference Goopy, Donaldson and Hegarty 10 ), ratites( Reference Frei, Hatt and Ortmann 11 ) and non-ruminant foregut fermenters( Reference Vendl, Frei and Dittmann 12 , Reference Vendl, Frei and Dittmann 13 ). This has also been reflected in models for the prediction of ruminant enteric CH4exhal that included MRT (or passage rate) as a predictive factor( Reference Janssen 14 , Reference Huhtanen, Ramin and Cabezas-Garcia 15 ). In addition, a lower CH4exhal was measured in steers (Bos taurus domesticus) after the placement of weights in their reticulum, which decreased MRT( Reference Okine, Mathison and Hardin 16 ). It has been shown that increased MRT of digesta is associated with increased CH4exhal in humans( Reference Attaluri, Jackson and Valestin 17 , Reference Soares, Lederman and Fagundes-Neto 18 ). The CH4 breath test has been used as a diagnostic aid in the investigation of ‘irritable bowel syndrome’, where patients with increased exhaled CH4 are ascribed to the ‘constipation type’ of the syndrome( Reference Chatterjee, Park and Low 19 , Reference Kunkel, Basseri and Makhani 20 ); however, see Di Stefano et al.( Reference Di Stefano, Mengoli and Bergonzi 21 ).
Although these studies suggest a causative relationship – that is, a reduction in CH4exhal due to a reduced MRT – the question whether the presence of CH4 itself influences digestive physiology, particularly factors affecting MRT such as gut motility, has received less attention. The presence of CH4 in the GIT was found to modulate peristalsis of the small intestine, inducing augmented contractile activity in the guinea pig (Cavia porcellus) ileum in vitro ( Reference Pimentel, Lin and Enayati 22 , Reference Jahng, Jung and Choi 23 ). It also apparently delayed intestinal transit of digesta through the jejunum of fistulated dogs (Canis lupus familiaris)( Reference Pimentel, Lin and Enayati 22 ). However, added CH4 had no effect on the guinea pig colon in vitro ( Reference Jahng, Jung and Choi 23 ). The overall interpretation in these studies is that CH4 in the GIT slows down intestinal transit. In humans, treatment with antibiotics decreased the concentration of CH4 in the breath (putatively due to eliminating methanogens) and alleviated obstipation-type irritable bowel syndrome exhibited in patients who produced CH4 before antibiotic administration( Reference Pimentel, Chatterjee and Chow 24 ). In ruminants, CH4exhal was reduced in sheep (Ovis aries) fed nitrate (an alternative H2-acceptor), and there was an associated reduction of fluid retention in the reticulorumen (RR)( Reference Nolan, Hegarty and Hegarty 25 ). In contrast, no changes were apparent in rumen retention times when reducing CH4exhal in cattle using chloroform( Reference Knight, Ronimus and Dey 26 ).
On the basis of all these reports, our objective was to determine the effects of CH4 insufflation (iCH4) or CH4 inhibition on digesta kinetics in non-lactating dairy cows. We hypothesised that the level of CH4 within the ruminant GIT has an effect on gut peristalsis, motility, digesta propulsion and consequently MRT in ruminants. It was expected that an increased presence of CH4 would delay passage from both the RR and the GIT. Such a relationship could indicate an evolutionary scenario in which the presence of methanogens (and therefore CH4) in the ruminant digestive tract increases MRT, and hence contributes to the well-documented physiological adaptations of ruminants that enhance their ability to utilise a fibrous diet by giving microbes more time to degrade fibrous nutrients.
Methods
Experimental design
This experiment was conducted at the University of Reading’s Centre for Dairy Research (CEDAR), UK. All procedures were licenced and monitored by the UK Home Office Animals (Scientific Procedures) Act 1986. In total, four rumen-fistulated, non-lactating, non-pregnant Holstein dairy cows were used in a 4×4 Latin square design experiment with 28-d periods, commencing with 7 d of individual tie-stall housing for adaptation to treatments (d 1–7), a 7-d measurement period (d 8–14), followed by a 14-d recovery period (d 15–28) with free-stall housing. The four treatments were (i) control (C), ruminal insufflation (mean 295 (sd 82) litre/d) of either (ii) CH4 gas (iCH4) or (iii) N2 gas (iN2) via the fistula and (iv) reduction of CH4 production via ruminal administration of bromochloromethane (BCM). Measurements during d 8–14 included individual DM intake (DMI), apparent total tract digestibility of feed components, digesta MRT, rumen pH, rumen motility, rumination and chewing activity, breath CH4exhal (determined three times daily using a GreenFeed unit (C-Lock)) and CH4 concentration in rumen fluid (CH4RRf).
Animals and housing
Animals aged 6–13 years, with an initial mean body mass (BM) of 712 (sd 81) kg, were surgically fitted with a rumen cannula (type #1C, 100-mm centre diameter; Bar Diamond Inc.) during a previous lactation. Cannulae plugs were fitted with airtight ports that allowed the placement of infusion lines or motility sensors into the rumen.
During adaptation and measurement periods (d 1–14), cows were kept in individual tie stalls and DMI was measured. Cows were bedded on rubber mats with a layer of wood shavings that was replenished twice daily. During the recovery period (d 15–28), cows were group housed in a cubicle yard with straw bedding. On d 1 of the recovery period, approximately 20 kg of the RR contents from the animal that had received the BCM treatment was removed via the fistula and replaced with a corresponding amount of RR content from the other three animals that had not received the BCM treatment (approximately 7 kg from each cow) to minimise carry-over effects of BCM.
Cows were fed the same diet for the entire experimental period consisting of chopped timothy (Phleum pratense L.) hay from an external supplier, a limited amount of a commercial pellet (Super Rearer 18 Nuts; BOCM Pauls Ltd)( Reference Hammond, Humphries and Crompton 27 ) at 863 (sd 6) g DM/cow per d and daily 100 g/cow of a mineral mixture (CW FA super dry cow; Countrywide Farmers); see Table 1 for nutrient composition of the ingredients and diets. The low crude protein (CP) content of the hay had not been intended but was confirmed in analyses of multiple sample replicates. Because of this low CP content, the ingested diets had CP levels of 54–56 g/kg DM (Table 1), which is below the recommendations for dry cows( 28 ). We had intended to restrict cows to intakes of 1·2×metabolisable energy (ME) requirements for maintenance( Reference Thomas 29 ) estimated on the basis of the supplier’s analysis of the hay, in order to minimise excessive weight gain and feed refusals. However, because of the large variation in intake displayed from the very beginning (with refusals ranging from 0·3 to 1·6 kg DM of hay/cow per d, possibly due to the low CP content of the hay), hay was offered ad libitum from the onset of the study. During the adaptation and measurement periods, cows were fed three times daily at 09.00, 15.00 and 21.00 hours, and during the recovery periods cows were fed once daily.
Table 1 Mean nutrient composition (g/kg DM) of the diets offered to the cows over the four experimental periods and of the respective consumed diets per treatment
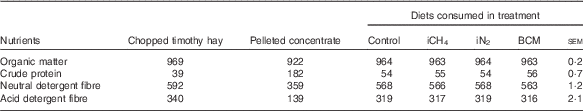
iCH4, methane insufflation; iN2, nitrogen insufflation; BCM bromochloromethane.
Insufflation treatments
Gaseous CH4 (treatment ii: iCH4) was insufflated continuously from an external bottle into the ventral rumen via the fistula. As a control for gaseous CH4, gaseous N2 (treatment iii: iN2) was insufflated using the same set-up as for iCH4. Both CH4 and N2 were insufflated at an average rate of 295 (sd 82) litre/d (205 (sd 57) ml/min; 276 (sd 31) litre/d for iCH4 and 313 (sd 37) litre/d for iN2), which was intended to double the amount of enteric CH4 gas produced from non-lactating dairy cows( Reference Hammond, Humphries and Crompton 30 ).
To control insufflation rates and to ensure that they were similar for iCH4 and iN2 treatments, CH4 and N2 gas cylinders (both British Oxygen Company; 99·5 % purity) were placed on electronic balances (GFK 150H; Adam Equipment). From the cylinders, gases flowed via flexible gas-tight tubing (PFA Flexible Tubing) to peristaltic pumps (LA 1 P; Omicron) and then through flow metres (GEC Marconi). From the peristaltic pumps, CH4 and N2 were insufflated into the rumen via tubes leading through the fistula. A chromium steel weight of about 100 g was fitted to the end of each insufflation tube for placement in the ventral rumen. The insufflation tubes used ended in a perforated coil covered with a nylon bag to prevent clogging. This solution was chosen on the basis of an in vitro pilot study during which aquarium bubble stones had been tested. The stones had already partly dissolved after 24 h in the rumen fluid, and digesta particles had clogged most holes, leading to bubbles rising from a single opening. All tubing and fittings were airtight and regularly checked for leaks. On the basis of bottle weight changes, masses of CH4 and N2 administered were recorded at least twice daily.
Methane-reduction treatment
To reduce CH4exhal, a dose of 0·45 g BCM/100 kg BM was administered directly into the rumen twice daily via the fistula (i.e., a total daily dose of 0·9 g/100 kg)( Reference Tomkins, Colegate and Hunter 31 ), at 08.00 and at 17.00 hours (treatment iv: BCM). The inhibitor compound was prepared by entrapping BCM in an α-cyclodextrin matrix( Reference May, Payne and Stewart 32 ), which was dissolved in a syringe containing 120 ml of warm water immediately before administration into the rumen via a silicone tube through the fistula. Before this study, the efficacy of the BCM compound was confirmed in vitro by the Hohenheim gas test( Reference Menke, Raab and Salewski 33 ) (data not shown).
Body mass, feed intake and whole-tract digestibility
Cows were weighed at the start and end of each treatment period. Feeds offered and refused as well as faeces were collected and weighed daily for 5 d from d 8 to 12 of each measurement period. Pooled composites from each individual cow for each measurement period were collected and frozen at −20°C before being thawed and sub-sampled for further analyses and determination of whole-tract digestibility. Total daily collection, sampling and processing of faeces were performed using previously described methods( Reference Reynolds, Humphries and Kirton 34 ).
Digesta kinetics and rumen pH
To measure MRT of particles and fluid, the following markers were used: three different-sized particle markers based on fibre from grass hay mordanted with Cr (<2 mm), La (5 mm) and Ce (8 mm) as particle markers and the water-soluble Co-EDTA( Reference Udén, Colucci and Van Soest 35 , Reference Schwarm, Ortmann and Wolf 36 ). For 3 d before administration of markers, 1 faecal sample/d and a single rumen fluid sample on the last of these days were collected to determine baseline marker concentrations for each animal. Markers were administered into the RR at 08.00 hours on d 8 via the fistula, where each cow received 7 g Co-EDTA and 70 g of each particle marker, soaked in warm water. After marker administration, a sample of faeces (at least 10 % of the total amount defaecated in the interval) was collected every 1–3 h on d 8, every 4 h on d 9, every 6 h on d 10, every 8 h on d 11–13 and every 12 h on d 14. Faecal samples were oven-dried immediately at 60°C for 48 h. In addition, rumen fluid was sampled every 1–3 h for 24 h after marker administration to determine the decline in Co concentration in the rumen. Rumen fluid samples (40 ml) were collected from the ventral sac via aspiration through a coarse filtered tube inserted vertically and approximately 40 cm into the rumen mat directly below the rumen fistula. Each rumen fluid sample was mixed gently, and pH was measured immediately (pH meter: HI2210; Hanna Instruments) before it was stored frozen at −20°C for analysis of Co concentration.
Rumen motility
Contractions of the RR were measured by a system developed by the Physiological Institute of the University of Veterinary Medicine Hannover (Hannover, Germany). Nitrile rubber balloons of approximately 7-cm diameter were attached to a pressure sensor via flexible tubing and placed in the dorsal part of the rumen. Rumen contractions were recorded continuously for approximately 8 h on d 4 or 5 of the measurement period. Balloons were placed in the RR only for the duration of these measurements. Data from the motility sensors were analysed using software developed by Itin+Hoch GmbH (available from the corresponding author) for the frequency of contractions, the length of individual contractions as well as the interval between contractions. Primary and secondary contractions could not be differentiated by this method.
Chewing activity
Jaw movement of the cows was recorded from d 8 to 13 using noseband sensors (RumiWatchSystem; Itin+Hoch GmbH)( Reference Zehner, Niederhauser and Nydegger 37 ). Data from the noseband sensors were analysed using RumiWatch Converter software (Itin+Hoch GmbH) to determine time spent ruminating and eating, the number of regurgitated boli per day as well as the chewing frequency during eating and rumination.
Methane levels in breath and rumen fluid
CH4exhal in the breath of cows (exhaled CH4) was measured three times daily at approximately 09.30, 13.00 and 16.30 hours from d 10 to 12 using a GreenFeed unit( Reference Hammond, Waghorn and Hegarty 38 ). The GreenFeed unit was mounted on wheels, which allowed positioning in the feed manger of each cow, where it was left in place long enough to allow each cow to consume a proportion of the daily allotment of pellets and obtain a CH4 measurement. The average rate of CH4exhal (g/min) from the three daily readings was converted into an emission rate per day, and the results are expressed as estimated daily CH4 production (litre/d) and yield (litre/kg DMI).
Ruminal CH4 concentrations were determined from rumen fluid samples collected once daily (at 11.00 hours) from d 9–11( Reference Reynolds, Crompton and Barratt 39 ). Samples of centrifuged rumen fluid (2 ml) were maintained in anaerobic conditions and added to an equal volume of lactic acid (13 m) in an evacuated headspace crimp top vial (22 ml; Perkin Elmer) and thoroughly mixed. The vials were returned to atmospheric pressure with N2, and the headspace was analysed for CH4 concentration using GC (Clarus 500; Perkin Elmer), fitted with a megabore capillary Elite PLOT Q column (Perkin Elmer) and a flame ionisation detector set at 350°C. A bracketed calibration using five gas standards was used with each batch of samples, and calibration samples of known concentration were included at regular intervals within each sample run. The concentration of CH4 liberated from samples was proportional and linear over the range of 0·5–3·0 ml of added rumen fluid.
Sample analyses
Pooled composite samples for digestibility measurements of feed offered, refused feed and faeces were analysed( 40 ) for DM and total ash (Association of Official Analytical Chemists (AOAC) no. 942.05), CP (AOAC no. 977.02), neutral detergent fibre (NDF, AOAC no. 2002.04 using α-amylase) and acid detergent fibre (ADF, AOAC no. 973.18). All fibre values were corrected for residual ash content, and all analyses were performed in duplicate.
Concentrations of Co, Cr, La and Ce in faecal and rumen fluid samples were analysed( Reference Frei, Ortmann and Reutlinger 41 ) after wet ashing with 4 ml nitric acid and 2 ml hydrogen peroxide in a microwave oven. The temperature was increased over 15 min to 170°C and over 20 min to 200°C, and then held at 200°C for 5 min. The wave-length was 12·25 cm, and the frequency was 2·45 GHz. Concentrations of Co, Cr, La and Ce in the samples were determined using an inductively coupled plasma optical emission spectrometer (model Optima 8000; Perkin Elmer). Co concentration from the rumen fluid sampled through the fistula was determined as described above, but without wet ashing.
Digesta kinetics calculations
RR liquid volume and MRT of solutes in the RR (MRTsoluteRR) were calculated from marker concentrations in rumen samples. All other MRT measures (for both GIT and RR) were obtained from faecal marker concentrations, including a second additional measure for MRTsoluteRR. The RR liquid volume was estimated by dividing the amount of Co-marker administered by the slope of the regression of ln-transformed Co concentrations over time( Reference Shipley and Clark 42 ). The MRT of digesta phases represented by the four markers (solutes, small, intermediate and large particles) were determined by a multi-compartmental model, using equation 8 of Dhanoa et al.( Reference Dhanoa, Siddons and France 43 ) for curve fitting and equation 12 of that publication for MRT calculation. Values were corrected for individual baseline concentrations (taken before the marker application). Values <1 % of the maximum concentration of a marker in the excretion curve were set to 0 to avoid an artificial increase in MRT by infinite excretion curves due to variation in baseline concentrations( Reference Bruining and Bosch 44 ).
Statistical analysis
The relative DMI (rDMI) was expressed per kg BM0·85 ( Reference Hackmann and Spain 45 , Reference Müller, Codron and Meloro 46 ). As rDMI typically shows a good correlation with MRT measures( Reference Clauss, Steuer and Erlinghagen-Lückerath 47 ), this measure is particularly suitable to control for the effect of feed intake across different-sized individuals. Data were averaged for each cow and treatment, and the effect of treatments was determined via mixed-model procedures that included treatment and treatment period (periods 1–4) as fixed factors, cow as a random factor and rDMI as a covariate. All statistical tests were carried out in R 3.0.2( 48 ) using the function lme from the package nlme, followed by the function drop1 to determine significances of the fixed factors by stepwise exclusion. As treatments had different effects on CH4exhal yield and CH4RRf, the same models (with rDMI as covariate) were additionally applied with exhaled CH4 yield (litre/kg DMI) or CH4 in rumen fluid (µg/ml) as covariates instead of treatment as a fixed factor. In addition, means of measures were compared between treatments using paired t tests with Holm–Bonferroni adjustment for multiple testing. Significance levels were set to P<0·05.
Results
Effects on intake, body mass change and methane measurements
Absolute DMI varied from 7·1 to 8·4 kg/d. There was an influence of treatment on rDMI (P=0·024), which was lowest for cows on the BCM treatment and highest for the control treatment (Table 2). Exhaled CH4 was affected by treatment (P<0·001) (Table 2). Compared with the control, exhaled CH4 yield (litre/kg DMI) was reduced on average by 82 % with BCM treatment (P<0·001), increased on average by 78 % with iCH4 treatment (P<0·001) and unaffected by iN2 treatment (P=0·96). CH4 in rumen fluid was similar across treatments, except for BCM treatment, which was lower than all other treatments (P<0·001) (Table 2), representing a reduction of 99 %.
Table 2 Treatment means and effect of treatments (Trx) and relative feed DM intake (rDMI) on methane (CH4) and measurements of digestive physiology

iCH4, methane insufflation; iN2, nitrogen insufflation; BM, body mass; BCM, bromochloromethane; DMI, DM intake; GIT, gastrointestinal tract; RR, reticulorumen; RUM, during rumination.
a,b,cUnlike superscript letters were significantly different (paired t test with Holm–Bonferroni adjustment; P<0·05) between treatments.
Effects on digesta kinetics
Both in the GIT and the RR, the MRT increased from the solute to the small, to the intermediate and to the large particle marker (P<0·001 in all cases). Treatment had an influence on both solute and particle MRT in the GIT (P≤0·045), but not in the RR (P≥0·16) (as determined either directly via rumen samples or indirectly via faeces) (Table 2). For MRT2 mmGIT and MRT5 mmGIT, there were trends for an increased MRT for the BCM treatment when compared with the control in pairwise comparisons (MRT2mmGIT: unadjusted P=0·022; MRT5mmGIT: unadjusted P=0·073). There was a negative influence of rDMI on particle MRT measurements in the GIT (P≤0·003) and RR (P≤0·052, with only a trend in MRT2mmRR) (Table 2). The MRTsoluteGIT and MRTsoluteRR measured via faeces were not influenced by rDMI, whereas the MRTsoluteRR measured via rumen samples was negatively influenced by rDMI (P=0·016), indicating a divergence between the two values (Table 2).
When exhaled CH4 yield (litre/kg DMI) was used as the covariate instead of treatment, there was a tendency for a negative effect on all MRT measures (P≤0·059), including those for the RR, except for MRTsoluteRR (from both faeces and rumen, P≥0·33) (Table 3). Using exhaled CH4 yield as the covariate, there was a negative influence of rDMI on all MRT measurements determined from faecal samples (P≤0·029) but not for MRTsoluteRR determined directly from rumen samples (P=0·29).
Table 3 Relation of methane (CH4) levels exhaled in breath and dissolved in rumen fluid to measures of digestive physiology in mixed models that included methane and the relative feed DM intake (rDMI) as covariates

DMI, dry matter intake; GIT, gastrointestinal tract; RR, reticulorumen; RUM, during rumination.
When CH4 in rumen fluid (µg/ml) was used as the covariate, there was a negative influence on all MRT measures in the GIT (P≤0·052), but no effect in the RR (P≥0·10). Relative DMI tended to have a negative effect on all MRT measures (P≤0·095) except for MRTsoluteRR (as determined indirectly from the faeces, P=0·82) (Table 3).
Effects on apparent whole-tract digestibility
Treatment had no effect on the apparent digestibility of DM, organic matter (OM) and ADF (Table 2). Treatment had an effect on the digestibility of CP and NDF (P<0·05), which were both highest for the BCM treatment.
Using exhaled CH4 yield (litres/kg DMI) as the covariate, there was no effect on the digestibility of DM, OM or ADF, but a negative effect on the digestibility of CP and NDF was found (P≤0·028) (Table 3). Using CH4 in rumen fluid (µg/ml) as the covariate also yielded a negative effect on CP and NDF digestibility (P≤0·006), and negative trends were observed on the digestibility of DM, OM and ADF (P≤0·075) (Table 3). Relative DMI tended to negatively influence NDF digestibility (P<0·070).
Effects on rumen fluid pH, DM content of the faeces and reticuloruminal volume
Treatment did not show an influence on rumen pH, the DM content of the faeces and the liquid volume in the RR (P≥0·16) (Table 2); rDMI negatively affected rumen pH (P=0·038).
Exhaled CH4 yield (litre/kg DMI) as the covariate had no significant influence on rumen pH, the DM content of the faeces and liquid volume in the RR (Table 3); rDMI negatively influenced rumen pH and liquid volume in the RR (P=0·007).
CH4 in rumen fluid (µg/ml) as the covariate negatively affected the DM content of the faeces (P=0·013) and tended to negatively affect the liquid volume in the RR (P=0·081) (Table 3); rDMI negatively affected rumen pH and liquid volume in the RR (P≤0·043).
Effects on chewing, rumination and rumen motility
Treatment affected all measures of rumination (P≤0·041), but had no influence on the animals’ eating behaviour (time spent eating and number of chews during eating; P≥0·13) (Table 2). Animals spent more time ruminating on BCM, compared with the control (P=0·002). Treatment affected the number of regurgitated boli per hour (P=0·006) and the number of chews per bolus (P=0·004), which were both highest on BCM (Table 2).
Applying either exhaled CH4 yield (litre/kg DMI) or CH4 in rumen fluid (µg/ml) as covariates had a negative influence on all rumination measures (P≤0·042), but no effect on eating measures (P≥0·40) (Table 3).
For rumen motility measurements, both treatment and rDMI affected the number of contractions and the interval between contractions (P≤0·038), with the fewest contractions and the longest interval between contractions on the BCM treatment, but there was no effect on the length of contractions (P≥0·97). Exhaled CH4 yield (litre/kg DMI) as the covariate was positively related to the number of contractions (P=0·003) and negatively related to the interval between contractions (P=0·008); rDMI influenced the latter measurement negatively (P=0·039). CH4 in rumen fluid (µg/ml) as the covariate tended to positively affect the number of contractions (P=0·082) and negatively affect the interval between contractions (P=0·071), with no influence of rDMI (Table 3).
Discussion
The present study investigated the effects of iCH4 or rumen CH4 inhibition on the digestive physiology of non-lactating dairy cows, including measurements of intake, digesta retention times, total tract digestibility, chewing activity and rumination, and rumen motility. When comparing treatments, a reduction in CH4exhal was accompanied by a decrease in DMI, an increase in MRT in the GIT, a reduction in rumen motility and an increase in NDF digestibility, whereas iCH4 or iN2 did not result in a clear pattern. As a change in DMI alone could have explained the patterns observed on reduced CH4exhal, variation in DMI had to be accounted for in the statistical analyses. When assessing effects as responses not to treatment but to either the concentration of CH4 in rumen fluid or exhaled CH4 yield and including rDMI as a covariable in the analyses, a systematic negative association of increased CH4 with MRT was indicated. These results must be considered with caution because of the unexpectedly low CP content of the hay provided. Although differences between the four MRT markers were as expected, with increasing MRT from solute to small, intermediate and large particles( Reference Lechner, Barboza and Collins 49 , Reference Dittmann, Runge and Ortmann 50 ), the absolute difference between the small particle marker (mordanted with Cr) and the two larger particle markers (marked with La and Ce) was possibly underestimated, because for a given particle size Cr mordants are typically retained longer than lanthanide markers( Reference Schwarm, Albrecht and Ortmann 51 ).
Intra-ruminal gas insufflation
Insufflation of gas into the rumen does not necessarily affect the concentration of gas dissolved in the rumen fluid, as evident, for example, from the absence of increased concentrations of dissolved ammonia in the ventral rumen fluid after insufflation of ammonia gas( Reference Davidovich, Bartley and Bechtle 52 ). In order to affect the concentration of a gas dissolved in fluid, mechanical treatment such as shaking is required( Reference Murray, Bryant and Leng 53 ). Correspondingly, iCH4 in the present study did not increase the measured concentration of CH4RRf. Similarly, a very low effect of insufflating H2 on CH4 production had been interpreted as a consequence of an assumed incomplete dissolution of the gas in rumen fluid( Reference Olijhoek, Hellwing and Weisbjerg 54 ). The increase in estimated daily CH4exhal via exhaled air, based on 3 short-term measurements/d, over 3 d, accounted for 60 % of the iCH4 rate. Although this recovery rate was lower than that reported for the GreenFeed system when compared with other methods of measuring CH4 ( Reference Hammond, Waghorn and Hegarty 38 ), it needs to be emphasised that when using the GreenFeed system, 3 d of spot sampled CH4 measurements are insufficient to accurately reflect an animal’s daily CH4exhal rate. Further, one has to consider CH4 losses via the fistula. However, for the purpose of this study, the spot measures were simply an indication of the CH4 emitted by each animal for a set time point across treatments at set times of the day. Corroboration of our results using respiration chambers would be welcome.
Gas insufflation has previously led to an increase in rumen contractions in various studies( Reference Louvier, Colvin and Ishizaki 55 ), but its effect has typically been investigated by enforcing an increase in intra-ruminal pressure by blocking eructation for a certain period of time. Eructation was prevented in a cow and different insufflation treatments of air, CH4 or H2 had no apparent effects on rumen contraction( Reference Dougherty 56 ). Another study did not detect a difference in rumen motility between insufflations of CO2:O2 (5:95), CO2:N2 (5:95) or CO2:CH4 (60:40) mixtures in decerebrated sheep( Reference Reid and Titchen 57 ). Intra-ruminal pressure was increased in sheep by blocking the trachea, and gases containing CO2 (i.e., both exhaled air and a CO2:CH4 (60:40) mixture) stimulated more primary rumen contractions during the pressure-release phase than N2 or compressed air( Reference Louvier, Colvin and Ishizaki 55 ). In bison (whose eructations were not impeded), an increase in secondary rumen contractions from 0·5 to 1·0/min was observed when N2 was insufflated at a rate of 3–5 litre/min( Reference Dziuk 58 ). In the animals of the present study, eructations were not impeded, and the insufflation rate was about 200 ml/min. Therefore, the effect of insufflation alone due to physical distension of the RR can be considered less relevant. H2 insufflation at 800 ml/min did not affect feed intake in cattle( Reference Olijhoek, Hellwing and Weisbjerg 54 ). To our knowledge, no studies on the effect of insufflation on MRT or whole-tract digestibility in ruminants exist.
Bromochloromethane treatment
A number of previous studies have demonstrated the CH4-suppressing effect of BCM in steers, sheep and goats( Reference Tomkins, Colegate and Hunter 31 , Reference Abecia, Toral and Martín-García 59 – Reference McCrabb, Berger and Magner 64 ). The present study confirms again, in non-lactating dairy cattle, that BCM substantially reduces CH4exhal. For the present study, this treatment efficiently created conditions of low CH4 production. The previous studies mentioned above produced different results with respect to the DMI-reducing effect of BCM. No reduction in DMI was reported for steers fed diets high in concentrates( Reference Tomkins, Colegate and Hunter 31 , Reference Johnson, Wood and Stone 60 ) or sheep and goats fed diets of hay and concentrate( Reference Abecia, Toral and Martín-García 59 , Reference Sawyer, Hoover and Sniffen 61 , Reference Lalu, Bhar and Das 62 ). In contrast, there was a reduction in DMI for steers fed both low-quality and medium-quality roughage diets( Reference McCrabb, Berger and Magner 64 ), similar to the situation with the low-quality roughage fed in the present study. As average daily BM gain did not differ in the steers, this corresponded to a higher feed conversion ratio( Reference McCrabb, Berger and Magner 64 ). In the present study, BM losses were concomitantly lowest on this treatment, possibly due to the increase in ME content of the diet owing to the massively reduced CH4 loss. In saying this, a complete understanding of the mechanism needs to be achieved. This includes investigation of other potential effects of BMC such as an increased proportion of propionate produced by microbial fermentation or increased nutrient availability. In another study( Reference Abecia, Toral and Martín-García 59 ), such an increase in ME content was also considered responsible for higher milk yield in BCM-treated goats, and similar effects were observed with other CH4 inhibitors( Reference Hristov, Oh and Giallongo 65 ).
Variability of DM intake
The variation in DMI measured in our study represents a constraint on its interpretation, because all aspects of digestive physiology are known to be influenced by feed intake, particularly measures of MRT( Reference Clauss, Streich and Schwarm 66 ), rumen motility and rumination activity( Reference Deswysen, Ellis and Pond 67 ). Despite efforts to minimise variation in intake, DMI varied in our study, both between cows and periods, and cows had lower intakes on the BCM treatment. Ruminants produce less CH4 on concentrate-dominated diets( Reference Beauchemin, Kreuzer and O’Mara 68 , Reference Hristov, Oh and Firkins 69 ), and hence any ME-sparing effects of BCM should be less pronounced on such diets, leading to less distinct differences in intake. Therefore, feeding the cows a concentrate-based diet in the present study would probably have resulted in less overall variation in both DMI and CH4 reduction, and hence might not have necessarily resulted in a clearer signal. Evidently, hay of better quality should have been used for the current study. The combined effect of both the level of DMI and the presence of CH4 translates into significant effects of treatment on many of the physiological variables measured when rDMI is included as a covariable in the analysis but no direct difference between treatments in pairwise comparisons that do not account for rDMI (Table 2).
Effects on digestive physiology
The CH4RRf was only significantly affected by the BCM treatment. As far as we are aware, this is the first report in the literature of an effect of BCM on rumen fluid CH4 concentration, and the magnitude of the effect (99 % reduction) is notable. For several measures of MRT in the GIT, total tract digestibility and faecal DM concentration, the BCM treatment produced outstanding numerical differences to the other treatments. The iCH4 treatment did not represent the opposing extreme (Table 2), and this might suggest that relevant physiological effects are mainly linked to dissolved CH4 and not the CH4 concentration in rumen gas that is eructated. Dissolved CH4 might act on receptors directly located in the digestive tract or after absorption into portal blood( Reference Reynolds, Crompton and Barratt 39 ) and distribution in the body. In particular, dissolved CH4 may pass into the lower digestive tract causing a change in its motility similar to that found in guinea pigs( Reference Pimentel, Lin and Enayati 22 , Reference Jahng, Jung and Choi 23 ). Gaseous CH4 cannot be expected to reach those sites (but may be produced in lower amounts in the large intestine). Whether the effects suggested in the present study are really triggered by CH4, or by some other factor associated with our treatments, for example, changes in dissolved H2, remains to be clarified.
The iCH4 treatment affected rumen motility and rumination activity in the opposite direction to BCM treatment (Table 2). Notably, exhaled CH4 yield, but not CH4 in rumen fluid, was significantly correlated with MRTparticleRR (Table 3). Although gaseous CH4 is most likely not absorbed in the GIT, gassing GIT segments with CH4 in vitro has led to the changes in peristalsis described in the introduction( Reference Pimentel, Lin and Enayati 22 , Reference Jahng, Jung and Choi 23 ). In addition, because a large proportion of the gas eructated from the rumen is inhaled into the lungs and then exhaled( Reference Dougherty 70 ), some CH4 can be recovered in the arterial blood flow from the lungs( Reference Reynolds, Crompton and Barratt 39 , Reference Dougherty, Allison and Mullenax 71 ) and can be distributed throughout the body in this way.
The findings of the present study suggest that dissolved and, possibly to a lesser extent, also gaseous CH4 had an effect on the digestive physiology of the cows. The effect was consistent across the various measures, whereby decreased CH4 was associated with (i) a decrease in rumen motility with a concomitant increase in rumination time and intensity, (ii) a longer MRT in the RR but mainly in the lower digestive tract (with concomitantly drier faeces) and (iii) an increased apparent digestibility of fibre and CP. In other words, less CH4 was associated with reduced motility, increased MRT and increased fibre and CP digestibility. A similar effect of another CH4 inhibitor on digestibility was documented in an experiment where there was no confounding between treatment groups from variation in DMI( Reference Hristov, Oh and Giallongo 65 ).
These findings were unexpected, given the general association of increased exhaled CH4 yield with longer digesta retention within and across various species including man as outlined in the introduction. However, the findings could be tentatively interpreted as an indication of a feedback mechanism, by which ruminants attempt to counteract the loss of ingested energy to CH4, or using CH4 production to adjust digestive physiology. In such a feedback system, the high CH4 levels could signal successful fermentation of plant fibre, and hence trigger digesta propulsion, whereas low CH4 levels could signal that fermentation has not yet occurred to a favourable extent, and hence delay of propulsion is indicated. Such a system would represent a fine-tuning of events that are otherwise mainly dominated by the level of feed intake, which controls digesta retention, digestion, and hence CH4 production( Reference Hammond, Pacheco and Burke 9 ). Such a hypothetical mechanism would also match the expectation that, over evolutionary time, some adaptations should evolve to counter the seemingly inevitable energetic losses due to the omnipresence of methanogens.
Experimental evidence on the effect of CH4 on gut motility in monogastric animals is, however, typically interpreted in the opposite direction: CH4 is assumed to delay digesta transit by increasing the amplitude of peristaltic contractions in and by decreasing their velocity of travel along the small intestinal segments in in vitro assays in guinea pig intestine( Reference Pimentel, Lin and Enayati 22 , Reference Jahng, Jung and Choi 23 ). However, extra CH4 did not change the rate at which an artificial digesta bolus was transported in the proximal or distal colon in such a setting( Reference Jahng, Jung and Choi 23 ). Another experimental approach consisted of measuring the recovery of a marker applied to a 150-cm small intestinal segment of fistulated dogs at a second, more distal fistula during a time period of 30 min, during which 2 ml of buffer was infused per minute (a total of 60 ml); recovery of the marker was apparently not calculated using the marker concentration in the total recovered amount of fluid, but from six 1-ml samples collected at 5-min intervals( Reference Pimentel, Lin and Enayati 22 ). With this method, calculated marker recovery was reduced when the subsequent segment of the intestine was insufflated with CH4. More experimental evidence for modulation of gut motility by CH4 is warranted.
Whether, in man, higher enteric CH4 levels are a cause or an effect of prolonged colonic retention times (and hence constipation-related disorders) remains controversial. Limited clinical trials with the application of antibiotics that primarily target methanogens led to a reduction in constipation-related symptoms( Reference Pimentel, Chatterjee and Chow 24 , Reference Pimentel, Chang and Chua 72 ). However, it was pointed out that the reduction in breath CH4 did not exactly match the reduction in clinical symptoms( Reference Sachdeva, Kondala and Dahale 73 ). In these studies, colonic digesta passage was not quantified. However, interventions that specifically targeted digesta retention both behaviourally and/or pharmacologically and/or even included the application of dietary fibre (a substrate for methanogenesis) not only led to a reduction in digesta retention but also in CH4 production( Reference Soares, Lederman and Fagundes-Neto 18 , Reference El Oufir, Flourié and Bruley des Varannes 74 , Reference Vega, Perelló and Martos 75 ). Whether colonic motility and retention can be influenced in a clinical setting by specifically changing CH4 production only or whether CH4exhal indicates changes in motility and retention achieved by other effects remains to be investigated.
The only existing evidence known to us for an effect of CH4 level on digesta retention in ruminants found both lower CH4 yields and shorter MRTfluidRR in sheep supplemented with nitrate as compared with a control group( Reference Nolan, Hegarty and Hegarty 25 ); however, as the study design did not include a cross-over or a record of baseline values, it remains an open question whether decreased MRT represented an effect of reduced CH4 or the natural variation in MRT and CH4 in sheep( Reference Goopy, Donaldson and Hegarty 10 ) with an independent effect on CH4 in the treatment group.
Thus, in conclusion, the present study opens the possibility that CH4 production in ruminants is not only affected by levels of food intake, digesta retention, diet composition or other anatomical or physiological traits influencing digestive physiology, but that the level of CH4 production itself could influence some of these processes in the sense of a feedback mechanism. These results raise questions about the exact feedback mechanisms (e.g., CH4 receptors), and represent an interesting contribution to the basic knowledge about ruminant and potentially general herbivore digestive physiology. For future studies, a constant food intake across treatments as well as the addition of dissolved CH4 to both the digestive tract and the vascular system would be desirable.
Acknowledgements
We thank Keith Bamford, Debby Cockman, Colin Green, Andy Hicks and Anna Thompson from CEDAR for their support during the experiments. The authors are grateful to Nigel Tomkins for sharing his experience in BCM usage, to Heidrun Barleben for the analysis of numerous retention time samples, to Gerhard Breves and Klaus-Werner Grunert for their support with the rumen motility measurements, to Neil Donovan for the analysis of CH4 in rumen fluid, to Joel Niederhauser and Nils Zehner for their support with the RumiWatch measurements and to two anonymous reviewers for their comments that greatly improved the manuscript.
This study was a part of the project 310030_135252/1 funded by the Swiss National Science Foundation (SNF). The SNF had no role in the design, analysis and writing of this article.
M. C., M. K., K.-H. S., C. K. R., D. J. H. and L. A. C. designed the study; M. T. D., K. J. H., P. K., D. J. H., L. A. C. and M. C. performed the study; L. A. C., S. O., T. H. M., K.-H. S., A. S., M. K. contributed analytical tools; M. T. D., K. J. H. and M. C. analysed the data; M. T. D., K. J. H. and M. C. drafted the first version of the manuscript; all authors contributed to further versions and approved the final version of the manuscript.
The authors declare that there are no conflicts of interest.






