Introduction
Hunting is considered to be a major threat to wildlife populations in tropical forest regions (Fa et al., Reference Fa, Peres and Meeuwig2002; Mallon et al., Reference Mallon, Hoffmann, Grainger, Hibert, van Vliet and McGowan2015; Benítez-Lόpez et al., Reference Benítez-López, Alkemade, Schipper, Ingram, Verweij, Eikelboom and Huijbregts2017), and mammals comprise the greatest portion of hunted species (Fa et al., Reference Fa, Peres and Meeuwig2002; Nasi et al., Reference Nasi, Taber and van Vliet2011). A meta-analysis has demonstrated that there have been significant declines (up to 83%) in mammal abundances in many regions as a result of hunting pressure (Benítez-Lόpez et al., Reference Benítez-López, Alkemade, Schipper, Ingram, Verweij, Eikelboom and Huijbregts2017). The unsustainable use of wild mammals in most tropical forest areas hinders the possibility that impoverished communities will be able to continue to feed on wild mammals in the future (Wilkie et al., Reference Wilkie, Wieland, Boulet, Le Bel, van Vliet and Cornelis2016).
After several decades of a so-called fences and fines approach to hunting there is now a wider recognition that sustainable-use approaches merit more consideration (CBD, 2016; Mayor et al., Reference Mayor, El Bizri, Bodmer and Bowler2017). Managing hunting sustainably must address the multiple needs and desires of societies without jeopardizing the options for future generations to benefit from the full range of goods and services provided by hunted species (van Vliet et al., Reference van Vliet, Fa and Nasi2015).
In ecological terms the ability of prey species to withstand various levels of harvest, without depletion, depends on the life-history traits and biological parameters of the species (Caughley, Reference Caughley1977). Population dynamics depend on intrinsic population growth, mortality and the dynamics of in–out migration based on spatial (e.g. dispersal rate, dispersal distance and territory size) and temporal (e.g. seasonality of reproduction) parameters (Novaro et al., Reference Novaro, Redford and Bodmer2000; Salas & Kim, Reference Salas and Kim2002; van Vliet et al., Reference van Vliet, Milner-Gulland, Bousquet, Saqalli and Nasi2010).
A thorough understanding of the dynamics of prey populations under hunting pressure is needed for robust management decision-making. In tropical forests one of the major impediments to sound estimates of population dynamics is the paucity of available biological and ecological data for hunted species (Milner-Gulland & Akçakaya, Reference Milner-Gulland and Akçakaya2001; van Vliet & Nasi, Reference van Vliet and Nasi2008; Weinbaum et al., Reference Weinbaum, Brashares, Golden and Getz2013; van Vliet et al., Reference van Vliet, Fa and Nasi2015; Mayor et al., Reference Mayor, El Bizri, Bodmer and Bowler2017), and when such data exist they are not available in a synthetic and comprehensive manner.
Here we synthesize all the available information regarding parameters pertinent to the sustainable use of two of the most hunted tropical species: the lowland paca Cuniculus paca (in the Neotropics) and the blue duiker Philantomba monticola (in Africa). These are common and generalist small-sized species, with a widespread geographical range, and are crucial for the livelihoods of many rural communities.
Study species
Cuniculus paca is a large nocturnal rodent that occurs in Mexico, Colombia, Venezuela, the Guianas, Ecuador, Peru, Bolivia, Paraguay and most of Brazil, and has been introduced into Cuba and the Antilles (Patton et al., Reference Patton, Pardiñas and D'Elía2015). The species occurs in a wide range of forest types in moist areas and is an important seed distributor, with scatter-hoarding behaviour (Eisenberg & Redford Reference Eisenberg and Redford1999). It is categorized as Least Concern on the IUCN Red List, based on its wide distribution, presumed large population, and occurrence in a number of protected areas, and because it is unlikely to be declining (Emmons, Reference Emmons2016). However, unsustainable hunting of the paca has been reported from several locations (e.g. Koster, Reference Koster2008; Zapata-Ríos et al., Reference Zapata-Ríos, Urgilés and Suárez2009; Valsecchi et al., Reference Valsecchi, El Bizri and Figueira2014) and has led to local depletion of the species. The paca is sought after for its taste, nutritional value and low fat content (Aguiar, Reference Aguiar1996; Cordón and de Ariza, Reference Cordón and de Ariza1999; Lemire et al., Reference Lemire, Fillion, Barbosa, Guimarães and Mergler2010; Gálvez et al., Reference Gálvez, Arbaiza, Carcelén and Lucas2014), and because of these attributes is one of the most hunted and consumed species in Latin America (Koster, Reference Koster2008; Read et al., Reference Read, Fragoso, Silvius, Luzar, Overman, Cummings and de Oliveira2010; El Bizri et al., Reference El Bizri, Morcatty, Lima and Valsecchi2015; Quiceno-Mesa et al, Reference Quiceno-Mesa, van Vliet, Moreno and Cruz-Antia2015; van Vliet et al., Reference van Vliet, Fa and Nasi2015; Gómez et al., Reference Gómez, Restrepo, Moreno, Daza, Español and van Vliet2016; Vanegas et al., Reference Vanegas, van Vliet, Cruz and Sandrin2016).
Philantomba monticola is an abundant ungulate, widely distributed in central, eastern and southern Africa, from the Cross River in Nigeria to south-west South Sudan and southwards to central Angola, and Zambia, Malawi, eastern Zimbabwe, parts of central Mozambique, and on the islands of Pemba, Zanzibar and Mafia (East, Reference East1999; Wilson, Reference Wilson2001; Hart & Kingdon, Reference Hart and Kingdon2013). It thrives in a wide range of forested and wooded habitats, including primary and secondary forests, gallery forests, dry forest patches, coastal scrub farmland, regenerating forest and degraded forest patches, even near human settlements (Hart & Kingdon, Reference Hart and Kingdon2013). The species is among the most hunted throughout its range and is an important source of meat and income for rural people (de Merode et al., Reference de Merode, Homewood and Cowlishaw2004; Kümpel, Reference Kümpel2006; van Vliet et al., Reference van Vliet, Nasi, Emmons, Feer, Mbazza and Bourgarel2007; van Vliet & Nasi, Reference van Vliet and Nasi2008). It withstands hunting pressure better than most of the larger duikers (van Vliet et al., Reference van Vliet, Nasi, Emmons, Feer, Mbazza and Bourgarel2007; Mockrin, Reference Mockrin2008) and is categorized as Least Concern on the IUCN Red List (IUCN SSC Antelope Specialist Group, 2016).
Methods
During March–April 2017 we carried out a bibliographic search using seven databases: ISI Web of Science, Science Direct, EBSCO, Scielo, Redalyc, Scopus and Google Scholar. The search used a combination of words in English, Spanish, Portuguese and French: (‘species scientific name’ OR ‘common name’) AND (reproducti* OR dispers* OR behaviour OR mortality OR gestati* OR offspring OR longevity OR ‘litter size’ OR ‘biological parameters’ OR ‘life-history traits’). For the blue duiker we used the scientific names Cephalophus monticola and Philantomba monticola, as both are commonly used in the literature.
We screened the references using titles and abstracts according to the following primary inclusion criteria:
(1) Only studies for which we were able to source the full text. We searched for the full online text or the PDF, contacting the authors if necessary. We were able to source full documents from as far back as 1900.
(2) Only studies with scientific merit. To ensure the scientific quality of the information reported, we selected only peer-reviewed documents such as scientific journal articles, published records of zoological data produced by zoos, book chapters or theses for Master's or PhD degrees.
(3) Only focused on the species of interest to this study. We selected only papers that provided information on C. paca or P. monticola.
We screened the resulting references based on the full text and used the following secondary criteria:
(1) Only studies that contained information on the following biological and demographic parameters useful for prey population management (Sutherland, Reference Sutherland2008): (i) Variables used in one-off intrinsic population growth models (Robinson & Redford, Reference Robinson, Redford, Robinson and Redford1991): age at first and last reproduction, gestation period, litter size, interval between births, sex ratio at birth (female/male), longevity, mortality rate. (ii) Variables used in spatially explicit demographic models (Novaro et al., Reference Novaro, Redford and Bodmer2000; Salas & Kim, Reference Salas and Kim2002; van Vliet et al., Reference van Vliet, Milner-Gulland, Bousquet, Saqalli and Nasi2010): mean territory size; dispersal distance, dispersal rate, dispersal age. (iii) Variables influencing temporal variations: seasonality in reproduction, dispersal and mortality.
(2) Only studies providing primary data. We selected studies only if they provided primary information on the biological and demographic characteristics described above. If no primary data were provided but the primary source was cited (e.g. in the AnAge database (Tacutu et al., Reference Tacutu, Craig, Budovsky, Wuttke, Lehmann and Taranukha2013) or in Weigl, Reference Weigl2005), then the primary source was searched and, if available, screened based on the same primary and secondary criteria described above.
For each of the studies that passed our filters (16 for P. monticola and 18 for C. paca) we extracted the information on each of the 15 variables (Tables 1 and 2), and recorded whether the information came from wild or captive populations, and the size of the sample studied, if available.
Table 1 Primary data available on basic biological and ecological parameters (reproduction, mortality, dispersal and seasonality) for the blue duiker Philantomba monticola. No data were available on age at last reproduction, mortality rate, seasonality of mortality and seasonality of dispersal. Blank cells indicate data were not provided in the source document.
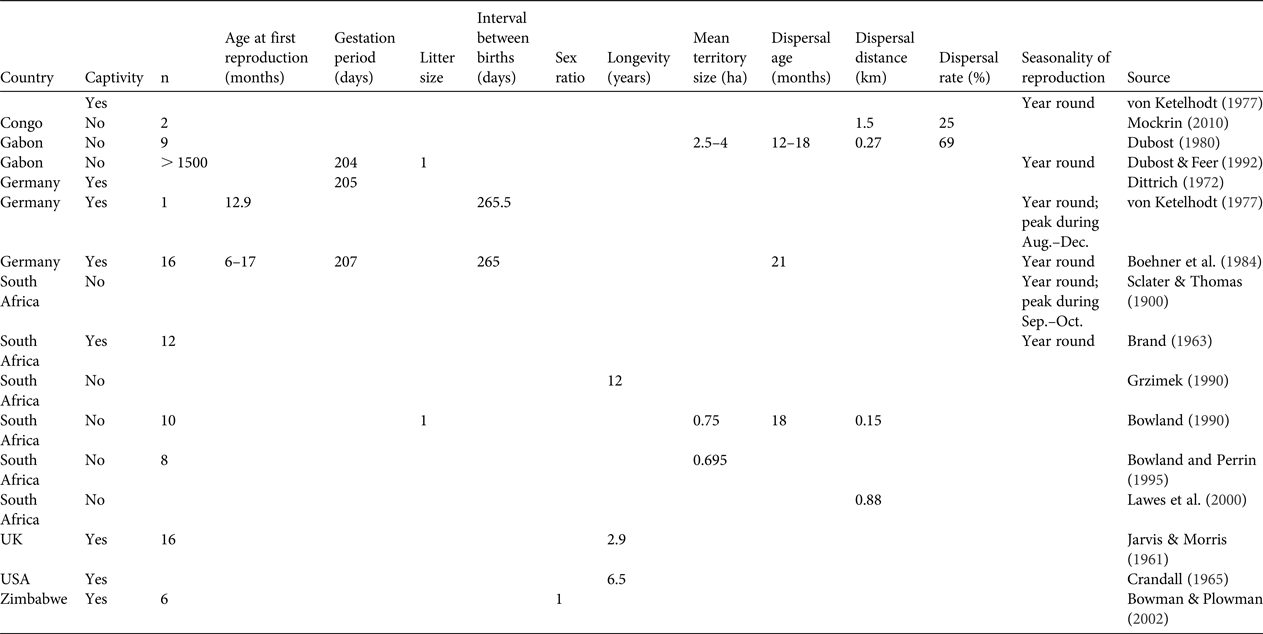
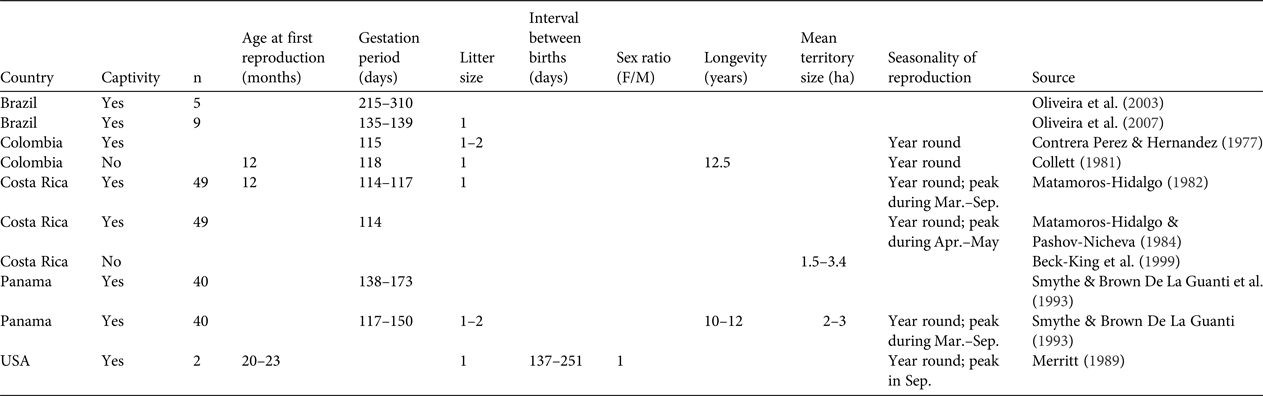
Table 2 Primary data available on basic biological and ecological parameters (reproduction, mortality, dispersal and seasonality) for the lowland paca Cuniculus paca. No data were available on dispersal (age, rate, distance, seasonality). Blank cells indicate data were not provided in the source document.
Results
Philantomba monticola
A total of 16 studies published during 1900–2010 were found to have generated primary data on biological and demographic parameters for P. monticola, of which eight were based on individuals in captivity, five on wild individuals from South Africa, two on wild individuals in Gabon and one on wild individuals in Republic of Congo. Most of the information for each variable was gleaned from 1–6 studies, mostly from individuals in captivity or from a limited number of wild individuals (1–16). No information was found on age at last reproduction, mortality rate, seasonality of mortality or seasonality of dispersal. The results are synthesized in Table 1.
Cuniculus paca
A total of 18 studies published during 1979–2016 were found to have generated primary data on the biological and demographic parameters of C. paca, of which 13 were based on individuals in captivity and five on wild individuals (in Peru, Colombia, French Guiana, Costa Rica). Reproduction variables were derived from sample sizes of 45–212 for individuals in the wild and 2–49 for individuals in captivity. For longevity, a sample of 40 individuals was used. For seasonality in reproduction, data were derived from samples of 2–49 individuals. We found no information on dispersal (age, rate, distance, seasonality). The results are synthesized in Table 2.
Discussion
We summarize the available published data on the main variables needed to assess productivity and management for the sustainable use of two common and non-threatened species used as a source of food in African and Neotropical forests. Researchers and managers may refer to the synthetic tables produced, in which we cite the primary sources of data, with information they provide on sample size, geographical origin of the assessment and whether the data originated from captive or wild individuals.
In general terms, our review suggests that both species reach maturity after c. 1 year following birth, reproduce twice per year all year round, for the whole duration of their mature life (c. 10 years) and give birth to one offspring per year. In addition, the available data suggest that P. monticola has the capacity to disperse and occupy available empty areas through a small-scale source–sink process (Mockrin, Reference Mockrin2010). This behavioural characteristic probably contributes to the resilience of the species to hunting (van Vliet et al., Reference van Vliet, Milner-Gulland, Bousquet, Saqalli and Nasi2010). No information on dispersal is available for C. paca. The resilience to hunting observed for this species may be linked to its generalist behaviour and capacity to exist in high population densities (83–96 individuals per km2; Eisenberg & Redford, Reference Eisenberg and Redford1999).
This review highlights the paucity of data available for the parameters needed to manage these species sustainably. There is little information available on parameters that influence demographic patterns, such as reproduction, dispersal, home range characteristics, mortality, longevity and seasonal variations. Most of the available data are from captive individuals, and most, particularly for P. monticola, are from studies conducted during the 1980s and 1990s or earlier.
The lack of robust data on life-history traits hinders efforts to propose sustainable management practices for these two species, which are both important nutritional assets for forest people. Nevertheless, scientists and decision makers continue to make decisions based on erroneous estimates of maximum sustainable yields. Without a significant investment in estimations of life-history traits under varying contexts, quota setting efforts are prone to failure as they will be based on best guesses rather than on sound scientific evidence.
We call for new assessments covering the possible variations in parameter values across the distribution range of these two species, and covering various anthropogenic contexts; for example, to address hypotheses on forms of density-dependent mortality and reproduction, and compensatory vs additive mortality effects in tropical harvested species (Weinbaum et al., Reference Weinbaum, Brashares, Golden and Getz2013). Life-history trait data are also needed for many other species, particularly those of conservation concern, as the use of mean values from a few studies is insufficient to assess intra-specific variations and adaptations of different populations in relation to environmental and anthropogenic gradients.
Methodological and technical innovations already developed could help produce new assessments; for example, participatory sampling involving hunters (e.g. to collect reproductive organs or monitor pregnant females) is an underestimated and under-used approach that could be an efficient means of gathering information about reproduction patterns (Mayor et al., Reference Mayor, El Bizri, Bodmer and Bowler2017; van Vliet et al., Reference van Vliet, Sandrin, Vanegas, L'haridon, Fa and Nasi2017). Technologies such as camera traps (Jędrzejewski et al., Reference Jędrzejewski, Puerto, Goldberg, Hebblewhite, Abarca and Gamarra2017), non-invasive DNA methods (Fusaro et al., Reference Fusaro, Conner, Conover, Taylor, Kenyon, Sherman and Ernest2017; Granjon et al., Reference Granjon, Rowney, Vigilant and Langergraber2017), injectable sensors or electronic tags (Bozkurt, Reference Bozkurt2017), passive integrated transponder tags (Ousterhoudt & Burkhart, Reference Ousterhout and Burkhart2017), micro-chip implants, vaginal implant transmitters (Newbolt et al., Reference Newbolt, Acker, Neuman, Hoffman, Ditchkoff and Steury2017), modern recording systems for acoustic monitoring (Crossin et al., Reference Crossin, Heupel, Holbrook, Hussey and Lowerre-Barbieri2017), and advanced telemetry systems using ultra-light global positioning systems (Alippi et al., Reference Alippi, Ambrosini, Longoni, Cogliati and Roveri2017) could be applied to forest mammals and contribute to a better understanding of their demographic parameters (Mathur et al., Reference Mathur, Habib and Mathur2017).
Acknowledgements
We acknowledge the contributions from Daniel Cruz, Jessica Moreno and Federico García in supporting the bibliographic search. This work was funded by the U.S. Agency for International Development through the Bushmeat Research Initiative of the Center for International Forestry Research and the Forest, Trees and Agroforestry Program of CGIAR.
Author contributions
NvV conceived the study, carried out the bibliographic search and wrote a first draft of the article. RN contributed extensively to revisions of the article.
Biographical sketches
Nathalie van Vliet’s research focuses on the links between wildlife and livelihoods. For the last 15 years she has worked on bushmeat and its contribution to food security and local economies in Central Africa. She has also developed research projects in the Amazon, where her team is analysing bushmeat market chains and consumption patterns. Working at local, national and international levels, her research aims to provide more visibility to current bushmeat use and provide objective data for innovative management policies that include ecological, cultural and socio-economic sustainability. Robert Nasi has been living and travelling extensively in Africa, Asia and the Pacific since 1982, undertaking research in the fields of ecology and management of tropical forests. He is interested in the various issues related to the sustainable use of forest resources, blending conservation and development.




