Biofuels produced more readily from cellulose-IIII
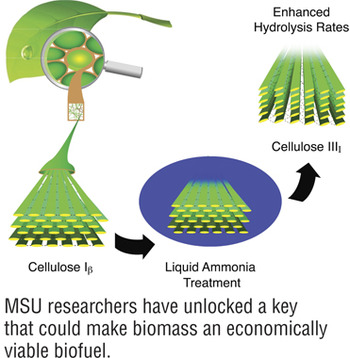
Hydrolyzing polymeric cellulose in plants to glucose is a crucial step in biofuels production, but the crystallinity of the naturally occurring cellulose Iβ structure of lignocellulose hinders the action of enzymes in promoting this conversion. Now a team of researchers led by Shishir Chundawat at Michigan State University has used experimental and computational methods to increase the rate of glucose formation fivefold by converting the cellulose from the Iβ to the IIII crystalline form using aqueous ammonia. “The main finding of our work is that by modifying the crystal structure of cellulose you’re actually changing the physicochemical characteristics of the substrate,” Chundawat said, “which eventually results in enzymes giving much higher rates of hydrolysis despite reduced overall binding to the substrate.” Insights the researchers have gained into the process of how the enzymes interact with the surface of cellulose have convinced Chundawat that “you have a better chance of engineering enzymes to further improve break down of cellulose-III than cellulose-I. I think that’s one practical implication of this work that we will be exploring next.”
DOE Report Highlights Computational Efforts in Materials Science and Energy
The U.S. Department of Energy’s (DOE) Innovative and Novel Computational Impact on Theory and Experiment (INCITE) program has just released In Review, a publication that highlights some of the best, most exciting work done in supercomputing since the program’s inception in 2003. Materials science and energy figure prominently in this overview. INCITE researchers have published 70 scientific papers on materials science and chemistry since 2008, the report notes. In Review includes articles with titles such as “Chemistry and Materials Computations Speed Clean Energy Production and Storage;” “Computational Scientists Help Superconductors Find the Path of Least Resistance;” and “Computing Yields Insights on Ways to Reduce Energy Dissipation in Transportation.”
Method produces a grid-structured transparent battery
Transparency can be a necessity in some parts of electronic devices, such as the screen of a cell phone. It can also be a cool design element if you can make the whole cell phone transparent. A major limitation in such a design is the battery, whose electrodes are thick films of inorganic active materials, carbon black nanoparticles, and an adhesive organic binder. One would think that any component containing carbon black would be largely opaque. But now, using a novel process they developed at Stanford University, researchers Yuan Yang, Yi Cui, and their colleagues have reported success in producing transparent Li-ion batteries. Using a microfluidics-assisted method to produce a grid-structured electrode whose gridlines cover only a small fraction of the area of the whole electrode, opaque components are not individually distinguishable. “If the feature dimension of the lines is comparable or less than the resolution of human eyes (50–100 microns),” the authors said, “the opaque electrode grid is indistinguishable from the transparent substrate.” The device is slightly colored rather than completely clear, but seeing through it is easy.
High energy density in Li-air batteries produces boost in capacity
Lithium-air batteries may one day compete with lithium ion (Li-ion) batteries, but researchers are still in the early stages of understanding how they operate. Robert Mitchell, Betar Gallant, and their colleagues at MIT recently developed a new oxygen electrode made of binder-free, all-carbon hollow nanofibers. They have achieved among the highest energy densities by weight reported to date for Li-air batteries, according to Mitchell, and in the process have observed the growth and disappearance of Li2O2 in the electrode during the battery’s discharging and charging stages, respectively. The projected energy density by weight for Li-air batteries is approximately three to five times that of Li-ion batteries because of their lightweight electrodes, the researchers noted.



