Clinical Implications
-
• For many patients with major depressive disorder, initial therapy with a monoaminergic agent does not sufficiently meet clinical need, as evidenced by low rates of remission and residual depressive symptoms.
-
• Various factors, including inflammation, metabolic disorders, and stress, contribute to depressive symptoms; thus, supplementation of usual care with adjunctive therapies that can address these factors may further increase response to treatment.
-
• Recent clinical trials have highlighted the involvement of folate in MDD pathophysiology, and the benefits of supplemental use of L-methylfolate, the biologically active form of folate, in patients with depression.
-
• Adjunctive therapy with L-methylfolate may be of particular benefit for patients with SSRI-resistant MDD, low folate levels, and/or identified biologic markers associated with inflammation/obesity and/or folate metabolism gene polymorphisms.
Introduction
Depression is one of the most prevalent mental disorders in the United States; more than 16% of individuals suffer from depression in the course of their lifetime.Reference Kessler and Wang 1 Globally, it is the leading cause of disability and impacts more than 300 million people. 2 Despite the prevalence and severity of the disease, its precise pathophysiology remains unclear. Because of considerable heterogeneity in the neurobiology and genetics of depression, there is a need for a variety of options in order to individualize treatment. Unfortunately, there are no clinically useful biomarkers to guide selection of optimal treatment.Reference McGrath, Kelley and Holtzheimer 3 The monoaminergic theory of depression, centered around insufficient levels of or abnormal neurotransmission of serotonin (5-hydroxytryptamine [5-HT]), was the most widely accepted explanation for the symptoms of depression for the latter part of the 20th century, and has evolved to encompass the tri-monoamine theory, which also implicates abnormalities of dopamine and norepinephrine.Reference Belmaker and Agam 4 , Reference Delgado 5 Indeed, research in recent decades has determined that specific monoaminergic neurotransmitters modulate various aspects of mood and behavior.Reference Nutt 6 For instance, norepinephrine plays a role in alertness, energy, and attention, and low levels may contribute to feelings of anhedonia, a lack of interest in taking pleasure from life. Dopamine, the hallmark neurotransmitter in the reward pathway, is implicated in attention, motivation, and pleasure, while serotonin is implicated in depressed mood, anxiety, and obsessive thoughts.Reference Nutt 6 More recently, folate deficiency has been identified as having a role in the pathology of depression.Reference Bottiglieri 7 L-methylfolate is thought to be the only form of folate that is able to pass through the blood–brain barrier and is involved in the regeneration of tetrahydrobiopterin (BH4; a critical cofactor for neurotransmitter synthesis), the levels of which are depleted with inflammation or oxidative stress.Reference Stahl 8 – Reference Subramaniapillai, Carmona, Rong and McIntyre 11 In major depressive disorder (MDD) patients who are unresponsive to antidepressants, L-methylfolate may be a viable treatment option.
This article highlights new advancements in our understanding of the heterogeneous pathogenesis of MDD, particularly those regarding the role of folate inflammation, stress, obesity, and related biomarkers. It also reviews the efficacy and safety data on a prescription form of L-methylfolate that support its clinical use in MDD.
Usual Care and Adjunctive Therapies
Usual care for MDD includes treatments such as selective serotonin reuptake inhibitors (SSRIs) or serotonin and norepinephrine reuptake inhibitors (SNRIs) that target monoamine neurotransmitters, increasing their presence in the synapse by blocking their reuptake by transporters. Despite decades of use, monoaminergic therapies leave several unmet needs for patients: treatment onset may be slow, and many patients do not respond or remit to symptom levels that allow normal function; side effects can be significant; and many patients find it difficult to continue treatment and even discontinue antidepressant use, resulting in withdrawal reactions and/or appearance of new depressive symptoms.Reference Davies and Read 12 – Reference Papakostas, Fava and Thase 16 The Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study, funded by the National Institutes of Mental Health, found that approximately 30% of patients experienced remission from depression and less than 50% of patients achieved a 50% or greater reduction in baseline depressive symptomology scores with initial SSRI monotherapy.Reference Trivedi, Rush and Wisniewski 15 Furthermore, rates of response and remission were found to decrease after every failed treatment.Reference Rush, Trivedi and Wisniewski 17 Of the patients who received secondary treatments after failure of initial SSRI therapy, 18%, 21%, and 25% achieved remission with sertraline, sustained-release bupropion, and extended-release venlafaxine treatment, respectively.Reference Rush, Trivedi and Wisniewski 17 Only 10% to 16% achieved remission in their third phase of treatment.Reference Fava, Rush and Wisniewski 18 Thus, treatment of MDD with monotherapy may be incomplete for many patients, and there is a clear need for effective alternative or adjunctive therapies.
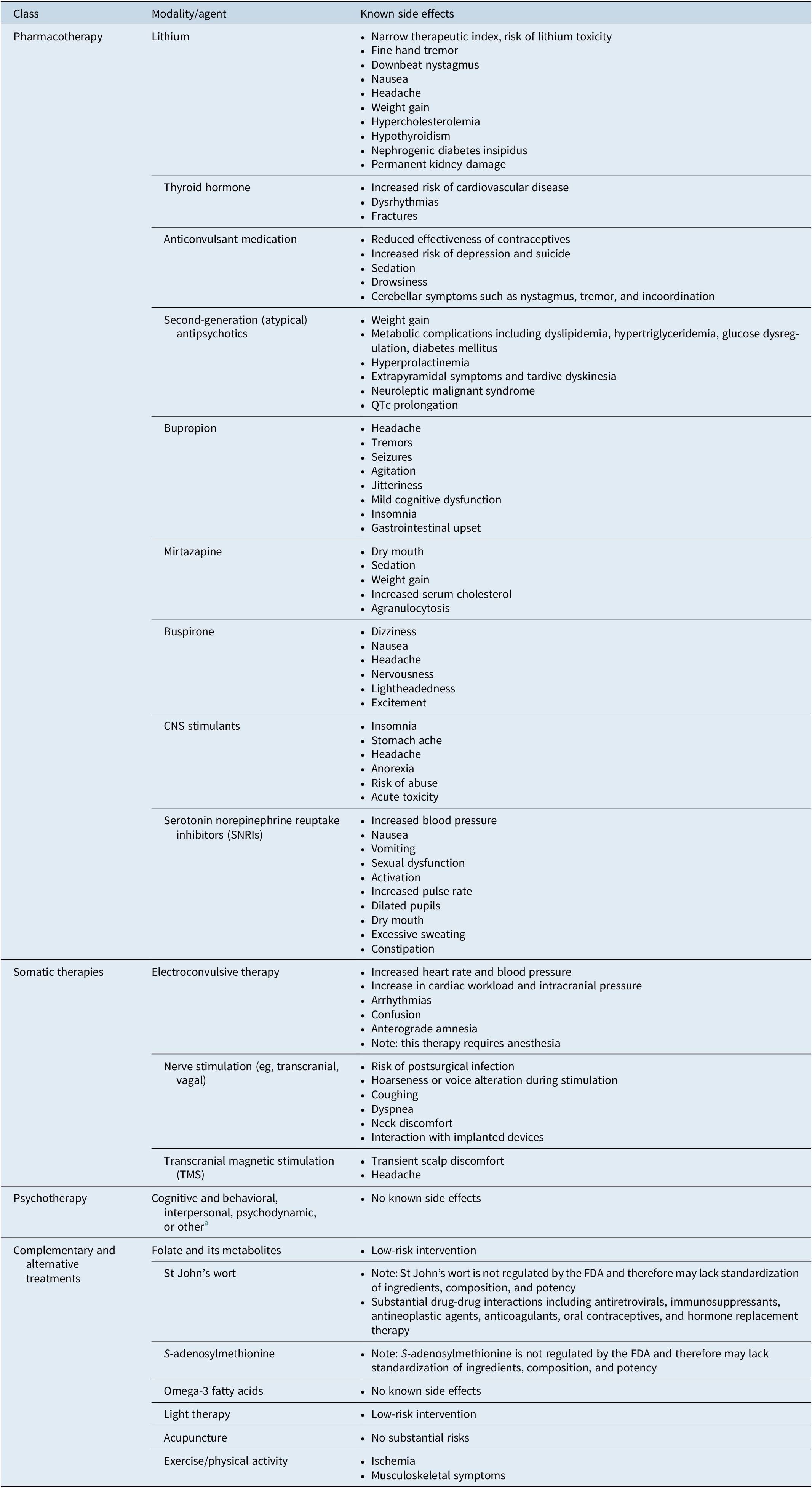
There are a number of commonly used adjunctive therapies available to patients for whom monoaminergic-based therapy is insufficient (Table 1). 19 – 25 Many of them are associated with potentially significant side effects that may put patients at higher risk for other serious health conditions. 19 – Reference Gelenberg, Freeman and Markowitz 23 While several are evidence-based, only the atypical antipsychotics aripiprazole, quetiapine, and brexpiprazole are currently approved by the FDA. 26 , 27 In selecting adjunctive therapies, clinicians should consider the risk–benefit profile of each agent and individualize treatment accordingly. Available evidence suggests that two-thirds of patients taking drugs for depression require the use of at least 1 adjunctive therapy, with many patients cycling through therapy combinations before finding reasonably effective treatment, often a combination of polypharmacy and nonpharmacologic treatments.Reference Warden, Rush, Trivedi, Fava and Wisniewski 28 In particular, patients with treatment-resistant depression are known to experience worse outcomes than those who respond to usual care therapies. Longer duration of untreated depression and the presence of residual symptoms are associated with worse outcomes, including greater cognitive and functional impairment, as well as higher risk of relapse, morbidity, and even mortality.Reference Blier 14 , 29 – Reference Kisely, Scott, Denney and Simon 32 It is therefore important to identify the proper adjunctive therapy early in the treatment process to improve the possibility of acute full remission and increase the likelihood of longer-term efficacy.Reference Zajecka, Fava, Shelton, Barrentine, Young and Papakostas 33 Advances in the understanding of depression etiology and pathophysiology may allow clinicians to better identify patients who will respond to particular therapies or adjunctive therapies.
Advances in Depression Pathophysiology Research
A growing body of literature has identified factors in depression etiology and pathophysiology that may predict response to certain treatments. These findings have largely implicated inflammatory markers, metabolic abnormalities, and stress as major categories contributing to depression symptoms in certain patients. In addition to uncovering their roles in causing and maintaining depression episodes, research into these processes has revealed diagnostic tools and biomarkers that can assist clinicians in determining the best adjunctive therapies for each individual patient.
Inflammation and inflammatory biomarkers
Inflammation and depression are directly correlated and form a bidirectional loop that plays a critical role in the mechanism behind depression in a subgroup of MDD patients,Reference Kiecolt-Glaser, Derry and Fagundes 34 potentially causing downstream metabolic and behavioral effects.Reference Gimeno, Kivimaki and Brunner 35 Increased inflammation causes the central nervous system (CNS) to elicit or intensify depressive symptoms such as negative mood, fatigue, anhedonia, increased pain sensitivity, an altered sleep pattern, and cognitive deficits.Reference Kiecolt-Glaser, Derry and Fagundes 34 , Reference Valkanova, Ebmeier and Allan 36 Depression also can promote inflammation by decreasing the sensitivity of the immune system to glucocorticoid hormones that stop the inflammatory response.Reference Gimeno, Kivimaki and Brunner 35 Furthermore, in MDD, there is a lack of parasympathetic activity to counter the continual sympathetic activity, which results in elevated norepinephrine and epinephrine levels and low acetylcholine levels, which ultimately results in release of inflammatory mediators from immune cells.Reference Won and Kim 37
The link between inflammation and depression lies in the cytokines, which elevate inflammatory signaling in the CNS, which subsequently leads to depressive symptoms.Reference Kiecolt-Glaser, Derry and Fagundes 34 Cytokines can activate indoleamine 2,3-dioxygenase (IDO), which converts tryptophan, a key component of serotonin, into kynurenine, thereby decreasing the production and availability of serotonin in the brain.Reference Kiecolt-Glaser, Derry and Fagundes 34 , Reference Miller, Haroon, Raison and Felger 38 Other factors involved in the bidirectional pathway of inflammation and depression include psychological stressors, sensitization of cells to neurotoxic peptides, and oxidative and nitrosative stress.Reference Valkanova, Ebmeier and Allan 36 Cytokines can affect production, metabolism, and transport of neurotransmitters that are responsible for mood (ie, dopamine, norepinephrine, serotonin, and glutamate).Reference Kiecolt-Glaser, Derry and Fagundes 34 , Reference Miller, Haroon, Raison and Felger 38 , Reference Anand and Charney 39 Additionally, cytokines may lead to a decrease in GABA release, which can further exacerbate inflammation in the CNS.Reference Miller, Haroon, Raison and Felger 38 Elevated levels of proinflammatory cytokines in the CNS may deleteriously influence neurotransmitters that are central to depression pathophysiology by increasing the activity of transporters that clear monoamine neurotransmitters from neuronal synapses, by decreasing the synthesis of monoamines, and by increasing excitatory and potentially neurotoxic glutamate activity through N-methyl-D-aspartate (NMDA) receptor activation and reduced astrocytic reuptake.Reference Miller, Haroon, Raison and Felger 38 Moreover, they are known to decrease neuroplasticity and cause oxidative stress by generating nitrogen and oxygen radicals, which can promote oxidative neurotoxicity.Reference Miller, Haroon, Raison and Felger 38 , Reference Blossom, Melnyk, Li, Wessinger and Cooney 40 , Reference Calabrese, Rossetti, Racagni, Gass, Riva and Molteni 41
Serologic markers of systemic inflammation are potentially useful tools that may inform clinicians about optimal treatment paradigms and antidepressant selection for individual patients.Reference Jha, Minhajuddin and Gadad 42 Antidepressants have been shown to affect the immune system and levels of proinflammatory cytokines.Reference Jha and Trivedi 43 Specific inflammatory biomarkers that have been implicated in MDD are detailed as follows and in Table 2.Reference Valkanova, Ebmeier and Allan 36 , Reference Jha, Minhajuddin and Gadad 42 , 44 – Reference Jha, Minhajuddin, Gadad and Trivedi 56
Table 2. Evidence for inflammatory biomarkers implicated in depression.
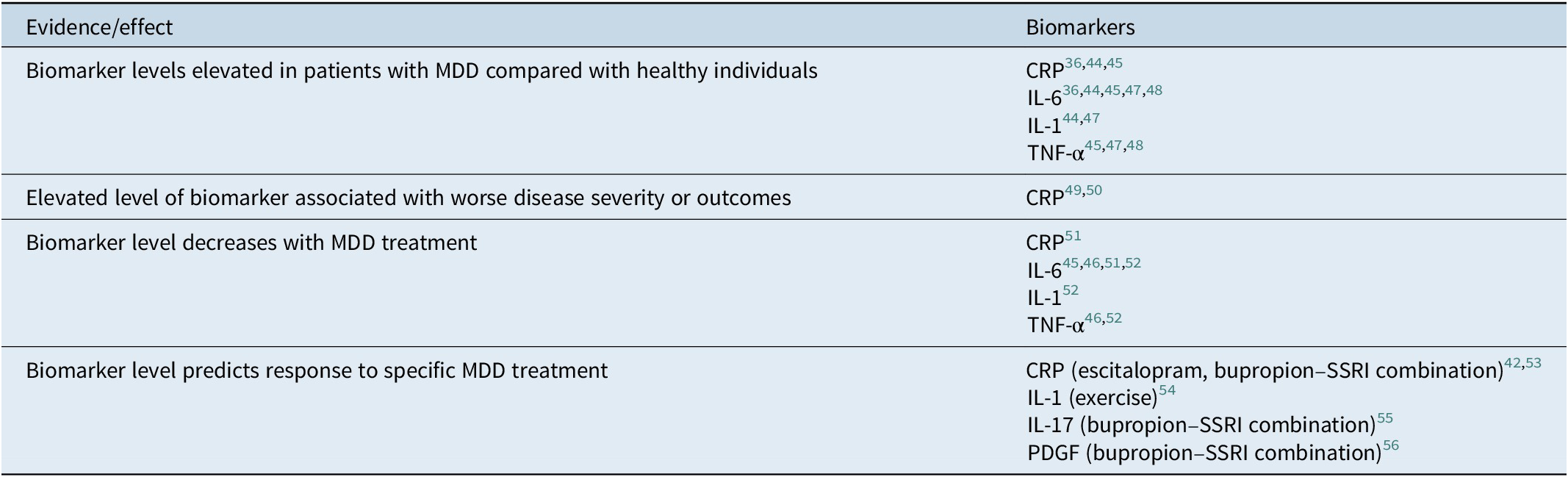
Abbreviations: CRP, C-reactive protein; IL, interleukin; MDD, major depressive disorder; PDGF, platelet-derived growth factor; TNF, tumor necrosis factor.
C-reactive protein (CRP) and interleukins 6 (IL-6) and 1 (IL-1)
IL-6 and IL-1, which are secreted by activated macrophages and are upstream of CRP, were initially hypothesized to be causative inflammatory agents in depression.Reference Maier and Watkins 57 Following decades of research, CRP—a nonspecific marker of inflammation as well as other acute-phase processes including tissue damage and infectionReference Pepys and Hirschfield 58 —has emerged as a major inflammatory biomarker in MDD. A meta-analysis of studies evaluating the potential relationship between CRP levels and depression found a significantly positive relationship overall (effect size, 0.22; 95% CI, 0.15–0.28; p < 0.001).Reference Howren, Lamkin and Suls 44 Similar findings were reported for IL-6 (effect size, 0.25; 95% CI, 0.18–0.31; p < 0.001) and IL-1 (effect size, 0.35; 95% CI, 0.03–0.67; p = 0.03). Additional meta-analyses have largely confirmed these results,Reference Valkanova, Ebmeier and Allan 36 , 45 – Reference Liu, Ho and Mak 48 with some suggesting a stronger link between CRP and depression, compared with IL-6 or IL-1. IL-6 and IL-1β levels may be particularly elevated in patients with late-life depression (ie, age >60 or >70 years).Reference Penninx, Kritchevsky and Yaffe 59 , Reference Thomas, Davis, Morris, Jackson, Harrison and O’Brien 60 Elevated CRP levels have also been associated with worse outcomes, more severe symptoms,Reference Wium-Andersen, Orsted, Nielsen and Nordestgaard 49 and higher rates of suicideReference Batty, Bell, Stamatakis and Kivimaki 50 in this clinical setting.
Some patients with MDD show signs of inflammatory response, including increased expression of proinflammatory cytokines and their receptors.Reference Miller and Raison 61 Inflammation has been associated with inadequate response to antidepressant treatment.Reference Miller and Raison 61 Conventional antidepressants increase synaptic availability of monoamines and also increase neurogenesis through brain-derived neutrophic factor (BDNF).Reference Miller and Raison 61 Cytokines impact synthesis, release, and reuptake of monoamines by decreasing serotonin and dopamine availability and increasing expression of monoamine reuptake transporters, thus weakening their signal.Reference Miller, Haroon, Raison and Felger 38 , Reference Miller, Maletic and Raison 62 , Reference Liu, Wang and Jiang 63 Nonresponsiveness to antidepressant treatment and increased inflammatory markers in treatment-resistant patients may be attributed to these effects of the cytokines.Reference Miller and Raison 61 Increasing evidence supports the role of CRP to guide anti-inflammatory treatment in depressed patients. In a trial conducted by Raison et al, it was shown that baseline concentrations of CRP >5 mg/L correlated with better response to infliximab.Reference Raison, Rutherford and Woolwine 64 Furthermore, this study demonstrated the effectiveness of anti-inflammatory medications for the treatment of depression in subjects with higher baseline CRP levels, as evidenced by greater improvement in Hamilton Depression Rating Scale (HDRS)-17 scores and improvement in symptoms.Reference Raison, Rutherford and Woolwine 64
Findings from meta-analyses have further detailed the relationship between CRP levels and MDD treatment.Reference Wiedlocha, Marcinowicz and Krupa 46 , Reference Hiles, Baker, de and Attia 51 Recently, using data from 2 independent prospective studies, researchers have shown that baseline CRP levels predict response to MDD treatment.Reference Jha, Minhajuddin and Gadad 42 , Reference Uher, Tansey and Dew 53 Uher et alReference Uher, Tansey and Dew 53 analyzed data from the Genome-Based Therapeutic Drugs for Depression study to evaluate whether baseline CRP level correlated with reduction in depression severity with escitalopram and nortriptyline. Patients with CRP levels of less than 1 mg/L had a greater reduction in depression severity with escitalopram versus nortriptyline (β, 3.27; 95% CI, 1.65–4.89; p < 0.001). In escitalopram-treated patients, the increase in baseline CRP level was associated with worsening of disease severity (p<0.001). In nortriptyline-treated patients, there was a trend toward improvement in severity with increased CRP level. Using data from the Combining Medications to Enhance Depression Outcomes (Co-MED) trial, Jha et alReference Jha, Minhajuddin and Gadad 42 also evaluated the potential relationship between biomarker levels and response to treatment with SSRI monotherapy compared with bupropion–SSRI combination. Overall, higher baseline CRP levels were associated with greater reduction in disease severity in patients treated with bupropion–SSRI combination (r = −0.63) compared with SSRI monotherapy (r = 0.40). In patients with a CRP level of less than 1 mg/L, there was a numerical trend toward improved outcomes with SSRI monotherapy versus bupropion–SSRI combination (p = 0.057).
Less is known about potential relationships between IL-6 or IL-1 and treatment response. Meta-analysesReference Strawbridge, Arnone, Danese, Papadopoulos, Herane Vives and Cleare 45 , Reference Wiedlocha, Marcinowicz and Krupa 46 , Reference Hiles, Baker, de and Attia 51 , Reference Hannestad, DellaGioia and Bloch 52 have demonstrated that IL-6 levels decrease with MDD treatment; however, there may not be a difference in outcomes by treatment response. A similar relationship has been shown for IL-1.Reference Wiedlocha, Marcinowicz and Krupa 46 , Reference Hannestad, DellaGioia and Bloch 52 Another study showed a positive correlation between change in IL-1β level and depressive symptoms in patients who underwent an exercise program to manage depression.Reference Rethorst, Toups and Greer 54
Tumor necrosis factor α (TNF-α)
Meta-analyses have also found levels of TNF-α to be higher in depressed patients than in nondepressed patients. 45 – Reference Liu, Ho and Mak 48 In a preclinical model, levels of TNF-α decreased after administration of bupropion.Reference Brustolim, Ribeiro-dos-Santos, Kast, Altschuler and Soares 65 Levels have been demonstrated to decrease with MDD treatments in some but not all clinical studies.Reference Wiedlocha, Marcinowicz and Krupa 46 , Reference Hannestad, DellaGioia and Bloch 52 Elevated TNF-α levels have been associated with failure to respond to antidepressant medications,Reference Strawbridge, Arnone, Danese, Papadopoulos, Herane Vives and Cleare 45 but have been shown to improve response to infliximab treatment (p < 0.05)Reference Raison, Rutherford and Woolwine 64 and exercise programs.Reference Rethorst, Toups and Greer 54
Interleukin 17 (IL-17)
Results from preclinical analyses and clinical studies have demonstrated that T-helper 17 cells accumulate in the brain and periphery during depressive states,Reference Beurel, Harrington and Jope 66 , Reference Chen, Jiang and Chen 67 suggesting a potential role for IL-17 cytokines produced by these T cells.Reference Jin and Dong 68 An analysis of the Co-MED trial data demonstrated that elevated levels of IL-17 at baseline were associated with greater effectiveness of bupropion and SSRI in combination, but not with SSRI monotherapy or venlafaxine–mirtazapine combination therapy.Reference Jha, Minhajuddin, Gadad, Greer, Mayes and Trivedi 55 These findings also suggested the converse, that patients with low levels of IL-17 had a poorer response to bupropion–SSRI in combination compared with other studied treatments.
Platelet-derived growth factor (PDGF)
PDGF signaling was recently shown to have a role in neuroinflammation.Reference Yang, Manaenko and Xu 69 It has been hypothesized that secretion of PDGF, a peripheral marker of neuroinflammation, increases following damage to the blood–brain barrier due to inflammation or stress.Reference Jha, Minhajuddin, Gadad and Trivedi 56 An analysis of data from the Co-MED trialReference Jha, Minhajuddin, Gadad and Trivedi 56 found that treatment with bupropion–SSRI combination therapy was associated with decreased severity of depression and anhedonia in patients with higher versus lower levels of PDGF at baseline.
Microglial cells and extracellular vesicles
Microglial cells are associated with depression, although the exact mechanism behind this is unclear.Reference Brites and Fernandes 70 , Reference Serafini, Rihmer and Amore 71 Elevated microglial cell numbers have been observed in patients with depression and depressive symptoms.Reference Brites and Fernandes 70 Microglial cells serve as the immunologic guards of the brain through their anti-inflammatory and neuroprotective role, and their abnormal activation results in release of inflammatory mediators, which may be a factor behind the immune response in depression.Reference Brites and Fernandes 70 , Reference Serafini, Rihmer and Amore 71 Activated microglia cells release microvesicles that contain IL-1β, IL-1β processing enzyme caspase-1, IL-6, TNF-α, the P2X7 receptor, reactive oxygen species, and reactive nitrogen species, and can cause inflammation in the brain.Reference Miller, Maletic and Raison 62 , Reference Brites and Fernandes 70 Extracellular vesicles are also released by reactive microglia and are important in intercellular communication and neuroinflammation through transport of mRNA, microRNA, and proteins.Reference Brites and Fernandes 70
Metabolic disorders
The prevalence of obesity is rising, which not only increases the risk of cardiovascular disease but also increases the rates of depression.Reference Benjamin, Blaha and Chiuve 72 Obese individuals have up to a 2-fold increased probability of developing depression, with significantly increased risk in those with higher body mass index (BMI) and in females. 73 – Reference de Wit, Luppino, van Straten, Penninx, Zitman and Cuijpers 76 A BMI of 30 kg/m2 or more, increased waist-to-hip ratio, and particularly abdominal fat have been shown to increase the risk of MDD and to predict lower response to usual care antidepressants.Reference Luppino, de Wit and Bouvy 75 , Reference Kloiber, Ising and Reppermund 77 , Reference Dunbar, Reddy and Davis-Lameloise 78 Inflammation and abdominal fat serve as the link between obesity and MDD, as well as worse antidepressant response. It has therefore been suggested that BMI measurements could be used to direct patient care in depression.
Metabolic disorders are not limited to obesity or high BMI: diabetes and insulin resistance are also associated with increased levels of depression.Reference Holt, de Groot and Golden 79 Insulin resistance is associated with increased CRP levels and BMI, specifically abdominal fat.Reference Preis, Massaro and Robins 80 In the Framingham Heart Study, the odds ratios of insulin resistance with abdominal obesity, as measured by subcutaneous adipose tissue and visceral adipose tissue, were 2.48 (95% CI, 2.24–2.74; p < 0.0001) and 3.46 (95% CI, 3.08–3.90; p < 0.0001), respectively.Reference Preis, Massaro and Robins 80 Improving dietary factors, addressing insulin resistance and/or diabetes, and promoting a regimen of exercise may reduce depressive symptomology.Reference Lim, Kim, Kim, Lee, Choi and Yang 81 , Reference Stubbs, Vancampfort and Hallgren 82 Physical exercise has many benefits, such as decreased risk of cardiovascular and metabolic disease; reduced inflammatory parameters, including CRP; improved psychological functioning and mood; and increased neurotransmitters such as serotonin, dopamine, and norepinephrine.Reference Stubbs, Vancampfort and Hallgren 82 – Reference Craft and Perna 84 In addition, markers of obesity, insulin resistance, and metabolic syndrome have been correlated with increased levels of CRP in patients with depression, as well as increased severity of depressive symptoms.Reference Delgado, Huet and Dexpert 85 , Reference Rethorst, Bernstein and Trivedi 86
Cardiovascular disease
Cholesterol is essential for CNS development and function, synapse formation, dendrite formation, and axonal guidance.Reference Parekh, Smeeth, Milner and Thure 87 Interference with any of these mechanisms can result in disruption in neurotransmission and diminished synaptic plasticity, both of which are found in patients with depression.Reference Parekh, Smeeth, Milner and Thure 87 Studies have demonstrated an increased risk of cardiovascular disease and cardiac-related death in patients who have depression.Reference Nicholson, Kuper and Hemingway 88 , Reference Meijer, Conradi, Bos, Thombs, van Melle and de Jonge 89 Though the exact link between cardiovascular disease and MDD has not yet been clearly defined, altered cholesterol metabolism and atherosclerosis have been thought to contribute to the risk of depression, as evidenced by abnormal cholesterol levels in MDD patients.Reference Parekh, Smeeth, Milner and Thure 87 , Reference Freedland and Carney 90 Patients with MDD have been observed to have lower high-density lipoprotein cholesterol levels and elevated low-density lipoprotein cholesterol levels, indicating a positive correlation between depression and cholesterol metabolism.Reference Dunbar, Reddy and Davis-Lameloise 78 , Reference Parekh, Smeeth, Milner and Thure 87 , Reference Gupta, Petkar, Jadhav and Dubey 91 , Reference Lehto, Niskanen and Tolmunen 92
Notably, serotonin plays an important role in platelet aggregation. Increased serotonin in the cardiovascular system may cause arrhythmia and subsequent heart block or valvular fibrosis.Reference Maurer-Spurej 93 During occlusive coronary thrombus formation, serotonin may increase clot stability and ischemia, as a result of its vasoconstrictive properties. Administration of SSRIs limits the uptake of blood serotonin by platelets, inhibits platelet aggregation, and increases risk of bleeding.Reference Meijer, Heerdink, Nolen, Herings, Leufkens and Egberts 94
Stress
Stress, both chronic and acute, may be neurotoxic in nature, associated with increased levels of inflammatory markers, weakening of the blood–brain barrier, and peripheral cytokine entry into the brain.Reference Menard, Hodes and Russo 95 Life stressors, in combination with genetic predisposition, put individuals at a higher risk for developing depression.Reference Southwick, Vythilingam and Charney 96 Stress may induce an inflammatory response in the brain (through an overstimulated immune system and overactivated sympathetic nervous system) and increase glucocorticoid levels.Reference Liu, Wang and Jiang 63 In some patients, activation of the hypothalamic–pituitary–adrenal axis is observed, which increases stress hormones such as corticotrophin-releasing hormone and adrenocorticotropic hormone.Reference Liu, Wang and Jiang 63
Proinflammatory cytokines and acute-phase reactants, such as CRP, IL-6, and TNF-α, are involved in stress-induced inflammation.Reference Liu, Wang and Jiang 63 Through increased inflammatory cytokine expression, stress can provoke depressive symptoms and lead to changes in behavior.Reference Liu, Wang and Jiang 63 Stress also leads to the release of hormones, such as cortisol and dehydroepiandrosterone (DHEA), that are associated with the development of depressive symptoms.Reference Southwick, Vythilingam and Charney 96 These factors can lead to hyperactivity of neural networks such as the hypothalamic–pituitary axis, which may be responsible for depressive symptomology resulting from chronic or acute stress.Reference Gunn, Brown, Lambert and Belelli 97 Stress may also cause reductions in 5-HT1A receptor binding and changes in serotonin activity, which may contribute to anxiety and depression.Reference Southwick, Vythilingam and Charney 96 Indeed, stressful life events, especially early life adversity are associated with a higher risk of depression. 98 – Reference Comijs, Beekman, Smit, Bremmer, van Tilburg and Deeg 100 Early life adversity, which includes abuse, neglect, distress, and negative experiences during the infancy/toddler age, has been shown to impact neurobiological development, resulting in depressive behavior.Reference Goff and Tottenham 99 Furthermore, maternal depression is a risk factor for depression in children and remission in the treatment of depressed mothers has been shown to reduce psychopathology in their children.Reference Weissman, Pilowsky and Wickramaratne 101
Key takeaways
The emerging evidence discussed here suggests that factors including inflammation, metabolic abnormalities, and stress may contribute to the pathophysiology of depression. Furthermore, it is possible that when these elements have contributed to an individual patient’s depression, that patient may be less likely to respond adequately to antidepressant monotherapy, especially therapies that are predominantly serotonergic, such as SSRIs or SNRIs. These patients may be better served by early adjunctive interventions.
The Role of Folate in Depression
Following the active uptake of the reduced form of folate across the blood–brain barrier, it is transported into neuronal cells through the cerebrospinal fluid and is involved in the methylation of homocysteine, synthesis of methionine and S-adenosylmethionine (SAMe), and other methylation-dependent pathways in depression.Reference Bottiglieri 7 A growing body of literature supports the importance of folate in cognition and the cognitive deficits that are associated with psychiatric conditions. Folate levels have been found to correlate with performance in various cognitive tasksReference de Lau, Refsum, Smith, Johnston and Breteler 102 and are inversely correlated with dementia and Alzheimer disease risk.Reference Luchsinger, Tang, Miller, Green and Mayeux 103 , Reference Smach, Jacob and Golmard 104 Antibodies against folate receptors have been found in patients with psychiatric conditions,Reference Frye, Sequeira, Quadros, James and Rossignol 105 and maternal folate deficiency increases the risk of psychiatric illness in children.Reference McClellan, Susser and King 106 In studies of folate and depression, higher folate intake was correlated with a lower risk of depression and anxiety, and folate levels were found to be inversely correlated with depression risk.Reference Jacka, Maes, Pasco, Williams and Berk 107 , Reference Nanri, Hayabuchi, Ohta, Sato, Mishima and Mizoue 108 In a prospective clinical study, folate levels were shown to be notably lower in patients with depression than in the control group (p < 0.01).Reference Bottiglieri, Laundy, Crellin, Toone, Carney and Reynolds 109 Furthermore, decreased levels of folate have been shown to be associated with lower cognitive performance, lower psychomotor speed, and greater depressive symptoms, suggesting a concentration–response relation.Reference de Lau, Refsum, Smith, Johnston and Breteler 102 , Reference Smach, Jacob and Golmard 104 , Reference Nanri, Hayabuchi, Ohta, Sato, Mishima and Mizoue 108 , Reference Beydoun, Shroff, Beydoun and Zonderman 110
Several mechanistic links may exist between folate and depression (Figure 1).Reference Bottiglieri 7 , Reference Stahl 8 , 111 – Reference Douaud, Refsum and de Jager 114 Folate has been associated with a reduction in gray matter loss, suggesting a neuroprotective effectReference Douaud, Refsum and de Jager 114 ; it is required for the health and functioning of DNA through its role in methylationReference Folate 115 ; and it is necessary for proper functioning of the 1-carbon metabolism cycle. Proper functioning of the 1-carbon metabolism cycle is critical for neural development, neural health in adulthood, and inflammation signaling pathway function,Reference Guest, Bilgin, Hokin, Mori, Croft and Grant 116 which has been implicated in mood regulation.Reference Haroon, Raison and Miller 117 Folate can positively impact lipid profiles and reduce oxidative stress, and folate-related genes can impact mood through stress-related mechanisms. 118 – Reference Lok, Bockting and Koeter 120 Furthermore, during inflammation or oxidative stress, folate may be involved in the biochemical reactions, leading to the synthesis of the monoaminergic neurotransmitters that are implicated in the pathophysiology of depression.Reference Haroon, Raison and Miller 117
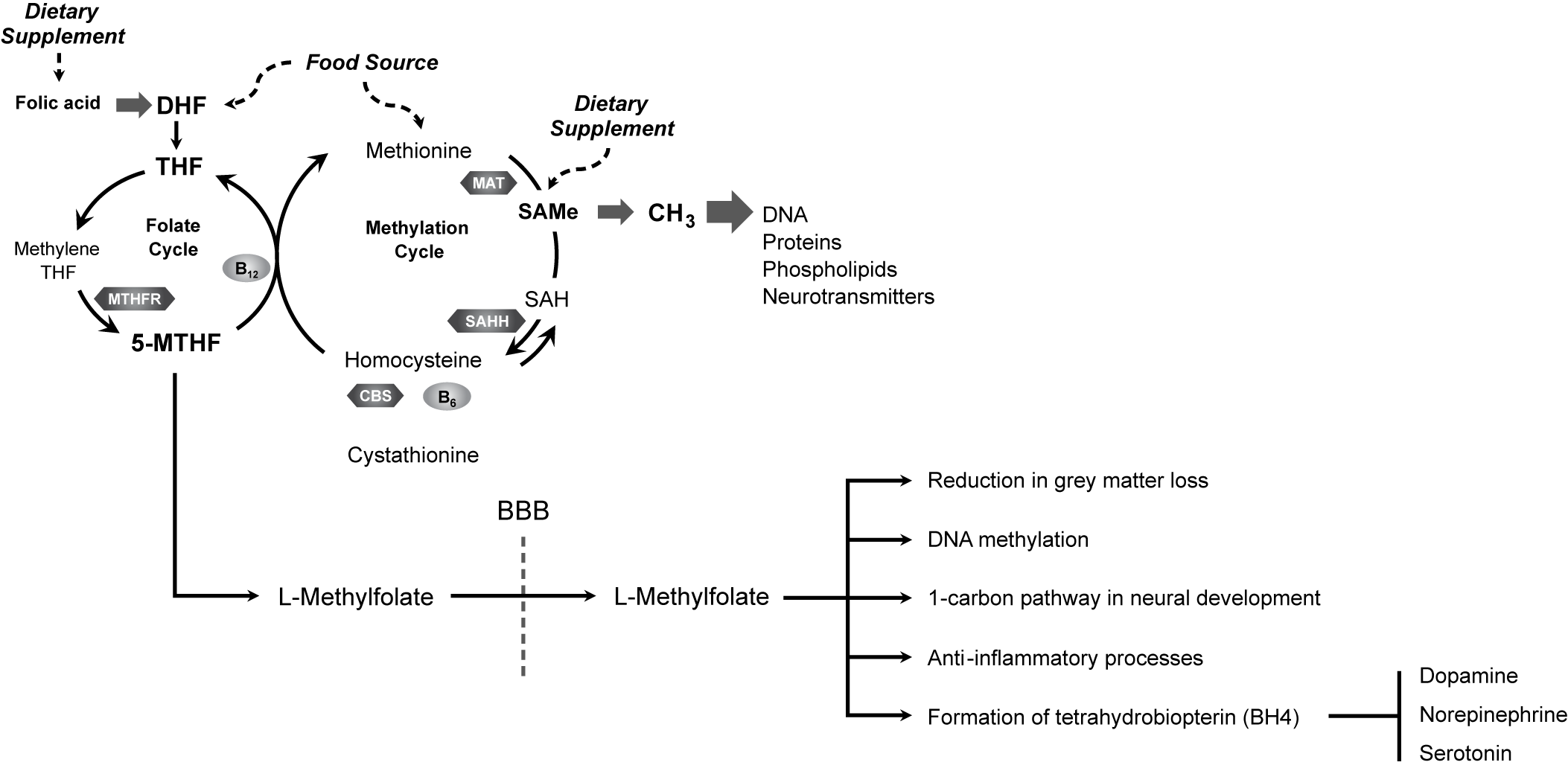
Figure 1. L-Methylfolate enters the brain and influences numerous neural processes to promote neurological health and function.Reference Bottiglieri 7 , Reference Stahl 8 , 111 – Reference Douaud, Refsum and de Jager 114 BBB, blood–brain barrier; CBS, cystathionine β synthase; DHF, dihydrofolate; MAT, methionine adenosyltransferase; 5-MTHF, 5-methyltetrahydrofolate; MTHFR, methyltetrahydrofolate reductase; MTR, methionine synthase; SAH, S-adenosylhomocysteine; SAHH, S-adenosylhomocysteine hydrolase; SAMe, S-adenosylmethionine; THF, tetrahydrofolate.
Underscoring the link between folate and depression are genetic mutations and polymorphisms to folate-related genes that have been identified in conjunction with neurological disorders.Reference Krajinovic 121 The gene encoding the enzyme methylenetetrahydrofolate reductase (MTHFR) of the 1-carbon pathway, and the genes encoding the enzymes methionine synthase (MTR) and methionine synthase reductase (MTRR),Reference Wang, Jiao, Wang, Sun and Dong 122 are necessary for converting dietary dihydrofolate or folic acid to L-methylfolate, the biologically active form of folate that can pass through the blood–brain barrier. L-methylfolate has a higher bioavailability than folic acid and is the only form of folate that is able to cross the blood–brain barrier.Reference Shelton, Sloan Manning, Barrentine and Tipa 123
MTHFR is an essential enzyme responsible for the irreversible final step in folic acid reduction to produce L-methylfolate, therefore, a mutation in the MTFHR gene cannot be bypassed by any of the intermediate metabolites, as this would require metabolism through MTHFR.Reference Pietrzik, Bailey and Shane 112 Numerous pharmacogenomics studies have revealed the strong correlation between MTHFR polymorphisms and depression, validating its importance in this clinical setting.Reference Lok, Bockting and Koeter 120 , 124 – Reference Wu, Ding and Sun 126 Furthermore, the precise polymorphism present may determine whether a patient demonstrates a normal, intermediate, or poor metabolizer phenotype.Reference Ellingrod, Miller, Taylor, Moline, Holman and Kerr 127 Patients with MTHFR variants may not synthesize adequate amounts of monoaminergic neurotransmitters, limiting the effectiveness of SSRI/SNRI antidepressants. Indeed, animal studies of folate in depression suggest that folate may improve symptoms of depression or augment the effects of antidepressants.Reference Budni, Zomkowski and Engel 128
Given its established role in the pathophysiology of depression, both the American Psychiatric AssociationReference Gelenberg, Freeman and Markowitz 23 and the British Association for PsychopharmacologyReference Cleare, Pariante and Young 129 have recommended folate in general, and L-methylfolate specifically, as augmentation/adjunctive strategies for patients with depression. According to the American Psychiatric Association, folate is recommended as a reasonable adjunctive strategy with little risk, as supported by modest evidence.Reference Gelenberg, Freeman and Markowitz 23 The British Association for Psychopharmacology recommends using L-methylfolate as the “next step” in patients who are not responsive to drugs for depression.Reference Cleare, Pariante and Young 129
Clinical Evidence for the Use of L-Methylfolate
A prescription formulation of L-methylfolate (Deplin®, Alfasigma USA, Inc.; Covington, LA) is a medical food with a recommended dosage of 15 mg/day for use under clinician supervision for the dietary management of depression, and meets distinctive nutritional requirements for patients with depression. 130 , 131 The efficacy of this formulation of L-methylfolate used adjunctively for the treatment of MDD has been analyzed in 2 double-blind, randomized, placebo-controlled clinical trials (Table 3),Reference Zajecka, Fava, Shelton, Barrentine, Young and Papakostas 33 , Reference Papakostas, Shelton and Zajecka 111 , Reference Fava, Papakostas and Shelton 113 , 132 – Reference Shelton, Pencina and Barrentine 134 which together represent the largest number of patients treated with any form of folate ever studied for MDD.Reference Zajecka, Fava, Shelton, Barrentine, Young and Papakostas 33 , Reference Papakostas, Shelton and Zajecka 111 , Reference Fava, Papakostas and Shelton 113 , Reference Ginsberg, Oubre and Daoud 132 , Reference Papakostas, Shelton and Zajecka 133 Results from these large-scale and best-designed clinical trials provide robust evidence regarding the safety and efficacy of various doses of L-methylfolate and its potential place in therapy for MDD. Furthermore, notable findings from these studies provide valuable insight into which candidates would achieve optimal outcomes and may benefit most from L-methylfolate treatment.
Table 3. Clinical evidence for the use of L-methylfolate in the treatment of depression.

Abbreviations: BMI, body mass index; CGI-S, Clinical Global Impressions-Severity; HDRS-28, 28-item Hamilton Depression Rating Scale; hsCRP, high-sensitivity C-reactive protein; MDD, major depressive disorder; MTHFR, methylenetetrahydrofolate reductase gene; MTR, methionine synthase gene; MTRR, methionine synthase reductase gene; N/A, not applicable; NNH, number needed to treat in order for 1 person to experience harm; NNT, number needed to treat in order for 1 person to benefit; SNRI, serotonin-norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor.
Importantly, adjunctive use of L-methylfolate was found to be efficacious and has been determined to work especially well in certain subsets of patients, including those with SSRI-resistant depression.Reference Papakostas, Shelton and Zajecka 111 Overall, adverse events with L-methylfolate and with placebo were similar (Table 4),Reference Papakostas, Shelton and Zajecka 111 with minimal changes in weight, supine and standing heart rate, and supine and standing diastolic and systolic blood pressure.
Table 4. Reported adverse events using adjunctive therapy to SSRIs with L-methylfolate or placebo in SSRI-resistant depression.Reference Papakostas, Shelton and Zajecka 111

Data are n (%).
a Ns are based on the total numbers of patients who received placebo or 15 mg of L-methylfolate, respectively, at some point during the trial.
In a post hoc analysis by Papakostas et al,Reference Papakostas, Shelton and Zajecka 111 , Reference Fava, Papakostas and Shelton 113 , Reference Papakostas, Shelton and Zajecka 133 changes from baseline in the 28-item HDRS-28 score were significantly greater with L-methylfolate versus placebo across all investigated plasma markers, which included a SAMe:SAH (S-adenosylhomocysteine) ratio of less than 2.71, high-sensitivity CRP (hs-CRP) levels of 2.25 mg/L or greater, and 4-hydroxy-2-nonenal blood levels of 3.28 μg/mL or less (p ≤ 0.05). There were also statistically significant improvements from baseline in most genetic markers in the L-methylfolate group (p < 0.05). Subgroups of patients with MTR 2756 AG/GG or MTRR 66 AG/GG genotypes had greater mean changes from baseline in HDRS-28 scores than those with homozygous dominant genotypes (p < 0.05). Combinations of biomarkers and/or genetic markers (eg, MTHFR 677 CT/TT+MTR 2745 AG/GG) had varying effect sizes but also were associated with marked improvements in HDRS-28 scores (p ≤ 0.05; number needed to treat in order for 1 person to benefit [NNT], 1–4). Another post hoc analysis by Shelton et alReference Shelton, Pencina and Barrentine 134 determined that patients with BMI ≥30 kg/m2 or elevated levels of IL-8 (p = 0.025) had significantly greater clinical responses with adjunctive L-methylfolate. After correction for multiple testing, pooled effects were demonstrated for patients with BMI ≥30 kg/m2 plus elevated levels of IL-8, IL-12, TNF-α, hsCRP, or leptin (p < 0.03).
Clinical Scenarios for L-Methylfolate use in MDD: Good, Better, and Best
The positive clinical trial findings for L-methylfolate use as an adjunctive treatment for depression suggest that certain patient characteristics may be especially predictive of response. These characteristics, along with the good, better, and best scenarios in which L-methylfolate use may be more or less likely to improve a patient’s symptoms, are summarized in Figure 2 Reference Stahl 8 , Reference Gelenberg, Freeman and Markowitz 23 , Reference Papakostas, Shelton and Zajecka 111 , Reference Fava, Papakostas and Shelton 113 , 130 , 131 , 133 – Reference Gilbody, Lightfoot and Sheldon 136 and described in detail below.

Figure 2. Good, better, best: candidates for the adjunctive use of L-methylfolate to treat depression.Reference Stahl 8 , Reference Gelenberg, Freeman and Markowitz 23 , Reference Papakostas, Shelton and Zajecka 111 , Reference Fava, Papakostas and Shelton 113 , 130 , 131 , Reference Papakostas, Shelton and Zajecka 133 ‑ Reference Gilbody, Lightfoot and Sheldon 136 MTHFR, methyltetrahydrofolate reductase; SSRI, selective serotonin reuptake inhibitor.
Good scenario
Overall, L-methylfolate and other forms of folate are considered to be low-risk adjunctive interventions in patients with MDD, and are associated with general health benefits.Reference Gelenberg, Freeman and Markowitz 23 Individuals who prefer nutritional products with limited side effects may find L-methylfolate an attractive adjunctive option,Reference Stahl 8 as well as those who attempt a holistic regimen including exercise inductionReference Gelenberg, Freeman and Markowitz 23 to manage more mild depressive symptoms. Patients with a partial response to SSRI/SNRIs may augment treatment with L-methylfolate to achieve better outcomes. In a retrospective study, patients who were treated with SSRI/SNRIs supplemented with L-methylfolate had greater improvements in depressive symptoms than patients who were treated with SSRI/SNRIs alone (p = 0.01),Reference Ginsberg, Oubre and Daoud 132 without alteration of the SSRI/SNRI side effect profile.
Better scenario
L-Methylfolate may be of better clinical utility in patients who have evidence of MDD treatment failure, particularly with SSRIs. As described above, use of L-methylfolate is associated with better efficacy in patients with SSRI-resistant depression.Reference Papakostas, Shelton and Zajecka 111 These outcomes are particularly improved in patients with the MTR 2756 AG/GG genotype, a low SAMe:SAH ratio, high BMI, or elevated levels of CRP or IL-8.Reference Fava, Papakostas and Shelton 113 , Reference Papakostas, Shelton and Zajecka 133 , Reference Shelton, Pencina and Barrentine 134 These findings are important because treatment considerations post–SSRI failure may include atypical antipsychotics, which are associated with a risk of tardive dyskinesia, potentially irreversible. Atypical antipsychotics might also be inappropriate in obese patients due to their class-related metabolic side effects (ie, weight gain; increased levels of insulin, glucose, and low-density lipoprotein cholesterol; diabetes mellitus; and hypertriglyceridemia).Reference Allison, Mentore and Heo 137 – Reference Lieberman 139 Use of these agents may also put patients at greater risk for cardiovascular complications, including stroke and coronary heart disease,Reference Lieberman 139 which are also associated with high CRP levels.Reference Pepys and Hirschfield 58
Best scenario
Patients with MDD and BMI ≥30 kg/m2, documented low levels of folate or its metabolites, and/or impaired MTHFR enzyme activity have a clear rationale for the adjunctive use of L-methylfolate.Reference Stahl 8 , Reference Fava, Papakostas and Shelton 113 , Reference Gilbody, Lightfoot and Sheldon 136 In some patients, low folate levels can result from alcohol abuse, hypothyroidism, eating disorders, pregnancy, gastrointestinal disorders, or from taking certain medications; thus, clinicians should consider adding L-methylfolate to the treatment regimen of patients with any of these factors.Reference Stahl 8 Findings from the post hoc analysis of clinical trials with L-methylfolateReference Papakostas, Shelton and Zajecka 133 demonstrated the largest effect sizes versus placebo and NNT = 1 for L-methylfolate in patients whose depression failed to respond to SSRI therapy and had 2 of the studied biologic markers associated with inflammation/obesity and/or folate metabolism gene polymorphisms (eg, MTR 2756 AG/CG + COMT (rs4633) CC or BMI ≥30 kg/m2 + DRD2 TC/TT). Additionally, pooled effects have been shown for patients with BMI ≥30 kg/m2 plus elevated levels of IL-8, IL-12, TNF-α, hsCRP, or leptin.Reference Shelton, Pencina and Barrentine 134 Therefore, identification of more than 1 of these factors is an especially important indication that adjunctive L-methylfolate use may improve a patient’s clinical outcome.
When to avoid use of L-methylfolate
Although it is an effective, low-risk option, L-methylfolate may not be suitable for all patients. L-methylfolate should not be used in patients with hypersensitivity to the product. 130 , 131 In general, high folate levels may increase the risk for cardiovascular disease.Reference Sauer, Mason and Choi 135 Similarly, while moderate folate levels have been associated with reduction in the risk of several cancer types, the risk of adenoma recurrence and colorectal cancer may be higher with both low and high levels of folate.Reference Sauer, Mason and Choi 135 Patients who exhibit signs of manic, hypomanic, or mixed episodes should have their diagnosis and treatment reevaluatedReference Gelenberg, Freeman and Markowitz 23 and should not necessarily initiate or continue folate supplementation. In particular, findings from a clinical study in bipolar depressionReference Geddes, Gardiner and Rendell 140 suggest potential dampening of the effects of lamotrigine, which is known to inhibit dihydrofolate reductase and the formation of L-methylfolate,Reference Mischoulon, Zajecka, Freeman and Fava 141 when coadministered with folate. Augmentation with L-methylfolate or folinic acid, which do not require conversion by dihydrofolate reductase, may mitigate this effect.Reference Mischoulon, Zajecka, Freeman and Fava 141
Additional considerations
Promotion of wellness
Considerations for adding L-methylfolate to a patient’s treatment include its use in addition to diet and exercise modifications and its introduction early in the course of treatment. Dietary supplementation with folate alone is not sufficient for treatment, since it requires enzyme conversion and must be reduced to the active form.Reference Pietrzik, Bailey and Shane 112 As previously mentioned, genetic mutations in the enzymes involved in the folate pathway may impair enzymatic conversion to L-methylfolate and affect L-methylfolate levels, especially in patients with depression and people with higher risk for low folate levels (eg, pregnant women, people who abuse alcoholic, people with eating disorders). In this subset of patients, supplementation with L-methylfolate would be beneficial. L-methylfolate, as opposed to atypical antipsychotics, is not known to produce fatigue,Reference Papakostas, Shelton and Zajecka 111 and therefore may be conducive to starting or maintaining exercise regimens and to supporting general wellness initiatives for patients with MDD. Because physical activity has recently been recognized by the European Psychiatric Association as being therapeutic for people with severe mental illness, including depression,Reference Stubbs, Vancampfort and Hallgren 82 and because exercise has clearly demonstrated antidepressant effects,Reference Belvederi Murri, Amore and Menchetti 142 – Reference Blumenthal, Sherwood and Babyak 144 consideration of L-methylfolate use aligns well with recommendations for diet/exercise and promotion of wellness in patients with MDD.
Safety profile
Importantly, L-methylfolate is well tolerated, and its safety profile is similar to that of placebo when used as adjunctive therapy in MDD.Reference Zajecka, Fava, Shelton, Barrentine, Young and Papakostas 33 , Reference Papakostas, Shelton and Zajecka 111 , Reference Ginsberg, Oubre and Daoud 132 Use of L-methylfolate is not associated with the sexual, cardiovascular, metabolic (ie, weight gain), and neurologic side effects associated with atypical antipsychotics and SSRI/SNRIs.Reference Gelenberg, Freeman and Markowitz 23 , Reference Papakostas, Shelton and Zajecka 111 , Reference Spielmans and Gerwig 145 In addition to metabolic and weight gain side effects, atypical antipsychotics have been associated with movement disorders such as akathisia, extrapyramidal symptoms, and tardive dyskinesia.Reference Salem, Nagpal, Pigott and Teixeira 146 , Reference Rasimas and Liebelt 147
Quality of life and patient satisfaction
Patients who used L-methylfolate as augmentation for the management of MDD reported major improvements in functioning at work, at home, and in social situations.Reference Shelton, Sloan Manning, Barrentine and Tipa 123 The percentage of patients who had reported functioning being very difficult or extremely difficult decreased from 50% to 13% following L-methylfolate treatment. Furthermore, patient satisfaction reached a rating of 7 out of 9 after treatment from 5.2 prior to treatment, with 1 being “not at all satisfied” and 9 being “very satisfied.”
Availability and interpretation of genetic testing
Pharmacogenetic testing was recently shown to improve treatment outcomes, identify patients likely to have treatment resistance, reduce side effect burden, and facilitate selection of genetically appropriate medications in depression management.Reference Greden, Parikh and Rothschild 148 , Reference Macaluso and Preskorn 149 These tests may provide clinicians with better insight in the management of depression by identifying genetic markers, such as mutations in enzymes involved in the metabolism of folate, that may guide treatment decisions. Overall, while the concept of pharmacogenetic testing is promising in MDD, more research is needed before clinical use may become routine.Reference Macaluso and Preskorn 149
Considering the positive correlation between MTHFR polymorphisms and the risk of depression, genetic testing may be a promising avenue for identifying patients who may benefit from L-methylfolate supplementation in this clinical setting.Reference Gilbody, Lewis and Lightfoot 124 Salivary or blood genetic testing kits for MTHFR variants, typically C677T and A1298C, are commercially available 150 and can be performed in most conventional laboratories.Reference Jade 151 The salivary genetic test is available through companies that offer complete genetic profiling; however, it is not recommended to be used for the diagnosis of depression.Reference Jade 151
Conclusions
L-methylfolate has been well studied in multiple clinical trials, and findings support its consideration for use as an adjunctive therapy in any depression management program, and especially in patients with characteristics suggestive of potential responsiveness, such as low folate levels, mutations in genes coding enzymes involved in the metabolism of folate, BMI greater than 30 kg/m2, and elevated markers of inflammation, including CRP. Use of L-methylfolate in the clinical setting of MDD should be considered in conjunction with other treatments and forms of holistic management, in a multimodal team approach. Supplementation with L-methylfolate fits well with the changing paradigm of MDD management, with the ultimate goal of producing wellness instead of focusing solely on symptom reduction. Future studies with L-methylfolate could help to further confirm its role in the management of MDD.
Acknowledgments
Medical writing assistance was provided by Peloton Advantage, LLC, an OPEN Health company, Parsippany, NJ and was funded by Alfasigma USA, Inc. The authors are entirely responsible for the scientific content of the article.
Funding
This work was funded by Alfasigma USA, Inc. The authors did not receive payment for their participation.
Disclosures
Rakesh Jain has the following disclosures: Consultant: Acadia, Alfasigma, Allergan, Eisai, Evidera, Impel, Janssen, Lilly, Lundbeck, Merck, Neos Therapeutics, Neurocrine Biosciences, Osmotica, Otsuka, Pamlab, Pfizer, Shire, Sunovion, Supernus, Takeda, Teva. Speaker/Promotional Honoraria: Alkermes, Allergan, Janssen, Lilly, Lundbeck, Merck, Neos Therapeutics, Neurocrine Biosciences, Otsuka, Pamlab, Pfizer, Shire, Sunovion, Supernus, Takeda, Teva, Tris Pharmaceuticals. Advisory Board: Alkermes, Janssen, Lilly, Lundbeck, Merck, Neos Therapeutics, Neurocrine Biosciences, Otsuka, Pamlab, Pfizer, Shire, Sunovion, Supernus, Takeda, Teva. Research Grants: Allergan, Lilly, Lundbeck, Otsuka, Pfizer, Shire, Takeda. Sloan Manning has the following disclosures: Consultant and Speakers Bureau: Sunovion and Otsuka. Consultant: Allergan, Acadia, Alkermes, Lundbeck. Andrew Cutler has the following disclosures: Consultant: Acadia, Alfasigma (Pamlab), Alkermes, Allergan, Avanir, Axsome, IntraCellular Therapies, Janssen, Lundbeck, Neurocrine, Novartis, Otsuka, Sage, Shire, Sunovion, Supernus, Takeda, Teva. Speaker/Promotional Honoraria: Acadia, Alfasigma (Pamlab), Alkermes, Allergan, Avanir, Janssen, Lundbeck, Neurocrine, Otsuka, Shire, Sunovion, Takeda, Teva. Research Grants: Acadia, Alkermes, Allergan, Axsome, IntraCellular Therapies, Janssen, Lundbeck, Neurocrine, Novartis, Otsuka, Shire, Sunovion, Supernus, Takeda. Board Member: Neuroscience Education Institute.