Strawberries are an important horticultural crop in the United States, with an estimated production value of $2.86 billion in 2014 (USDA 2015). Within the United States, Florida is the second highest producer, with an estimated production value of $306.5 million in 2014 (USDA 2015). Florida strawberries are produced during the winter months in a plasticulture system, typically consisting of cultivation and bed formation in August followed by the application of fumigants, insertion of the drip tape, and covering with plastic mulch. Holes are punched in the plastic and strawberries are transplanted approximately 1 mo after fumigation, which corresponds with mid-September to mid-October in northern and central Florida, respectively, while in southern Florida, planting occurs from October to December (Whitaker et al. Reference Whitaker, Boyd, Peres and Smith2015). Harvest occurs from approximately late December to late March.
Weed management is an important consideration in strawberry production. Plasticulture provides excellent broadleaf and grass weed control in the bed, with the exception of the planting-holes created for transplants. Two of the most problematic broadleaf weeds in Florida strawberry production are black medic (Medicago lupulina L.) and Carolina geranium (Geranium carolinianum L.) (Webster Reference Webster2014). These weeds escape current fumigant and PRE herbicides and emerge approximately midcycle, corresponding to late-November for black medic (Sharpe Reference Sharpe2017). Seeds survive the fumigation treatment and germinate when the fumigant has dissipated and the efficacy of the herbicide has diminished.
The only chemical alternative to hand weeding for POST broadleaf control within the bed for strawberry plasticulture production is the herbicide clopyralid. Clopyralid is registered for use on strawberries in Florida and has no detrimental effects on reproductive growth or harvested yield when applied 7 to 17 weeks after transplant at registered rates (140 to 280 g ae ha−1) (Boyd and Dittmar Reference Boyd and Dittmar2015). However, growers often report incomplete control of black medic and Carolina geranium with clopyralid, which is consistent with the label (Anonymous 2011). Optimal control of black medic occurs when plants are sprayed directly at the 0.5- to 1-cm stem length stage (Sharpe et al. Reference Sharpe, Boyd and Dittmar2016), which places the plant in the planting hole below the strawberry canopy. Canopy shielding may be a major obstacle in controlling black medic (McMurray et al. Reference McMurray, Monks and Leidy1996), as poor penetration of spray droplets into the canopy may result in incomplete coverage on the target vegetation. Incomplete coverage is further problematic, as clopyralid has demonstrated limited translocation between black medic stems (Sharpe Reference Sharpe2017).
Spray coverage is an important consideration for controlling weeds with herbicides applied POST. Crop canopy structure affects spray penetration and is largely species and growth-stage dependent. In soybean (Glycine max (L.) Merr.), fungicide spray coverage was significantly reduced in the early pod development stage when compared with other growth stages (Hanna et al. Reference Hanna, Conley, Shaner and Santini2008). In potato (Solanum tuberosum L.), spray deposition efficiency was significantly reduced in the bottom when compared with the top of the canopy (Nansen et al. Reference Nansen, Vaughn, Xue, Rush, Workneh, Goolsby, Troxclair, Anciso, Gregory, Holman, Hammond, Mirkov, Tantravahi and Martini2011). Similar canopy shielding likely occurs with strawberry, with reduced herbicide exposure and uptake and subsequent weed control.
Methods to increase spray penetration through the canopy to enhance weed control include increasing the application volume and changing the nozzle type. Air induction nozzles and high volumes (281 L ha−1) consistently increased spray coverage of Palmer amaranth (Amaranthus palmeri S. Wats.) in peanut plantings when compared with flat nozzles and lower volumes (94 and 187 L ha−1) (Berger et al. Reference Berger, Dobrow, Ferrell and Webster2014). In a direct application study, while nozzle type had no effect, increasing application volume increased control of three grass species (Ramsdale and Messersmith Reference Ramsdale and Messersmith2001). Nozzle type effectiveness is also influenced by spray solution formulation (Miller and Butler Ellis Reference Miller and Butler Ellis2000), which may affect spray penetration through the crop canopy. The objective of this research was to determine if strawberry cultivar affects spray penetration through the canopy and whether nozzle type and application volume can reduce the impacts of crop shielding.
Materials and Methods
Cultivar Canopy Effects on Penetration
The study was conducted at two locations: the Gulf Coast Research and Education Center in Balm, FL, and the Florida Strawberry Growers Association field site in Dover, FL. Planting beds were 66 cm wide and 30.5 cm high. Transplants of three commercially important Florida cultivars, ‘Florida Radiance’, ‘Strawberry Festival’, and Sensation™ ‘Florida 127’, were planted on October 8, 2013, in Dover and October 11, 2013, in Balm. For both locations, there were two rows per bed with 38-cm spacing between plants.
The experimental design was a complete block with four blocks. The main factor was strawberry cultivar type. There was a restriction on randomization, as cultivars were previously planted in rows, so randomization within the block was not possible. The experimental unit was a 3-m by 66-cm plot contained within one bed, with 16 plants per plot. The experiment was conducted on April 4, 2014, in Dover and April 21, 2014, in Balm, when plants were fully matured and producing fruit.
Strips of water-sensitive paper (WSP) (45 by 2.5 cm, Syngenta Crop Protection AG, Basel, Switzerland) were cut into three 15- by 2.5-cm sections. Three pieces were placed around two (Dover site) or three (Balm site) randomly selected plants per plot. WSP strips were positioned to radiate outwards from the crown. Two strips were oriented towards the nearest bed edge and one strip towards the middle of the bed.
Water was sprayed over the top of the strawberry canopy with a handheld CO2-pressurized sprayer (Bell-spray Inc., Opelousas, LA) with a single-nozzle boom using a traditional flat fan nozzle (TP8002EVS, TeeJet, Spraying Systems Co., Wheaton, IL), an application volume of 281 L ha−1, and a pressure of 276 kPa. The boom height was 70 cm above the ground. No surfactant was added to the water to mimic label recommendations with clopyralid. The WSP was permitted to dry and then was collected. Environmental conditions at the time of application in Dover were 27 C, 65% relative humidity (RH), and wind speed of 7 km h−1. In Balm, conditions were 20 C, 76% RH, and wind speed of 11 km h−1.
After WSP collection, each sample plant was measured (height and width), the number of leaves was counted, groundcover was estimated, and plants were destructively harvested to determine aboveground dry weight. Groundcover was determined using a 30.5- by 30.5-cm square quadrat that was partitioned into a grid with 2.5-cm cell spacing. The quadrat was held 30 cm above the bed top. Groundcover was calculated by the number of instances the strawberry plant was counted within each subsection of the quadrat, expressed as a percentage of the total. For dry weight, strawberry plants were cut at the soil line through the crown, dried, and weighed.
The 15-cm WSP strips were scanned digitally in grayscale at a resolution of 600 dots per inch (DPI). For data analysis, strips were divided into the following distances from the crown: 0 to 5 cm (beside the crown), 5 to 10 cm, and 10 to 15 cm (canopy edge). Images were analyzed using DepositScan™ software to calculate percent coverage, droplet number (no. cm−2), and deposition volume (µl cm−2). Distance along the length of the WSP was marked on the image using the software and was considered a spatially repeated measure for the analysis.
Volume and Nozzle Selection
A study was initiated at the Gulf Coast Research and Education Center in Balm, FL, to examine the effect of application volume and nozzle selection on spray penetration through the strawberry canopy. Formed beds were 66 cm wide and 30.5 cm high. The strawberry cultivar at this site was Florida Radiance and was planted in two rows per bed with 38 cm between plants. Strawberry transplants were planted on October 8, 2015.
The experimental design was a randomized complete block with a factorial arrangement of treatments containing four blocks. The experimental unit was a 2-m-long by 66-cm-wide plot, contained in a single bed containing 12 plants. The study occurred on March 31, 2016, and the strawberry crop was fully mature and producing fruit. The experiment was repeated twice in the same field, but on different plants. Plant height, groundcover, and the number of leaves per plant were recorded for twenty plants randomly selected across the study site.
The two main factors were nozzle type and application volume. Three nozzle types were tested: 1) TeeJet Even Flat Spray Tip (TP8002EVS) (EVS), 2) DG TeeJet Drift Guard Flat Spray Tip (DG8002EVS) (Drift Guard), and 3) TwinJet Even Flat Spray Tip (TJ60-8002EVS) (TwinJet) (TeeJet, Spraying Systems Co., Wheaton, IL). The EVS nozzles were selected as the industry standard; the other nozzles have characteristics that may increase canopy penetration: Drift Guard nozzles produce a larger droplet size and TwinJet nozzles have a different spray angle from the industry standard. The TwinJet nozzles apply liquid through two streams at a 60° angle compared to a single stream for the industry standard. Application volumes were 187, 375, and 740 L ha−1. These volumes were selected as the industry standard (187 L ha−1), twice the industry standard but still within the labeled range for clopyralid (375 L ha−1), and a high volume similar to that used to apply fungicides in strawberries (740 L ha−1).
Four strips of WSP (7.6 by 2.5 cm, Syngenta Crop Protection AG, Basel, Switzerland) were randomly placed in each plot below the canopy radiating out from the crown towards the canopy edge. No distinction was made regarding where in the bed the paper was placed, but a limit of one piece of WSP per plant was imposed. Water was applied with a handheld CO2-pressurized sprayer (Bell-spray Inc., Opelousas, LA) with a single nozzle boom according to treatment and a pressure of 276 kPa. The boom height was 70 cm above the ground. At the time of application for the first run of the experiment, environmental conditions were 29 C, 65% RH, and wind speed 17 km h−1. For run two, the conditions were 30 C, 56% RH, and wind speed 20 km h−1. No surfactant was used, per label recommendations with clopyralid. The strips were allowed to air-dry before being collected.
WSP was scanned in grayscale at 600 DPI to produce a digital image. Images were analyzed using DepositScan™ software (U.S. Department of Agriculture, Agricultural Research Service, Wooster, OH) to calculate percent coverage, droplet number (no. cm−2), and deposition volume (µl cm−2). Limits for detection by DepositScan™ are linked to the scanner resolution, where the maximum droplet size for detection at 600 DPI is 42.3 µm diam, the size of a single pixel (Zhu et al. Reference Zhu, Salyani and Fox2011). If a droplet lands among two, three, or four pixels, it is calculated as occupying all of them, leading to a miscalculation in size (Zhu et al. Reference Zhu, Salyani and Fox2011). The relative error was found to decrease with increasing droplet size (Zhu et al. Reference Zhu, Salyani and Fox2011).
Data for both experiments were subjected to analysis of variance utilizing PROC MIXED in SAS version 9.4 (SAS Institute Inc., 100 SAS Campus Drive, Cary, NC). Block and trial run were considered random variables. For the strawberry canopy experiment, distance from the crown was considered a spatial repeated measure using the REPEATED statement, specifying TYPE=AR(1). Model assumptions of normality and constant variance of the residuals were verified. Mean separation of treatments was conducted using Tukey’s HSD (α=0.05).
Results and Discussion
Strawberry Canopy
There were no differences among cultivars in groundcover (P=0.13) or plant dry weight (P=0.89). Leaf number ranged from 37 to 52, plant width ranged from 30 to 36 cm, and plant height ranged from 18 to 22 cm (Table 1). Spray penetration through the strawberry canopy with a flat-fan nozzle, measured as percent coverage, droplet number, or deposition volume, was not affected by cultivar (Table 2). This was unexpected, given the relatively more open growth habit of Florida Radiance compared to the robust (denser canopied) Strawberry Festival or the compact habit of Sensation™ Florida 127 (Chandler et al. Reference Chandler, Santos, Peres, Jauquand, Plotto and Sims2009; Whitaker et al. Reference Whitaker, Chandler and Peres2014; Whitaker et al. Reference Whitaker, Boyd, Peres and Smith2015). It is likely that the bulk biomass of the fully developed strawberry plants overshadowed any subtle differences among growth habits. This suggests that differences in canopy architecture between cultivars of strawberry has minimal influence on spray penetration and subsequent exposure of herbicides to weeds.
Table 1 Canopy parameters of three commercially important strawberry cultivars used in spray penetration studies at Balm and Dover, FL, in 2014.Footnote a
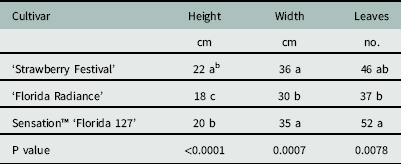
a Data were pooled across both sites and values are least square estimates (n=8).
b Different letters within a column indicate a significant difference (α=0.05) by Tukey’s HSD.
Table 2 Analysis of variance for POST water droplet penetration through three strawberry cultivar canopies measured at various distances from the crown at Balm and Dover, FL, in 2014.Footnote a

a Data were generated from image analysis of scanned water sensitive paper. The area measured was 5 by 2.5 cm. Analysis included both runs with trial as a random variable (n=72).
b Cultivar refers to ‘Florida Radiance’, ‘Strawberry Festival’, and Sensation™ ‘Florida 127’.
c Distance refers to the distance from the crown to the edge of the strawberry plant, measured below the canopy. Three distances were measured: 0 to 5 cm, 5 to 10 cm, and 10 to 15 cm.
d Interaction refers to the interaction between cultivar and distance for the analysis of variance.
There was an effect of distance from the crown on percent coverage, droplet number, and deposition volume below the canopy (Table 2). Droplet coverage closest to the crown (0 to 5 cm) was only 8.1% (Table 3). Coverage increased to 14.8% at 5 to 10 cm from the crown, with a maximum of 27.0% coverage at the canopy edge (10 to 15 cm). The compressed stem, or crown, is the central growing point and site of attachment for strawberry leaves at the nodes (Darnell Reference Darnell2003). The compact growth at the crown was likely an influential factor affecting spray penetration.
Table 3 Effects of distance from strawberry crown on spray coverage, deposition number, and deposition volume for water droplets penetrating a strawberry canopy at Balm and Dover, FL, in 2014.Footnote a
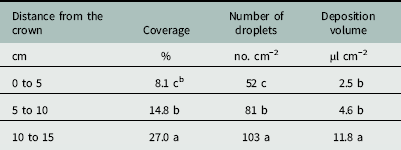
a Strawberry plants were fully mature at 18 to 22 cm in height, 30 to 36 cm in width, and 37 to 52 leaves per plant. Data were generated from image analysis of scanned water sensitive paper. Data were pooled across trial run and cultivar (n=24 per distance from the crown). Data were averaged across cultivars.
b Different letters within a column indicate a significant difference (α=0.05) by Tukey’s HSD.
The ANOVA showed an effect of distance from the crown on the number of spray droplets that penetrated the strawberry canopy (Table 2). The number of droplets increased from 52 droplets cm−2 beside the crown to 81 droplets cm−2 under the canopy middle (Table 3). The number of droplets increased again to 103 cm−2 at the canopy edge. This trend was similar to increased coverage with distance from the crown.
The ANOVA showed an effect of distance from the crown on the volume of spray deposited below the canopy (Table 2). There was no difference in the volume of spray deposited below the canopy between 0 to 5 and 5 to 10 cm from the crown (Table 3). This was contrary to the trend with both coverage and the droplet number (Table 3), and was likely due to the habit of the strawberry plant, where all of the leaves attach to the central crown at the nodes (Darnell Reference Darnell2003). Water deposited on these leaves could move down the petioles toward the crown and ultimately pool at the planting hole. The phenomenon of water deposition at the planting hole beside the crown was not observed consistently in all strips and the standard deviation for the 0 to 5 cm distance was larger than that of any other distance. Depending on the growth habit of black medic, this may lead to increased exposure within the planting hole if black medic is prostrate, as the spray likely pooled on the ground before being absorbed into the soil. There was an increase in the deposition volume from 4.6 µl cm−2 at the middle portion to 11.6 µl cm−2 at the canopy edge.
Averaged across all cultivars, the area beside the crown (0 to 5 cm) received only 8% coverage and a volume of 2.5 µl cm−2 deposited (Table 3). Within the strawberry production system, black medic primarily emerges from the soil at the planting hole as it is unable to penetrate the plastic mulch. It is unknown if black medic at the 0.5- to 1-cm stem length stage can be controlled with such low spray penetration through the canopy, and further experimentation is required. Although spray coverage increased to 27.0% at the canopy edge, controlling black medic with stem lengths of 10 to 15 cm may be problematic, given that plants with stems at 2 to 5 cm were more tolerant to directly applied clopyralid than plants with stems 0.5 to 1 cm in length (Sharpe et al. Reference Sharpe, Boyd and Dittmar2016). Methods to increase spray penetration to overcome strawberry crop shielding may be beneficial in controlling problematic weeds originating at the planting hole.
Volume and Nozzle Type
The Florida Radiance strawberry canopy averaged 25 cm high, with 80% ground-cover and 41 leaves per plant across the trial. There was no effect of nozzle type on coverage below the strawberry canopy (P=0.24), but application volume did affect coverage (P < 0.0001) (Table 4). As application volume increased from 187 to 375 L ha−1, coverage below the canopy increased from 21.9% to 39.7% (Table 5). Increasing the application volume to 740 L ha−1 increased coverage to a maximum of 52.9%. Thus, doubling the application volume overcame the strawberry shielding effects and funneling dynamics of the plant canopy (Table 5). This was consistent with previous findings for direct application onto WSP using conventional and Drift Guard nozzles across lower application volumes (47, 94, and 190 L ha−1) (Ramsdale and Messersmith Reference Ramsdale and Messersmith2001).
Table 4 Analysis of variance for the spray penetration of water through ‘Florida Radiance’ strawberry canopy as affected by various nozzles and application volumes at Balm, FL, in 2016.Footnote a
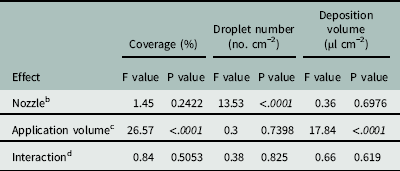
a Data were generated from image analysis of scanned water sensitive paper. The area measured was 7.6 by 2.5 cm from the crown towards the plant edge. Analysis included both trial runs as random variables (n=72).
b Nozzle refers to the type of nozzle used for application of water over the canopy. The three nozzle types were TeeJet Even Flat Spray Tip (TP8002EVS), DG TeeJet Drift Guard Flat Spray Tip (DG8002EVS) and TwinJet Even Flat Spray Tip (TJ60-8002EVS).
c Application volume refers to the volume of water sprayed per unit area over the top of the strawberry canopy. Three volumes, 187, 375, and 740 L ha−1, were tested.
d Interaction refers to the interaction between nozzle type and application volume in the analysis of variance.
Table 5 Effect of application volume on the coverage and water volume deposited below the canopy of ‘Florida Radiance’ at Balm, FL, in 2016.Footnote a
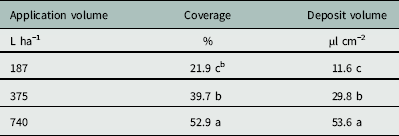
a The area measured was 7.6 by 2.5 cm radiating lengthways outward from the crown. Data were generated from image analysis of scanned water sensitive paper. Data were pooled across trial run and nozzle selection (n=24 per application volume).
b Different letters within a column indicate a significant difference (α=0.05) by Tukey’s HSD.
There was no effect of nozzle type on the deposition volume below the canopy (P=0.70) (Table 4). Neither the larger droplets produced by the Drift Guard nozzles nor the change in angle with the TwinJet nozzles increased the overall volume of water calculated to have penetrated through the canopy. However, there was a significant effect of application volume on the deposition volume below the canopy (P < 0.0001) (Table 4). As the application volume increased from 187 to 375 L ha−1, the volume of spray deposited below the canopy increased from 11.6 µl cm−2 to 29.8 µl cm−2 (Table 5). As the application volume increased to 740 L ha−1, the spray volume deposited below the canopy reached a maximum of 53.7 µl cm−2. Again, this was likely due to the increased volume of water overcoming the natural shielding of the strawberry leaves.
There was no effect of application volume on the number of droplets penetrating the canopy (P=0.74) (Table 4). However, there was an effect of nozzle type (P > 0.001) on the number of droplets. Drift Guard nozzles significantly reduced the number of droplets (72 droplets cm−1) compared to the EVS (109 droplets cm−1) and TwinJet nozzles (131 droplets cm−1) when averaged across all application volumes. The difference was likely due to the larger droplets produced by the Drift Guard nozzles during application. The reduced number of droplets did not affect the coverage nor the volume of water deposited below the canopy (Table 4). Overall, there was no positive effect of changing nozzles on increasing spray droplet penetration; however, increasing application volume increased the number of droplets that reached below the canopy.
In previous work, no interaction was found between nozzle type (Drift Guard and conventional nozzles) and application volume (47, 90, and 190 L ha−1) on herbicide efficacy of either glyphosate or paraquat on grass control (Ramsdale and Messersmith Reference Ramsdale and Messersmith2001). Similarly, there was no interaction between application volume (94 and 140 L ha−1) and nozzle type (extended range, air induction extended range, Turbo TeeJet, and Turbo TeeJet air induction) on herbicide efficiency between the bottom and middle of a soybean canopy (Legleiter and Johnson Reference Legleiter and Johnson2016). This supports our work, suggesting that nozzle type and application volume effects are independent of each other.
Spray penetration through the strawberry canopy is a major obstacle for POST weed control within the bed. Droplet coverage below the canopy was maximal at the canopy edge (10 to 15 cm from the crown), although control of black medic at this distance from the planting hole may be problematic because poor efficacy was demonstrated when stems were longer than 1 cm (Sharpe et al. Reference Sharpe, Boyd and Dittmar2016). The growth and maturity of the strawberry plant at current application times further compounds the problem, shielding weeds that may be present and decreasing coverage (8%) of spray droplets penetrating the strawberry canopy at the planting hole. The increased tolerance of black medic to clopyralid as plant size of black medic increases, combined with strawberry plant shielding, is the major issue to overcome for controlling this problematic midcycle-emerging broadleaf weed species with POST herbicides.
Increasing application volume from 187 to 375 L ha−1 increased coverage from 22% to 40%. This increase in application volume is within the limits of the clopyralid label (Anonymous 2011) and represents an easy alteration to management practices. Further study is required to determine herbicide efficacy in controlling black medic in field settings. Alternatively, future studies altering application pressure instead of application volume may increase canopy penetration. Application of clopyralid earlier in the strawberry production cycle when the strawberry plant is less developed may increase spray penetration, but strawberry crop tolerance to early clopyralid applications requires further study.
Acknowledgements
The authors would like to acknowledge the Florida Strawberry Festival and the Florida Strawberry Research and Education Foundation for supporting and funding this project.







