Protein–energy malnutrition is the most prevalent form of nutritional disorder among children in developing countries. It is estimated that more than 3·7 million deaths in 2000 could be attributed to underweight1. Protein malnutrition often occurs during gestation, lactation and the first 2 years of lifeReference Desai, Garcia Tavares, Dutra de Oliveira, Douglas, Duarte and Dutra de Oliveira2. Despite an overall decrease of stunting in developing countries, child malnutrition still remains a major public health problemReference De Onis, Frongillo and Blossner3.
Barker et al. Reference Barker, Martyn, Osmond, Hales and Fall4 have associated low birth weight with diseases related to the metabolic syndrome (diabetes, obesity and hypertension) in adulthood. This association has been denominated programming, which is defined as the basic biological phenomena that putatively underlie relationships among nutritional experiences of early life and adult diseasesReference Lucas5–Reference Moura and Passos7.
We have shown that adverse situations early in life, such as malnutrition and hormonal changes during lactation, could affect permanently the progenyReference Passos, Ramos and Moura8–Reference Lins, Moura, Lisboa, Bonomo and Passos16.
Previously we showed that the milk of protein-restricted (PR) mothers had low protein and lipid concentrationsReference Passos, Ramos and Moura8 and serum albumin is lower in the offspring at the end of lactation, which confirms that these animals during lactation were malnourished.
Our data concerning the adult offspring whose mothers were submitted to protein restriction during lactation support the hypothesis of a hypermetabolic status programming. We demonstrated that protein restriction during lactation programmed the lower body weight, despite no change in food intake in adult offspringReference Passos, Ramos and Moura8, but direct measurements of body composition have not been reported under this condition. Furthermore, those animals showed a hyperthyroid statusReference Passos, Ramos, Dutra, Mouco and Moura9, Reference Dutra, Passos, Lisboa, Santos, Cabanelas, Pazos-Moura and Moura12, Reference Vicente, Moura, Lisboa, Costa, Amadeu, Mandarim-de-Lacerda and Passos15, Reference Moura, Lisboa, Custódio, Nunes, Souza and Passos17 that could be partly responsible for the hypermetabolic status in the adult age.
The aim of the present study was to characterise the phenotype of these programmed PR animals through the evaluation of their body composition and of some key hormones, such as catecholamines, corticosterone and insulin, that could help us to understand better this hypermetabolic status.
Experimental methods
Animals
Wistar rats were kept in a room with controlled temperature (25 ± 1°C) and with artificial dark–light cycles (lights on from 07.00 to 19.00 hours). Virgin female rats, aged 3 months, were caged with one male rat at the proportion of 3:1. After mating, each female was placed in an individual cage with free access to water and food until delivery. The use of the animals according to our experimental design was approved by the Animal Care and Use Committee of the Biology Institute of the State University of Rio de Janeiro, which based its analysis on the principles described in the Guide for the Care and Use of Laboratory AnimalsReference Bayne18.
At birth, ten lactating rats were randomly assigned to each one of the following groups: control group (n 5), with free access to a standard laboratory diet (23 % protein); PR group (n 5), with free access to an isoenergetic and low-protein diet (8 % protein). Generally, pregnant rats produced ten to twelve pups, and so to avoid the influence of the litter size in the programming effect we only used mothers whose litter size was ten offspring. On the first day of birth, litter adjustment was performed and six male pups were kept per control or PR dam, because it has been shown that this procedure maximises lactation performanceReference Fishbeck and Rasmussen19. Malnutrition was started at birth and ended at weaning (21 d).
Table 1 shows the composition of the diets, which follow recommended standards. The PR diet was made in our laboratory using the control diet and replacing part of its protein with maize starch. The amount of starch was calculated so as to make up for the decrease in energy content due to protein reductionReference Passos, Ramos and Moura8, Reference Dutra, Passos, Lisboa, Santos, Cabanelas, Pazos-Moura and Moura12, Reference Vicente, Moura, Lisboa, Costa, Amadeu, Mandarim-de-Lacerda and Passos15.
Table 1 Composition of the diets
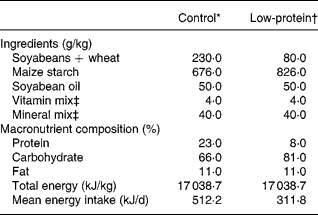
* Standard diet for rats (Nuvilab-NUVITAL Nutrientes LTDA, Paraná, Brazil).
† The low-protein diet was prepared in our laboratory using the control diet and replacing part of its protein with maize starch. The amount of the latter was calculated so as to make up for the decrease in energy content due to protein reduction.
‡ Vitamin and mineral mixtures were formulated according the AIN-93G recommendation for rodent dietsReference Reeves, Nielsen and Fahey46.
After weaning (21 d lactation), control and PR offspring received a normal diet until they were age 180 d. Both groups of rats were killed by decapitation, to collect blood, adrenal glands, visceral fat mass and carcasses. We chose decapitation, since this a quick method and also to avoid catecholamine and glucocorticoid changes induced by anaesthetics commonly used for rats.
Nutritional evaluation
During lactation, each pup's body weight was monitored daily. From weaning until day 180, body weight and relative food intake (g/100 g body weight) were monitored every 4 d.
Body composition
Visceral fat mass was excised and weighed for the evaluation of central adiposity – mesenteric, epididymal and retroperitonealReference Hansen, Han, Nolte, Chen and Holloszy20.
Body composition (fat and protein mass, total body water) was determined by carcass analysisReference Leshner, Litwin and Squibb21, Reference Toste, Moura, Lisboa, Fagundes, Oliveira and Passos22. After the rats were killed, control and PR animals were eviscerated, the carcasses were weighed, autoclaved for 1 h and homogenised in distilled water (1:1). The homogenate was stored at 4°C for analysis.
Homogenate (3 g) was used to determine fat content gravimetricallyReference Stansbie, Browsey, Crettaz and Demton23. Samples were hydrolysed in a shaking water-bath at 70°C for 2 h with 30 % KOH and ethanol. The total fatty acids and non-esterified cholesterol were removed with three successive washings with petroleum ether. After drying overnight in vacuum, all tubes were weighed and data were expressed as g fat/100 g carcass.
Protein content was determined in 1 g homogenate. Tubes were centrifuged at 2000 g for 10 min. The total protein concentrations were determined by the Lowry methodReference Lowry, Rosebrough, Farr and Randall24. Data were expressed as g protein/100 g carcass.
Total body water was determined by drying 1 g homogenate (duplicate), overnight, at 90°C to a stable weightReference Pace and Rathbun25. Data were expressed as g water/100 g carcass.
Serum hormone levels
Blood samples were centrifuged (3000 rpm for 20 min, at 4°C) to obtain serum, which was individually kept at − 20°C until assay. All measurements were performed in one assay.
Insulin was determined by RIA, using a commercial kit (ImmuChemTM 125I, coated tube; ICN Biomedicals Inc., Aurora, OH, USA) with an assay sensitivity of 0·1 ng/ml and a range of detection of 0·1–10 ng/ml. The intra-assay variation was 8·9 %.
Total corticosterone was measured by a specific murine RIA kit (ImmuChemTM 125I, double antibody; ICN Biomedicals, Inc.). The intra-assay variation was 7·1 %.
Glucose measurement
Glycaemia was determined in blood samples from the tail vein of fasting rats using a glucosimeter (ACCU-CHEK® Advantage; Roche Diagnostics, Mannheim, Germany).
Insulin resistance
The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated according to the formula: insulin (μIU/ml) × serum glucose (mmol/l)/22·5Reference Matthews, Hosker, Rudenski, Naylor, Treacher and Turner26.
Catecholamine assays
The total catecholamine (adrenaline and noradrenaline) content in adrenal medulla was measured by the trihydroxyndole fluorescence methodReference Kelner, Levine, Morita and Pollard27, Reference Martins, Souza, Shio, Mathias, Lelkes and Garcia28.
Left adrenal glands were homogenised in 500 μl 10 % acetic acid using an ultrasonic processor and centrifuged (10 000 g for 1 min). To assay, 50 μl supernatant fraction was mixed with 250 μl 0·5 m-buffer phosphate (pH 7·0) and 25 μl potassium ferricyanate (0·5 %), followed by incubation (20 min). The reaction was stopped with 500 μl ascorbic acid–10 m-NaOH (1:19 proportion). The fluorometer parameters were: 420 nm to excitation and 510 nm to emission.
Results were obtained by plotting the values into a linear regression of the standard adrenaline curve. Data were expressed as μm catecholamines/mg gland. Protein concentration was determined by the Bradford methodReference Bradford29.
Statistical analysis
Results are reported as mean values with their standard errors. Statistical significance was determined by mixed-model ANOVA to analyse body weight and food intake. The other experimental data were analysed by Student's unpaired t test and differences were considered significant at P < 0·05.
Results
Nutritional evaluation
Body weight and food intake of pups whose mothers were submitted to protein restriction during lactation are shown in Fig. 1. PR offspring showed lower body weight than control animals (F(9, 162) = 10·95; P < 0·0001) from lactation until adulthood (Fig. 1 (A) and (B)), but no change in food intake (Fig. 1 (C)), as previously reported by our groupReference Passos, Ramos and Moura8, Reference Teixeira, Passos, Ramos, Dutra and Moura11, Reference Dutra, Passos, Lisboa, Santos, Cabanelas, Pazos-Moura and Moura12, Reference Vicente, Moura, Lisboa, Costa, Amadeu, Mandarim-de-Lacerda and Passos15.

Fig. 1 Body weight and food intake evolution. (A) Body weight during lactation of pups whose mothers were fed a control (■) or protein-restricted (PR; ○) diet. (B) Body weight after weaning until adult age of rats whose mothers were fed a control (■) or PR (○) diet during lactation. (C) Food intake after weaning until adult age of rats whose mothers were fed a control (■) or PR (○) diet during lactation. Values are means for thirty animals per group, with standard errors represented by vertical bars.Mean values for the PR animals were significantly different to those of the control animals: * P < 0·05, † P < 0·0001 (ANOVA).
As shown in Fig. 2 (A), the PR offspring aged 180 d showed lower content of visceral fat mass ( − 33 %; P < 0·01). Adult PR animals also presented lower ( − 33 %; P < 0·01) body fat mass (Fig. 2 (B)), but no change was observed in body protein mass (Fig. 2 (C)) and body water content (Fig. 2 (D)).
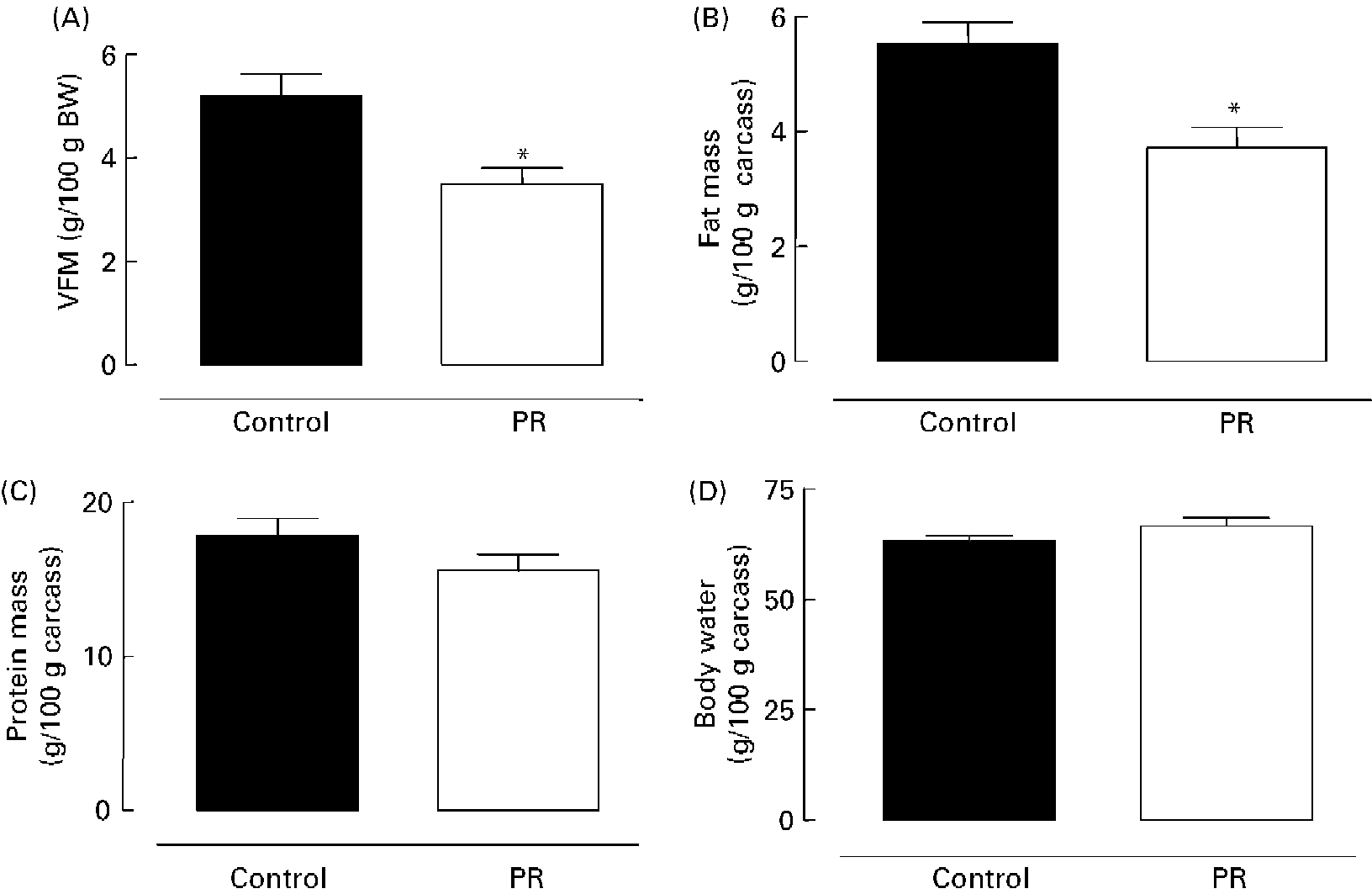
Fig. 2 Body composition of adult offspring. (A) Visceral fat mass (VFM) (g/100 g body weight (BW)), (B) body fat mass, (C) body protein mass and (D) body water of adult animals whose mothers were fed a normal (control; ■) or protein-restricted (PR; □) diet during lactation. Values are means for ten animals per group, with standard errors represented by vertical bars. *Mean value was significantly different from that of the control group (P < 0·01).
Glucose homeostasis
Fig. 3 shows serum glucose (Fig. 3 (A)) and insulin (Fig. 3 (B)) in 180 d old offspring whose mothers were submitted to protein restriction during lactation. The PR group presented lower glycaemia ( − 7 %; P < 0·05) and insulinaemia ( − 26 %; P < 0·05) than control animals. The evaluation of insulin resistance is shown in Fig. 4. There was a trend for the PR group to have a lower HOMA index ( − 20 %), suggesting higher insulin sensitivity.

Fig. 3 Glycaemia and insulinaemia of adult offspring. (A) Serum glucose and (B) insulin of adult animals whose mothers were fed a normal (control; ■) or protein-restricted (PR; □) diet during lactation. Values are means for ten animals per group, with standard errors represented by vertical bars. *Mean value was significantly different from that of the control group (P < 0·05).
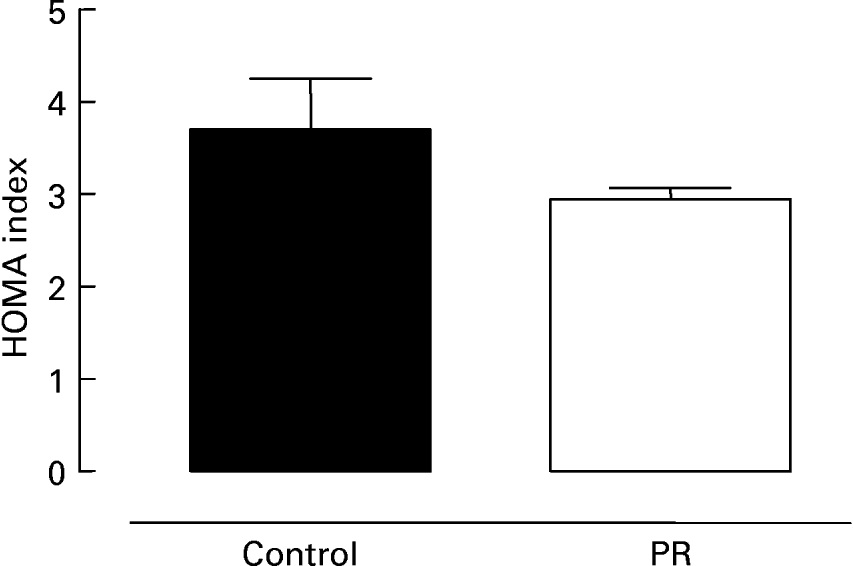
Fig. 4 Homeostatic model assessment (HOMA) index of adult offspring. HOMA index of adult animals whose mothers were fed a normal (control; ■) or protein-restricted (PR; □) diet during lactation. Values are means for ten animals per group, with standard errors represented by vertical bars.
Adrenal hormones
Serum corticosterone and intra-glandular catecholamine content of offspring aged 180 d whose mothers were submitted to protein restriction during lactation are shown in Fig. 5 (A) and Fig. 5 (B), respectively. PR rats showed higher corticosteronaemia (+51 %; P < 0·05) and adrenal medulla catecholamine content (+90 %; P < 0·05).

Fig. 5 Corticosteronaemia and catecholamines of adult offspring. (A) Serum corticosterone levels and (B) adrenal catecholamine content of adult animals whose mothers were fed a normal (control; ■) or protein-restricted (PR; □) diet during lactation. Values are means for ten animals per group, with standard errors represented by vertical bars. *Mean value was significantly different from that of the control group (P < 0·05).
Discussion
The present study showed that protein restriction during lactation programmed lower visceral fat mass and total body fat, which are responsible for the lower body weight in the adult offspring, without altering food intake, reinforcing previous studies from our groupReference Passos, Ramos and Moura8, Reference Teixeira, Passos, Ramos, Dutra and Moura11, Reference Dutra, Passos, Lisboa, Santos, Cabanelas, Pazos-Moura and Moura12, Reference Vicente, Moura, Lisboa, Costa, Amadeu, Mandarim-de-Lacerda and Passos15. In addition, those PR animals present, at age 180 d, hypoinsulinaemia, hypercorticosteronaemia and higher total catecholamine content, which may inform us about their energetic metabolism profile and suggested a metabolic dysfunction. Also, our previous findings showed that PR animals presented lower pituitary growth hormone mRNA expression ( − 29 %) and lower body length ( − 20 %) at weaning, as well as at age 90 d ( − 19 and − 5 %, respectively), but no changes in body length when they were age 180 d, even continuing with lower ( − 17 %) growth hormone mRNA expressionReference Moura, Lisboa, Custódio, Nunes, Souza and Passos17. Strangely, those animals had lower fat mass, even though growth hormone deficiency is associated with higher adiposityReference De Boer, Blok and Van der Veen30, Reference Malmlof, Din, Johansen and Pedersen31. Further, we also showed that neonatal protein restriction programmes for hyperthyroidism in adult offspringReference Passos, Ramos, Dutra, Mouco and Moura9, Reference Dutra, Passos, Lisboa, Santos, Cabanelas, Pazos-Moura and Moura12, evidencing that our programmed animals become hypermetabolic, which could increase cardiovascular risk.
Concerning catecholamines, the present findings corroborate another studyReference Petry, Dorling, Wang, Pawlak and Ozanne32, which showed an increase in serum catecholamines in rats at age 90 d whose mothers were fed a PR diet during gestation and lactation. However, the increase in medullary catecholamines could be due to a decrease in their secretion. Thus, a more direct evaluation turns out to be necessary, such as experiments of in vitro secretion or measurement of serum catecholamines or their metabolites.
Evidence that reinforces our main hypothesis that catecholamines could be increased is the fact that body fat mass of PR animals is lower. In addition, glucocorticoids stimulate catecholamine synthesis, mainly through the effect upon the enzyme phenylethanol amine-n-methyl transferase, responsible for noradrenaline to adrenaline conversion in the cytoplasm of chromaffin cellsReference Wurtman33. Thus, the hypercorticosteronaemia detected in these animals could contribute to the increase of adrenal medullar catecholamine content in PR animals.
The adult programmed PR animals showed lower serum insulin levels and a slight lower glycaemia than control animals. Furthermore, there was a trend for HOMA index to be lower, which may indicate that despite hypoinsulinaemia, there is normal or even higher insulin sensitivity, since a higher HOMA index indicates insulin resistanceReference Matthews, Hosker, Rudenski, Naylor, Treacher and Turner26, Reference Haffner, Kennedy, Gonzalez, Stern and Miettinen34. These results corroborate previous studiesReference Moura, Caldeira Filho, Mathias and Franco de Sá35–Reference Sampaio de Freitas, Garcia De Souza, Vargas da Silva, da Rocha Kaezer, da Silva Vieira, Sanchez Moura and Barja-Fidalgo37 about lower insulin secretion and its higher sensitivity in adult rats submitted to severe postnatal protein restriction (0 or 4 % protein content). Recently, Zambrano et al. Reference Zambrano, Bautista, Déas, Martínez-Samayoa, González-Zamorano, Ledesma, Morales, Larrea and Nathanielsz38 also described a lower glycaemia and higher sensitivity in adult offspring whose mothers were submitted to protein restriction (10 % protein content) during lactation.
A rat model of maternal protein restriction (80 g/kg v. 200 g/kg control) during gestation and lactation showed that offspring were born smaller and were programmed for hyperinsulinaemia and tissue insulin resistance, developing diabetes in adult lifeReference Fernandez-Twinn, Ozanne, Ekizoglou, Doherty, James, Gusterson and Hales39. The main difference between this model and the present data, concerning insulin levels and its sensitivity, seems to be the different period of undernutrition, which suggests that during gestation organogenesis can also be affected.
The slight decrease in glycaemia associated with higher insulin sensitivity in programmed PR rats could be causing a contra-regulatory response to insulin, suggested by hypercorticosteronaemia and higher tissue catecholamines shown in the present study. Thus, it is possible that the result of this hormonal profile generates lipolysis. At the present moment, we do not know when those hormonal changes begin during development of the programmed offspring. With this purpose, further studies involving temporal evolution are being performed.
The hypoinsulinaemia can also be explained by an increase of catecholamines, since these hormones inhibit insulin secretion through their action on the α1-adrenoreceptors of the Langerhans β-cellsReference Ahren, Lundquist and Jarhultm40.
In models of programming by neonatal stress, a lower body weight and elevated catecholamine and glucocorticoid levels in adult animals have also been describedReference Petry, Dorling, Wang, Pawlak and Ozanne32, Reference Benediktsson, Lindsay, Noble, Seckl and Edwards41–Reference Holmes, Abrahamsen, French, Paterson, Mullins and Seck43, but, contrary to our findings, hyperglycaemia and hyperinsulinaemia were presentReference Holmes, Abrahamsen, French, Paterson, Mullins and Seck43–Reference Dalziel, Walker, Parag, Mantell, Rea, Rodgers and Harding45.
In conclusion, maternal protein restriction during lactation programmes body composition and key hormones that control the intermediary metabolism of offspring in adulthood. Therefore, perhaps these programmed PR animals present a hypermetabolic state, which could explain their lower adiposity and body weight.
Acknowledgements
Research was supported by the National Council for Scientific and Technological Development (Conselho Nacional de Desenvolvimento Científico e Tecnológico; CNPq), Coordination for the Enhancement of Higher Education Personnel (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior; CAPES) and the State of Rio de Janeiro Carlos Chagas Filho Research Foundation (Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro; FAPERJ). A.T. S. F. was recipient of a CNPq fellowship. E. O. and F. P. T. were recipient of a CAPES fellowship. I. T. B. and I. H. T. were recipient of a FAPERJ fellowship. All authors are grateful to Ms Lauciene Andrade and Mr Carlos Roberto for technical assistance.








