The WHO classifies as small-for-gestational age (SGA) babies born below the 10th percentile of birth weight and length by sex in relation to a completed gestational age of a given reference population(1). Approximately 23·3 million infants (19·3 % of live births) in low- and middle-income countries are born SGA(Reference Lee, Kozuki and Cousens2). Being SGA is an important public health problem given that it is associated with an increased risk of mortality, morbidity and adverse functional consequences, including delays in cognition and educational performance, an increased risk of poor linear growth and a long-term risk of nutrition-related chronic diseases in adulthood(Reference Lee, Kozuki and Cousens2–Reference Christian, Lee and Angel5).
Deprived parental education, lack of access to health care with quality, maternal infections and other morbidities, young maternal age, smoking during pregnancy and poor maternal nutrition are the main modifiable risk factors for SGA(Reference Mosites, Dawson-Hahn and Walson4,Reference Monteiro, Benicio and Conde6–Reference Imdad and Bhutta8) . Some studies have explored associations between maternal diet and babies’ birth weight, but focusing on individual foods or nutrients(Reference Grieger and Clifton9). However, it may be difficult to assess the specific influence of foods or nutrients as determinants of birth size because they are consumed as part of a whole diet(Reference Englund-Ögge, Brantsæter and Juodakis10). Dietary pattern (DP) analysis constitutes a statistical method which determines food intake patterns and accounts for the cumulative and interactive effects of a diet, reflecting better the actual dietary consumption(11).
So far, there is limited research about women’s pre- or during pregnancy DP and their influence on the risk of delivering small babies(Reference Englund-Ögge, Brantsæter and Juodakis10,Reference Chen, Zhao and Mao12) . Some studies have shown that a prudent or healthful DP during pregnancy is associated with a decreased risk and Western or ‘wheat products’ pattern with an increased risk of having a SGA newborn(Reference Chen, Zhao and Mao12–Reference Okubo, Miyake and Sasaki14), while others found no such association(Reference Poon, Yeung and Boghossian15). Despite the recognition that the nutritional health of the mother before pregnancy may be as important as her nutrition throughout pregnancy(Reference Thompson, Wall and Becroft16,Reference Retnakaran, Wen and Tan17) , few studies have described the association between pre-pregnancy DP and newborn outcomes(11–Reference Knudsen, Orozova-Bekkevold and Mikkelsen13,Reference Grieger, Grzeskowiak and Clifton18) .
The promotion of healthy diets before pregnancy is an opportunity to prevent newborns’ under-nutrition and, consequently, disadvantages throughout their lives(Reference Hawkes, Ruel and Salm19). Therefore, to design more effective nutritional interventions to improve both maternal and child health, it is important to better understand how various food patterns within a population may affect the prevalence of babies born SGA. Thus, the objective of this study was to determine how specific pre-pregnancy DP are associated with the risk of delivering babies born SGA, defined as weight and/or length below the 10th percentile by sex in relation to a completed gestational age of a given reference population.
Materials and methods
Study design
We analysed data from the birth cohort ProcriAr (‘The Influence of Nutritional Factors and Urban Air Pollutants on Children’s Respiratory Health: A Cohort Study in Pregnant Women’), which was conducted with women who attended to four primary health care units from the west region of São Paulo – Southeast/Brazil. The primary required sample size was 400 individuals, aiming to detect a change of ≥5 % in pulmonary functional parameters with a study power of ≥80 %(Reference Friedrich, Pitrez and Stein20). Between March 2011 and December 2013, all women were invited to participate in the study as soon as they had a positive β-human chorionic gonadotropin blood test in the Basic Health Unit (n 619). Fifty-nine of those women did not meet the study eligibility criteria (single fetus, gestational age <14 weeks and absence of pre-existing chronic diseases). Further exclusions consisted of women who did not have the ultrasonography examination (n 42), women who had a miscarriage or no embryo (n 45) and women who dropped out of the study or moved out to another place in the beginning of the study (n 19). The cohort final sample was constituted of 454 women, and none of them was excluded from the study due to medical complications during pregnancy. The present study consists of 299 pregnant women who completed the baseline face-to-face home interview and were followed until the baby’s delivery (299/454: 65·9 %).
Ethical standards
This study was approved by the Ethical Committee of Municipal Department of Health of São Paulo (no. 430/10), the Ethical Committee of School of Medicine, University of São Paulo (no. 0068/10) and the Ethical Committee of School of Public Health, University of São Paulo (no. 1.501.677/16). Written informed consent was obtained from all participating women.
Exposure assessment
A validated 110-item quantitative FFQ was used to assess the pre-pregnancy food intake of the population(Reference Selem, Carvalho and Verly-Junior21,Reference Fisberg, Colucci and Morimoto22) . In the beginning of their pregnancy (median gestational age 11·1 weeks, 95 % CI 10·9, 11·6 weeks), women were asked about their frequency of food intakes in the previous 12 months, as well as the portion size consumed (small, medium or large). Brazilian manuals were used to convert foods and recipes from the FFQ into g(Reference Pinheiro, Lacerda and Benzecry23). Daily intakes were calculated by multiplying the portion size by the frequency of intake (1–10) and dividing it by the days.
Four distinct DP during pre-pregnancy were previously identified using factor analysis with principal component’s estimation(Reference Teixeira, Castro and Grant24). High loadings of food items intake were defined as >0·25 or <−0·25. DP1: ‘Lentils, whole grains and soups’ – positive high loadings for lentils, wheat bread and brown rice, soups, popcorn, cereal ready to eat and oats, white cheese, desserts with fruits and jelly, simple cakes, soya beverages, beef jerky, nuts, crackers, soya sauce, tea (sweetened), beef, stuffed pasta, feijoada, fruits, yogurt (whole milk), and negative high loading for French bread and white rice. DP2: ‘Snacks, sandwiches, sweets and soft drinks’ – positive high loadings for processed meats, sandwiches and snacks, sandwich sauces, desserts and sweets, soft drinks, pasta with meat sauce, stuffed pasta, yogurt with flavour, pork and frankfurters, bakery with filling, fried beef and fried chicken, fried egg or omelette, potato salad, with vegetables and mayonnaise, alcoholic beverages, chocolate milk, feijoada, potato or cassava, mozzarella cheese and negative high loadings for yogurt. DP3: ‘Seasoned vegetables and lean meats’ – positive high loadings for potato salad, with vegetables and mayonnaise, vegetables, oil (for salad dressing), salt, lean meats and fish, potato or cassava, fruits, French bread and white rice, and unsweetened juices (natural or artificial). DP4: ‘Sweetened juices, bread and butter, rice and beans’ – positive high loadings for sweetened juices (natural or artificial), butter or margarine, French bread and white rice, beans, whole milk, yogurt, fried egg or omelette, potato or cassava (fried), and negative high loadings for unsweetened juices (natural or artificial) and alcoholic beverages. Together, these four DP explained 25·5 % of the variance in food intake in this population(Reference Teixeira, Castro and Grant24). Individually, the DP explained the following variance in food intake: 9·9 % (Lentils, whole grains and soups), 6·6 % (Snacks, sandwiches, sweets and soft drinks), 4·7 % (Seasoned vegetables and lean meats) and 4·3 % (Sweetened juices, bread and butter, rice and beans).
Daily intake of energy, carbohydrate, protein, fat, caffeine, Fe, Ca, vitamin D, dietary folate equivalents, natural folate, synthetic folate, methionine, choline, betaine, vitamin B6, vitamin B12, n-3 and DHA was analysed using Nutrition Data System for Research software version 2.0 (2007), which was developed by the Nutrition Coordinating Center, University of Minnesota. The foods used had an 80–120 % match for energy and macronutrients between the NDSR and the Brazilian composition tables(25). The amount of folic acid added to fortified foods was corrected due to differences among US and Brazilian fortification policies. The choice of nutrients investigated was based on recommendations for healthy pregnancy outcomes(Reference Chen, Zhao and Mao12,26–28) . The adjustment of the nutrients for energy was performed by the residual method(Reference Willett and Stampfer29).
Outcome assessment
Information on newborn sex and anthropometric measurements were retrieved from the child health handbook in the first postpartum visit by researchers engaged in this study. The newborn measures of weight (in g) and length (in cm) were assessed by the health care teams of the different hospitals where the women gave birth using, respectively, paediatric scales and paediatric length boards or measuring mats. The estimate of the conception date was based on the last menstrual period and confirmed by ultrasonography performed in the first trimester of pregnancy. In case of a discrepancy between the last menstrual period and the ultrasound reading, it was considered the conception date as the one estimated by the ultrasound. Gestational age was determined by the difference between conception and childbirth dates.
We have standardised birth weight and length by gestational age and sex using as reference The International Fetal and Newborn Growth Consortium for the 21st Century study (INTERGROWTH-21st)(Reference Villar, Altman and Purwar30). Newborns with birth weight and/or birth length by gestational age and sex below the 10th percentile were considered as being SGA(1).
Covariates assessment
Information on maternal sociodemographic and lifestyle factors were collected during the baseline face-to-face interview. In addition, preconception weight and height were self-reported by the pregnant women. Maternal BMI was calculated and classified in accordance with WHO criteria(31). Due to the low prevalence of underweight in this population (n 6, 2·0 %), it was included in the BMI eutrophic category as follows: eutrophic ≤24·9 kg/m2; overweight 25·0–29·9 kg/m2 and obese ≥30·0 kg/m2.
Sociodemographic and health behaviours previously associated with fetal growth were chosen to describe the population, according to data availability(Reference de Andrade, Pellegrini Filho and Solar32,Reference Hill and Davies33) . Therefore, the following maternal characteristics were used to describe the population: age (years), education (< or ≥8 years), BMI (eutrophic, overweight or obese), status of formal work (yes or no), relationship status (married or not), smoking status (yes or no), sedentary behaviour (< or ≥2 h watching television/d), number of previous births (0, 1 or ≥2), unplanned pregnancy (yes or no), and vitamin/mineral supplement use (yes or no). Newborn characteristics such as gestational age (weeks), sex (male/female), preterm birth (yes if gestational age was <37 weeks or no), weight (g) and length (cm) were also described.
Statistical analyses
Variables were described as percentages and medians and 95 % CI. The Kruskal–Wallis test for continuous variables and the χ 2 test or Fisher’s exact test for categorical variables were implemented to compare the population of this study (n 299) with the original study sample (n 454). These tests were used to determine if any significant differences existed between the newborns classified as <10th or ≥10th percentile for birth weight and/or birth length by gestational age and sex, according to maternal and newborn characteristics.
Multivariate Poisson regression models with robust error variance were performed to examine the relationship between newborns born SGA and women’s DP, adjusted by maternal characteristics. Newborn size at birth (SGA: yes or no) was the outcome variable. The component scores of each maternal DP were categorised into quintiles and considered the exposure variable in this analysis. Maternal age was assessed as continuous variable (in years). Eight or more years of education, the lack of formal work, being married and smoking (pre-pregnancy period) were treated as dichotomous variables (yes or no options). Overweight and obese were defined in relation to eutrophic, using a dummy variable for BMI. None or two or more previous births were defined in relation to one previous birth using a dummy variable for number of previous births. Each multivariate regression model with one specific DP was adjusted by the other three DP.
Multivariate linear regression models were used to examine associations between energy and nutrient intakes and the scores of DP, both variables analysed as continuous variables. All models were adjusted by maternal age, education, BMI, work status, relationship status, smoking status and number of previous births.
All analyses were performed using Stata Statistical Software (release 14, 2015, StataCorp LP)(34). Two-sided statistical significance was determined at P < 0·05.
Results
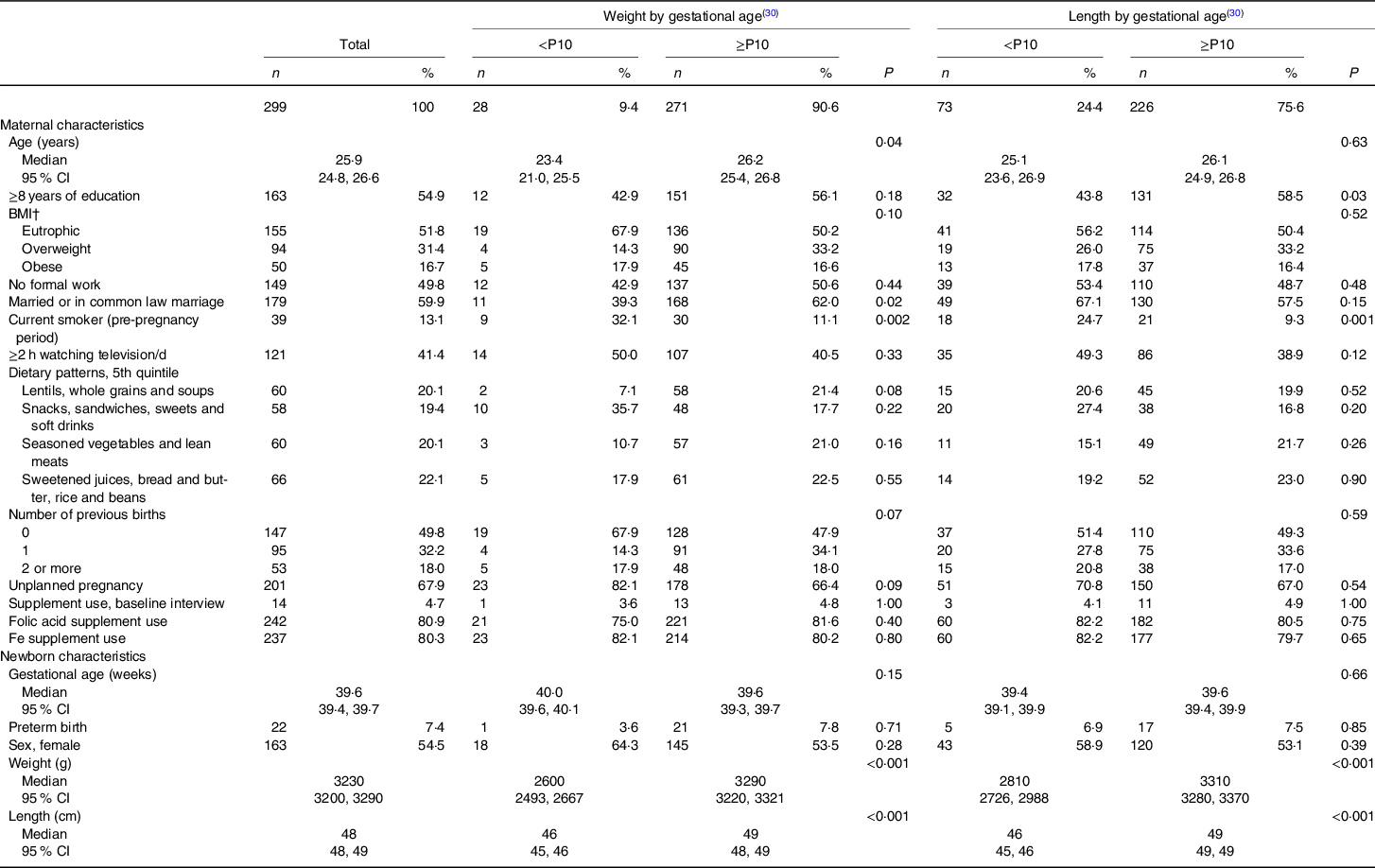
The studied women were assumed to be a random subsample of the original study population as there were no statistically significant differences in terms of sociodemographic and lifestyle factors, except for the number of previous births (online Supplementary Table S1). This population had, in median, 25·9 years old, more frequently were eutrophic (51·8 %), had no formal work (49·8 %), were married or in common law marriage (59·9 %), non-smoker (86·9 %), watched television for <2 h/d (58·6 %), had given previous birth (50·2 %), reported unplanned pregnancies (67·9 %), were not using vitamin/mineral supplements in the baseline interview (95·3 %), used folic acid (80·9 %) and Fe (80·3 %) supplements during pregnancy and had female babies (54·5 %) (Table 1).
Table 1. Maternal and newborns’ characteristics according to newborns’ weight and length by gestational age and sex, ProcriAr study – Sao Paulo/Brazil*
(Numbers and percentages; medians and 95 % confidence intervals)
P10, 10th percentile.
* Kruskal–Wallis test (continuous variables) or χ 2 test/Fisher’s exact test (categorical variables) was used to determine if any significant differences existed amongst the groups according to maternal and newborns’ characteristics (n 299).
† BMI; underweight and eutrophic ≤24·9 kg/m2; overweight 25·0–29·9 kg/m2 and obese ≥30·0 kg/m2. Prevalence of underweight was 2·0 % (n 6).
The majority of the newborns had birth weight classified between the 10th and 90th percentiles (79 %). The prevalence of birth length below the 10th percentile was almost three times higher (25 %) than the prevalence of birth weight below the 10th percentile (9 %) (Fig. 1). Babies with birth weight <10th percentile had younger mothers whom more frequently were smokers in the pre-pregnancy period and were not married or in common law marriage, when compared with mothers of babies with birth weight ≥10th percentile for the INTERGROWTH-21st standards (Table 1). In relation to mothers of newborns with birth length ≥10th percentile, mothers of babies with birth length <10th percentile were more frequently smokers in the pre-pregnancy period and had more frequently <8 years of formal education (Table 1).
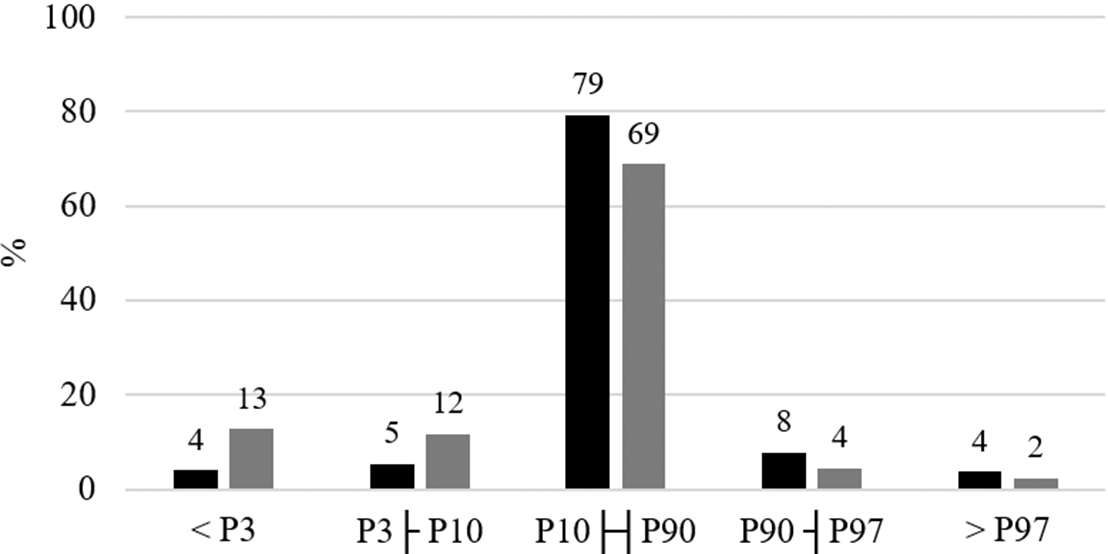
Fig. 1. Distribution of newborns according to birth weight and length percentiles adjusted by gestational age and sex, ProcriAr study – Sao Paulo/Brazil. P3, 3rd percentile; P10, 10th percentile; P90, 90th percentile; P97, 97th percentile. Birth weight and length percentiles were assessed using INTERGROWTH-21st standards(Reference Villar, Altman and Purwar30). ![]() , Weight;
, Weight; ![]() , length.
, length.
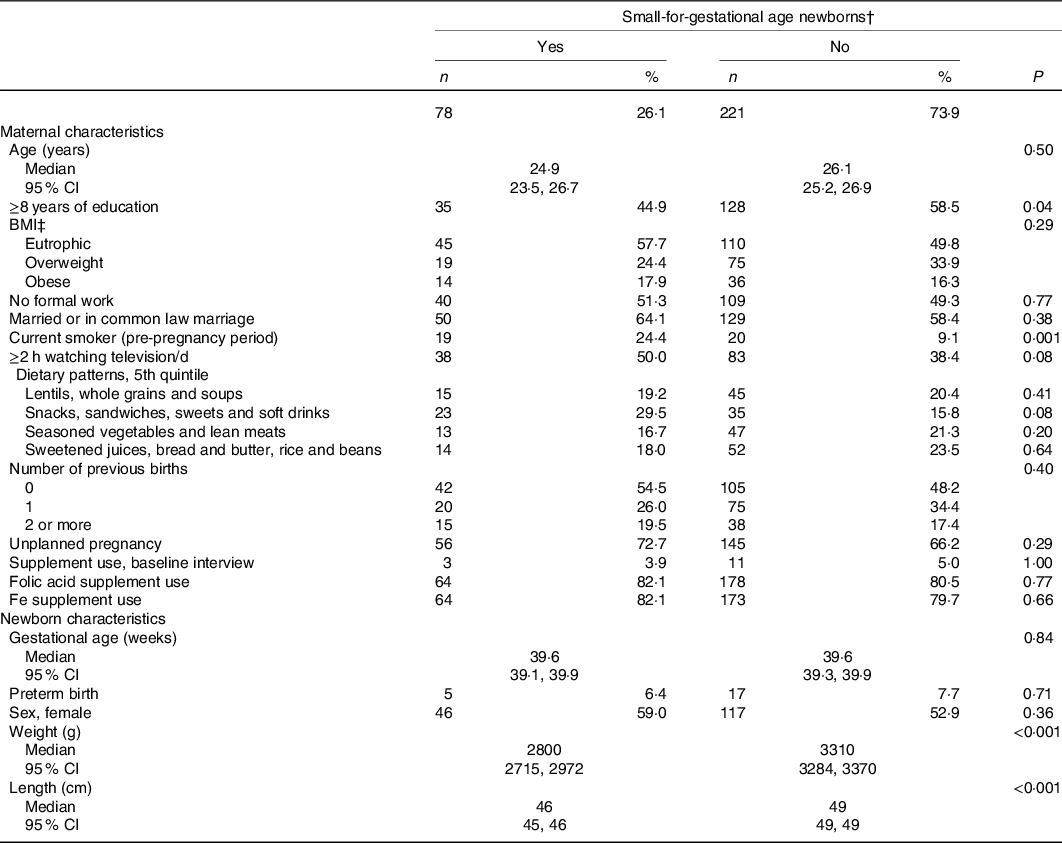
Overall, the prevalence of SGA newborns was 26·1 % (78/299), where 29·5 % (23/78) of them presented both birth weight and length <10th percentile, 64·1 % (50/78) presented only birth length <10th percentile and 6·4 % (5/78) presented only birth weight <10th percentile. When compared with mothers of non-SGA newborns, mothers of SGA newborns had less years of formal education and were more frequently smokers in the pre-pregnancy period (Table 2). When compared the SGA newborns with the ones who were not, no differences in the proportion of sexes and prematurity were verified. As expected, smaller median values of birth weight and length were verified for the SGA newborns in relation to the non-SGA ones (Table 2).
Table 2. Maternal and newborns’ characteristics according to newborns’ size at birth, ProcriAr study – Sao Paulo/Brazil*
(Numbers and percentages; medians and 95 % confidence intervals)
P10, 10th percentile.
* Kruskal–Wallis test (continuous variables) or χ 2 test/Fisher’s exact test (categorical variables) was used to determine if any significant differences existed amongst the groups according to maternal and newborn characteristics (n 299).
† Weight and/or length by gestational age below the 10th percentile of the INTERGROWTH-21st standard(Reference Villar, Altman and Purwar30), total agreement: 80·6 % (length <P10, n 50; weight <P10, n 5; length and weight <P10, n 23).
‡ BMI; underweight and eutrophic ≤24·9 kg/m2; overweight 25·0–29·9 kg/m2 and obese ≥30·0 kg/m2. Prevalence of underweight was 2·0 % (n 6).
In multivariate Poisson regression models, offspring of mothers in the fifth v. first quintile of the ‘Snacks, sandwiches, sweets and soft drinks’ DP had a significant increased relative risk of being SGA, independently of maternal characteristics (RR 1·92; 95 % CI 1·08, 3·39). It was also verified a trend effect of adherence to DP ‘Snacks, sandwiches, sweets and soft drinks’ among the SGA newborns (P = 0·041). There were no significant associations between being born SGA and the gradient scoring in the quintiles of the other three DP (Table 3).
Table 3. Adjusted associations between maternal dietary patterns and small-for-gestational-age (SGA) newborns in the ProcriAr study – Sao Paulo/Brazil*
(Relative risks (RR) and 95 % confidence intervals)
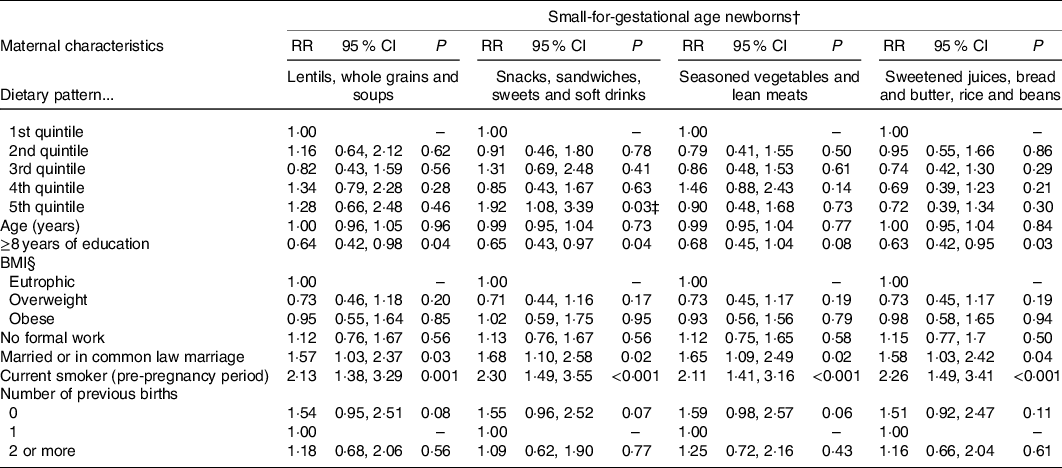
* Poisson regression models with robust error variance were used to determine the association between maternal dietary patterns and newborns’ SGA. All models were adjusted by the other dietary patterns. Loss of five individuals in the models due to missing information for maternal education (2), smoking status (1) and parity (2).
† Weight and/or length by gestational age below the percentile 10th percentile of the INTERGROWTH-21st standard(Reference Villar, Altman and Purwar30).
‡ Trend effect: P = 0·041.
§ BMI; underweight and eutrophic ≤24·9 kg/m2; overweight 25·0–29·9 kg/m2 and obese ≥30·0 kg/m2. Prevalence of maternal underweight was 2·0 % (n 6).
The only DP negatively associated with the intake of carbohydrate, Ca, vitamin D, dietary folate equivalents and natural folate was the ‘Snacks, sandwiches, sweets and soft drinks’. Likewise, the one and only positive association was found between ‘Snacks, sandwiches, sweets and soft drinks’ and fat (Table 4).
Table 4. Adjusted associations between maternal dietary patterns and intakes of energy and nutrients in the ProcriAr study – Sao Paulo/Brazil†
(β-Coefficients and 95 % confidence intervals)
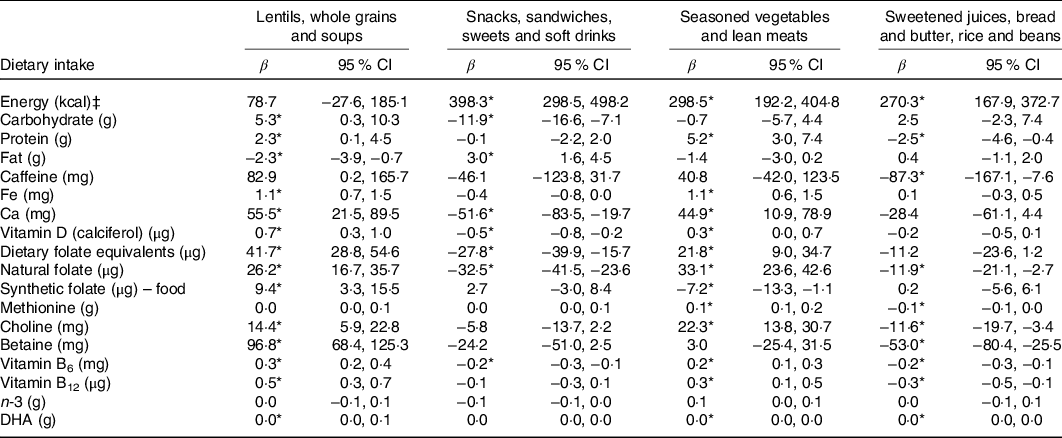
* P < 0·05.
† Linear regression models were used to determine the association between maternal dietary patterns and intakes of energy and nutrients. All models were adjusted by maternal age, education, BMI, work status, relationship status, smoking status and parity. Loss of five individuals in the models due to missing information for maternal education (2), smoking status (1) and parity (2).
‡ To convert kcal to kJ, multiply by 4·184.
Discussion
Many low- and middle-income countries are now experiencing the nutrition transition that is accompanied by an increased prevalence of obesity and a decreased prevalence of underweight(Reference Conde and Monteiro35). However, 19 % of children in low- and middle-income countries are born SGA(Reference Lee, Kozuki and Cousens2). It is well established that SGA is associated with poor environmental and socio-economic, illness and/or inappropriate feeding practices(Reference Mosites, Dawson-Hahn and Walson4,Reference Monteiro, Benicio and Conde6–Reference Imdad and Bhutta8) , but specifics on maternal preconception diet are not well known. Results from this analysis of the ProcriAr cohort study showed that women’s adherence to an unhealthful DP before pregnancy was significantly associated with delivering a SGA newborn, independently of maternal sociodemographic and health behaviour characteristics.
Researches have been published on maternal diet during gestation and the impact it has on subsequent SGA newborns(Reference Black, Allen and Bhutta3,Reference Chen, Zhao and Mao12) . However, to our knowledge, there are only two studies on the influence of pre-pregnancy DP on offspring’s weight and/or length by gestational age and sex(Reference Chen, Zhao and Mao12). A population-based birth cohort study with 847 pregnant Dutch women suggested that increasing adherence to a periconceptional energy-rich DP (higher intakes of bread/breakfast cereals, margarine, nuts, snacks/sweets and non-sweetened nonalcoholic beverages) was associated with increased babies’ crown–rump length, measured in the first trimester (linear trend analyses P = 0·015), while no association was revealed with estimated fetal weight, nor with birth weight(Reference Bouwland-Both, Steegers-Theunissen and Vujkovic36). The second study, involving 309 pregnant women from Adelaide, Australia, found that a high-fat/sugar/takeaway DP was associated with shorter birth length (adjusted regression coefficient –0·5; 95 % CI –0·8, –0·1; P = 0·004), but there were no relations between low birth weight and SGA with any of the three DP derived(Reference Grieger, Grzeskowiak and Clifton18). Our results extend this research given that we were able to assess newborn size using weight and length by gestational age, a more refined indicator of nutritional status(Reference Chen, Zhao and Mao12).
Potential explanations for the results reported in this study are most likely related to the fact that reduced bioavailability of key nutrients before pregnancy limits important developmental processes in utero, with potential long-term impact on the offspring health outcomes(Reference Black, Alderman and Bhutta37,Reference Crozier, Inskip and Godfrey38) . A prospective study of 3207 Caucasian pregnant mothers in Rotterdam, the Netherlands, found that the degree of adherence to a ‘Mediterranean’ DP, characterised by higher intakes of fruit, vegetables, vegetable oil, fish, pasta and rice, and lower intakes of meat, potatoes and fatty sauces, was positively associated with plasma folate and serum vitamin B12 concentrations and showed an inverse relationship with homocysteine and high-sensitivity C-reactive protein plasma concentrations (P < 0·05)(Reference Timmermans, Steegers-Theunissen and Vujkovic39). In the same study, lower adherence to the ‘Mediterranean’ DP in early pregnancy was associated with lower birth weight (g)(Reference Timmermans, Steegers-Theunissen and Vujkovic39). In the present study, the ‘Snacks, sandwiches, sweets and soft drinks’ was the one and only DP negatively associated with intakes of Ca, vitamin D, natural folate and dietary folate equivalents. Although it does not necessarily indicate an insufficient intake of these nutrients, the other three DP have not presented negative associations with these nutrients, maybe explaining why the DP ‘Snacks, sandwiches, sweets and soft drinks’ was associated with newborn size and the other DP were not. However, it is important to note that SGA is a very complex indicator of nutritional status and may be influenced by a number of dietary and environmental factors(Reference Mosites, Dawson-Hahn and Walson4,Reference Monteiro, Benicio and Conde6–Reference Imdad and Bhutta8) .
Considering the relationship between maternal periconceptional DP and offspring growth requires an interdisciplinary approach given the interactions that occur between diet and other maternal sociodemographic and health behaviour factors that promote or limit babies’ growth. We found that women with lower maternal education (<8 years of formal education) or who smoked in the pre-pregnancy period had a higher risk of having SGA babies compared with women with higher level of education or non-smokers. This finding is consistent with previous research in which women with lower educational levels and/or unhealthy behaviours (smoking and high intake of food rich in energy and poor in micronutrients) were more likely to have babies with growth deficits(Reference Chen, Zhao and Mao12,Reference Knudsen, Orozova-Bekkevold and Mikkelsen13) . This pattern constitutes what is termed as the vicious cycle of poverty, under-nutrition and poor growth. Poverty and low income lead to maternal under-nutrition and poor dietary intake, increasing the risk of poor growth of their babies(Reference Rajagopalan40,Reference Hoffman and Klein41) . Further babies’ nutritional insults contribute to prolonged growth delays, resulting in stunting in childhood and even in adulthood, both of which are associated with physical and cognitive delays, reduced human capital and potential income, that is, social disadvantages throughout life(Reference Hoffman and Klein41,Reference Darnton-Hill and Mkparu42) . Thus, improvement of women dietary intake presents an important opportunity to contribute to the disruption, even in part, of this unfair process. If policymakers want to effectively improve maternal and child health in developing countries, they should not disregard the potential of policies that will promote more equitable access to education and natural healthy food to women of childbearing age.
We acknowledge the limitations of this study. In the ProcriAr study, we were able to follow-up 65·9 % of the study sample and the subsample described in this paper (n 299) had previous births less frequently when compared with the total sample, indicating that the sample studied was possibly more concerned about health. Loss to follow-up is a problem in most cohort studies and may lead to bias and loss of statistical power(Reference Deeg43). It is worth noting that the associations between higher adherence to the pre-pregnancy DP ‘Snacks, sandwiches, sweets and soft drinks’ and babies born SGA were statistically significant even with a loss to follow-up (which could have contributed to a decrease in the statistical power of the study and the magnitude of the association found). In addition, this study used a FFQ to assess the dietary intake of women and this tool has been reported to provide misclassifications in dietary intakes(Reference Chen, Zhao and Mao12), which could bias the magnitude of the observed effects towards the null hypothesis. It is also important to highlight that the women’s food intake in the previous 12 months was assessed when they were in the first trimester of pregnancy. However, literature indicates that little or no dietary changes are expected in this first 3 months of pregnancy(Reference Crozier, Robinson and Godfrey44,Reference Cucó, Fernández-Ballart and Sala45) , which reassures the timing appropriateness of the pre-pregnancy diet in our study. Another point to consider is that the use of Brazilian food composition tables was not possible, as they have a limited number of nutrients available. Yet, the pregnant women had their deliveries in different maternity wards/hospital in the western region of Sao Paulo city, and the measurements were performed within their service routine. Thus, we cannot confirm that indeed paediatric length boards were used rather than measuring mats to assess birth length. Despite that, hospitals in Sao Paulo, in general, have reliable equipment and provide training to health teams to measure newborns’ weight and length. Furthermore, being born SGA can results from many other interacting factors apart from diet, such as paternal size, maternal morbidity, pregnancy complications, poor hygiene, low access to clean water, violence, or psychological aspects, which could not be assessed in this study(46).
A major strength of this study was the use of the INTERGROWTH-21st standards to evaluate the newborn weight and length by gestational age and sex. INTERGROWTH-21st is a multicentre, multiethnic, population-based project conducted in eight sites, including Brazil(Reference Villar, Altman and Purwar30). INTERGROWTH-21st used the same conceptual framework of WHO Multicentre Growth Reference Study(Reference Garza and de Onis47) to produce the prescriptive standards for newborn size(Reference Villar, Altman and Purwar30). We are confident that the results are sound given that a reputable standard for newborn size was our main outcome of interest. However, it is important to highlight that newborns classified as SGA may be simply constitutionally small, and, in this case, birth weight and/or length below the 10th percentile are not necessarily indicative of a growth restriction.
There are a number of important public health implications that merit discussion. Energy-dense, nutrient-poor food environment is harmful not only to the generation exposed to it but also to the next generation(Reference Chen, Zhao and Mao12), and our study findings reinforce the latest. Therefore, strategies to improve the quality of food intake should be implemented, especially for women of childbearing age. One approach is to improve the dietary diversity of a woman’s diet to increase micronutrient intakes without adding expenses generally associated with the use of micronutrient supplements(Reference Potdar, Sahariah and Gandhi48). A policy-based approach to supporting dietary diversity was modelled by the Dietary Guidelines for the Brazilian Population in 2014 in which food-based guidelines encourage a whole-foods diet with minimal intake of ultraprocessed foods(49). Diets with a higher intake of fruits, vegetables, legumes and fish, instead of higher intake of processed, ultraprocessed and takeaway foods, have positive pregnancy outcomes in general, and this evidence should be communicated not only to women but also to their social support(Reference Chen, Zhao and Mao12).
In conclusion, we found that a pre-pregnancy DP characterised by energy-dense, nutrient-poor foods increases the risk of having a SGA newborn. These findings provide an important foundation upon which dietary recommendations can be made or modified to ensure that rapid changes in the food environment do not promote poor fetal outcomes in countries undergoing rapid development. Investments in education and improved access to healthful food and nutritional information should be prioritised due to its potential impact on child health. However, further studies are warranted to identify specific metabolic pathways that may be underlying these associations and to more fully understand how measurements of paternal size, maternal morbidity and psychological aspects interact with diet to limit fetal growth.
Acknowledgements
We would like to acknowledge the participants and the technical team of the ProcriAr study. We are grateful to the relevant contributions of Ms Ana Lúcia da S. Castro in the study’s data management.
This study was supported by the Sao Paulo Research Foundation (FAPESP), Brazil (grant 2009/17315-9), and by the National Council for Scientific and Technological Development (MCT/CNPq/FNDCT/CAPES/FAPESP process no. 2008/57717-6). J. A. T. was supported by the Sao Paulo Research Foundation (2014/12647-1). The funding sources had no role in the design, analysis or writing of this article.
S. R. D. M. S., S. E. V. and R. P. V. F. were involved in the conception and design of the study. J. A. T. conducted the data analysis. J. A. T. and D. M. M. contributed to data interpretation. J. A. T. and D. J. H. drafted the manuscript, together with T. G. C. All authors participated in critically revising the manuscript and approved the final version.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114520004778








