CVD is the leading cause of death in developed countries. Annually, more than 1 100 000 Americans suffer a myocardial infarction (MI) and this cardiac event is responsible for one half of all cardiovascular deaths among Americans. Coronary artery disease is the leading cause of MI, and more than 12·4 million Americans have clinically significant coronary artery disease. Epidemiological studies, prospective randomised clinical trials, and laboratory investigations have reported a decrease in morbidity and mortality from heart disease in patients with diets supplemented with n-3 PUFA (reviewed in ref(Reference Siddiqui, Harvey and Zaloga1)) In contrast, it has been estimated that partially hydrogenated fat, the major dietary source of trans-fatty acids (TFA), may be responsible for 30 000–100 000 premature coronary deaths per year in the US alone(Reference Ascherio, Hennekens and Buring2).
Historically, much of the understanding of the beneficial health effects of fish oil has come from dietary studies in populations that have diets rich in n-3 PUFA, particularly EPA (C20 : 5, EPA) and DHA (C22 : 6, DHA)(Reference Ascherio3). n-3 PUFA exert many of their beneficial effects upon the cardiovascular system via their impact on several cellular processes(Reference Siddiqui, Harvey and Zaloga1). One example is the ability of n-3 PUFA to improve the plasma lipid profile. Short-term treatment (2–6 weeks) with n-3 PUFA results in a low to moderate lowering of TAG, lowering of LDL cholesterol and/or elevation of HDL cholesterol (HDL-C)(Reference Harris4, Reference Mori, Burke and Puddey5). Long-term treatment (4–6 months) with n-3 PUFA results in a significant reduction in TAG with a decrease in total cholesterol:HDL ratios and a modest increase in HDL-C(Reference Abe, El-Masri and Kimball6, Reference Harris, Ginsberg and Arunakul7). In addition to n-3 PUFA effects on improving lipid profiles, these fatty acids are anti-atherogenic(Reference Mori, Beilin and Burke8, Reference Thies, Garry and Yaqoob9) and improve vascular functions(Reference Geleijnse, Giltay and Grobbee10, Reference Mori11). n-3 PUFA are also known for their anti-inflammatory activity in the vasculature. Several studies have described effects of n-3 PUFA on endothelial cell adhesion molecule expression. For example, De Caterina et al. (Reference De Caterina, Liao and Libby12) provided evidence that DHA reduced endothelial expression of vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1 (ICAM-1) and E-selectin in response to IL-1, IL-4, TNF-α and bacterial lipopolysaccharide treatments. Similarly, Chen et al. (Reference Chen, Esselman and Jump13) have shown that pretreatment of human retinal vascular endothelial cells with DHA remarkably inhibited cytokine-induced ICAM-I and VCAM-1 expression. Furthermore, DHA attenuated ox-LDL-induced expression of adhesion molecules and the adhesion of monocytes to human coronary endothelial cells. In summary, the reported evidence suggests that n-3 PUFA generally have inhibitory effects on cytokine-induced adhesion molecule expression(Reference Chen, Li and Chen14). These effects may represent important mechanisms for modulating endothelial activation and for anti-atherosclerotic effects of n-3 PUFA or fish oils.
In contrast to n-3 PUFA, the consumption of TFA from processed fats has no known health benefits. In fact, TFA are felt to be associated with harm, even at low levels of intake. TFA occur naturally at relatively low levels in meat and dairy products as a by-product of fermentation in ruminant animals; however, during the process of partial hydrogenation of vegetable oils, some cis double bonds are converted to trans, some become saturated and others migrate along the acyl chains resulting in a wide range of unnatural geometric and positional fatty acid isomers(Reference Emken15). As compared with the consumption of an equal number of calories from saturated or cis unsaturated fats, the consumption of TFA raises levels of LDL cholesterol, reduces levels of HDL-C and increases the ratio of total cholesterol to HDL-C, a powerful predictor of the risk of CHD(Reference Lichtenstein, Ausman and Jalbert16). Recent studies have shown that TFA were independently associated with an increased risk of CVD, and contributed to disease through multiple mechanisms(Reference Ascherio3). For example, TFA (1) influence PG balance promoting thrombogenesis(Reference Kinsella, Bruckner and Mai17), (2) perturb essential fatty acid metabolism by inhibiting the conversion of linoleic acid to arachidonic acid and to other n-6 PUFA, which cause changes in the phospholipid fatty acid composition in the aorta(Reference Kummerow, Zhou and Mahfouz18), (3) activate systemic inflammatory responses, including substantially increased levels of IL-6, TNF-α, TNF receptors and monocyte chemoattractant protein(Reference Mozaffarian, Rimm and King19), (4) increase levels of several markers of endothelial dysfunction, including soluble ICAM-1 (sICAM-1), soluble VCAM-1 and E-selectin(Reference Lopez-Garcia, Schulze and Meigs20), and (5) impair endothelial function, as reflected by a reduction in brachial artery flow(Reference de Roos, Bots and Katan21). These observations suggest that TFA play a substantial role in the development of CHD. Clearly, TFA consumption causes a pro-inflammatory response within the vascular system.
Stimulation of vascular endothelial cells by inflammatory cytokines causes activation of the NAD(P)H oxidase system, which plays a crucial role in generating reactive oxygen species, which in turn causes NF-κB activation leading to enhanced gene transcription of adhesion molecules(Reference Chin and Dart22). Several studies suggest that n-3 PUFA have inhibitory effects on pro-inflammatory adhesion molecule (ICAM-1, VCAM-1 and E-selectin) expression(Reference Abe, El-Masri and Kimball6, Reference Chin and Dart22). A few studies indicate that regulation of adhesion molecule expression by n-3 PUFA occurs through their effects at the transcriptional level via NF-κB(Reference Chen, Esselman and Jump13, Reference De Caterina, Spiecker and Solaini23, Reference Dichtl, Ares and Jonson24). Effects of TFA on the NAD(P)H oxidase system are at present not known, but it is possible that TFA would act in an antagonistic manner to n-3 PUFA and induce oxidative stress. Increased production of O2 reacts with NO and generates peroxynitrite (ONOO− ). Peroxynitrite can not only uncouple and cause dysfunction of endothelial NO synthase, but also can effectively nitrate tyrosine, tryptophan, cysteine, or methionine-free amino acids or amino acid residues in proteins(Reference Alvarez, Ferrer-Sueta and Freeman25). Nitration of proteins usually results in functional impairment and is an indicator of oxidative stress(Reference Turko and ad26).
In the present studies, we extend the scientific information related to dietary effects of n-3 PUFA and TFA upon vascular disease development and progression. We determined the dietary effects of n-3 PUFA and TFA on sudden cardiac death and aortic fatty streak formation following MI in an in vivo rat model as well as determining their effects on vascular remodelling in a mouse model of hindlimb ischaemia. In addition, we performed in vitro studies with human aortic endothelial cells in order to understand the mechanism by which n-3 PUFA prevent and TFA cause vascular dysfunction and CHD.
Materials and methods
Animals
The coronary artery ligation studies were performed using male Wistar rats weighing 250–300 g (Harlan, Indianapolis, IN, USA), whereas femoral artery ligations were performed using male C57/BL6 mice weighing 30–35 g (Harlan). Both studies were approved by Methodist Research Institute's Animal Research Committee and conform to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
Coronary artery ligation
Rats were anaesthetised using isoflurane (1·5–2 %), intubated and placed upon a mechanical ventilator. Surgery was performed using sterile techniques. The rat's chest was shaved and prepped with betadine and alcohol. A left thoracotomy was performed, the heart exposed and the left anterior descending coronary artery ligated using 6-0 prolene sutures approximately 1 mm distal to the take-off of the left main coronary artery. The chest was closed in two layers (ribs and muscle, skin). Animals were allowed to wake up following surgery and then extubated. Animals were maintained on a heating pad and closely observed until fully mobile. Animals received standard rat chow and ad libitum water for the first 24 h. Twenty-four hours following surgery (MI), rats were randomised to the n-3 PUFA or TFA chow diets. Rats were evaluated twice daily and followed for 6 months. Sudden death for the present study was based upon clinical observation and occurred if an animal that was completely healthy on previous examination (within 12 h) was found dead on the subsequent examination. In addition, at autopsy, no cause of death could be found upon gross examination.
Femoral artery ligation
Mice were shaved on the right leg after induction with 3 % isoflurane in the induction chamber. The anaesthesia was maintained by using inhalational 0·5–1·0 % of isoflurane. An incision (0·.5–1·0 cm) was made on the thigh. The right femoral artery was isolated and ligated twice with 3-0 sutures approximately 1 cm distal to the inguinal ligament. Incision on the skin was sutured by using 5-0 Polysorb suture. Animals were allowed to wake up following surgery and were maintained on a heating pad and closely observed until fully mobile. After femoral artery ligation, animals when awake and fully mobile were returned to their cages. Animals received standard rat chow and ad libitum water for the first 24 h and were then randomised to the n-3 PUFA or TFA chow diets for 3 weeks.
Diets
Animals were fed isocaloric diets that varied in the quantity of n-3 PUFA and TFA (Research Diets, New Brunswick, NJ, USA). The n-3 PUFA diet was formulated with a mixture of maize and fish oil (Sigma Chemical Co., St Louis, MO, USA) and was rich in EPA (20 : 5) and DHA (22 : 6), whereas the TFA diet was formulated with a mixture of maize and Primex shortening (Procter & Gamble, Cincinnati, OH, USA) and was rich in elaidic acid (trans-18 : 1) and linoelaidic acid (trans-18 : 2). The diets contained similar quantities of protein (22 %), carbohydrates (57 %) and fats (10 %) with standard mixture of minerals and vitamins. Fatty acid compositions of diets are shown in Table 1. Water was provided ad libitum.
Table 1 Fatty acid composition of diets and plasma
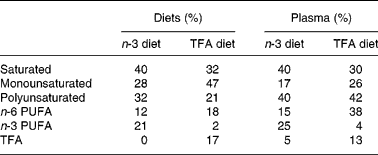
TFA, trans-fatty acid.
Analysis of fatty streaks deposition in aortic arch
The fatty streaks in aortas were stained with Sudan IV stain for 10 min and then washed several times with PBS until the non-specific stain washed out. Aortas were visualised under a dissecting microscope (Leica S8APO, Leica Corporation, Heerbrugg, Switzerland), and photographs for quantification of fatty streaks were captured with a digital camera (MagnaFire, Optronics, Goleta, CA, USA).
Plasma soluble intercellular adhesion molecule 1 levels
Rat blood samples were collected in tubes containing the anticoagulant, EDTA. Samples were centrifuged for 30 min at 1000 g, and the plasma was harvested for subsequent ELISA analysis (R&D Systems, Minneapolis, MN, USA). Plasma samples were diluted 50-fold in calibrator diluent as suggested by the manufacturer. To quantify the sICAM-1 plasma content, the diluted samples were analysed in comparison to a rat sICAM-1 standard curve as instructed by the manufacturer. Data are expressed as the means and standard deviations (n 5).
Fatty acid analysis
Fatty acids from plasma were extracted with chloroform–methanol (2:1) using the Folch method. The methyl esters of fatty acids were separated on a GC system (Shimadzu GC2010) equipped with an Rt 2560 column (100 m, 0·25 mm internal diameter, 0·2 mm). The oven temperature ramped from 100°C (4 min hold) to 240°C at 3°C/min (10 min hold) with a FID at 250°C to resolve fatty acids' peaks, which were identified using authentic standards (Restek Corp., Bellefonte, PA, USA). Data was analysed with Shimadzu's GC solutions software.
Analysis of vascular remodelling
The animals were sacrificed under anaesthesia and perfused through the terminal aorta at a constant pressure (approximately 100 mmHg) with a dilator solution (PBS+10 mm adenosine+1·0 mm sodium nitroprusside) containing 4 % paraformaldehyde and Microfil® (Flow Tech, Inc., Carver, MA, USA) vascular casting compound. The skin overlying the ventral hindlimb was removed, the primary collateral pathways were dissected out and photomicrographs of the collateral vessels were obtained under high magnification through a Leica dissecting microscope via a Spot Insight 4 digital camera (Diagnostic Instruments Inc., Sterling Heights, MI, USA).
Human aortic endothelial cell culture
A primary cell line derived from human aortic endothelial cells was maintained in EBM-2 medium containing 5 % fetal bovine serum and the bullet kit materials as specified by the manufacturer (Cambrex, East Rutherford, NJ, USA). Cells were maintained at 37°C in a humidified atmosphere in the presence of 5 % CO2. Only endothelial cell cultures of less than ten passages and 80–90 % confluency were utilised in the present study.
Adhesion molecule expression in endothelial cells
Trypsinised endothelial cells (1 × 105/sample) were washed in PBS containing 0·5 % bovine serum albumin and resuspended into a volume of 100 μl of this labelling buffer. Cells were labelled with 0·25 μg phycoerythrin-conjugated antibody for 20 min; subsequently, the cells were washed twice in PBS containing 0·5 % bovine serum albumin. An isotype control was established for each sample set to ensure specificity of the antibody binding. Analysis was performed on a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA, USA) equipped with an air-cooled argon laser emitting at a 488 nm wavelength. Fluorescence was detected through a 575 ± 26 band pass filter and quantified using CellQuest software (Becton Dickinson). Results indicate the mean fluorescent intensity of gated endothelial cells, which excluded cellular debris and particles.
Analysis of protein nitrosylation
Endothelial cells were cultured in media supplemented with n-3 PUFA or TFA, and nitrosylation of proteins was detected by Western analysis using anti-nitrotyrosine antibody (Upstate Biotechnology Incorporated, Lake Placid, NY, USA). Glyceraldehyde-3-phosphate dehydrogenase protein expression served as the loading control.
Statistical analysis
Data are presented as means and their standard deviations, and were compared between the n-6 and n-3 diet groups using two-sample two-sided Student's t tests. Survival was estimated using Kaplan–Meier procedures and was tested between diet groups using the log-rank test. P < 0·05 was considered significant.
Results
Plasma fatty acid composition reflects the dietary composition
Fatty acid compositions of the n-3 PUFA and TFA diets and plasma from animals fed these diets for 6 months are presented in Table 1. The n-3 PUFA diet contained 21 % n-3 fatty acids mostly containing EPA (C20 : 5) and DHA (C22 : 6), whereas the TFA diet contained 17 % TFA mostly containing elaidic acid (trans-18 : 1) and linoelaidic acid (trans-C18 : 2). Fatty acid compositions of plasma samples, particularly SFA, n-3 PUFA and TFA, reflect the composition of diets on which the animals were fed. However, on feeding either n-3 PUFA or TFA diets, the plasma levels of MUFA were decreased by 40–45 %, whereas levels of PUFA were increased by 25–50 % compared with levels present in the diets. The biggest increase was observed in n-6 PUFA levels in TFA-fed animals.
n-3 PUFA prevents sudden cardiac death following myocardial infarction
The data presented in Fig. 1 indicate that n-3 PUFA improved survival following a coronary artery ligation-mediated MI compared with a control (high n-6 PUFA diet) and TFA-enriched diet. It is evident from 6-month survival data that only 50 % of the animals survived on a TFA-enriched diet, whereas 85 % of the animals survived on an n-3 PUFA-enriched diet. Sixty-five percentage of the animals (control) survived consuming a diet closely resembling a typical Western diet (rich in n-6 PUFA; data replotted from a previous study, see ref.(Reference Zaloga, Ruzmetov and Harvey27)). These data clearly suggest that TFA diversely affect survival, whereas n-3 PUFA beneficially affect survival following MI. The animals did not show any gross differences in body weight, heart weight, left ventricle thickness and infarct size (data not shown). Because no detectable differences in infarct size and heart weight were apparent, these observations suggest that fatty acids may have induced other cellular and/or molecular changes in the vasculature of these animals. To further investigate, we colleted blood specimens and analysed the aortas from surviving animals fed n-3 PUFA- or TFA-enriched diets.

Fig. 1 Survival (%) over 180 d following myocardial infarction. Male rats were subjected to coronary ligation to induce myocardial infarction and were randomly assigned to trans-fatty acid (n 30) or n-3 (n 30) diets. Rats were fed corresponding diets for the next 6 months and observed twice daily for mortality. Mortality of animals from sudden cardiac death was recorded as described previously(Reference Zaloga, Ruzmetov and Harvey27). Data for animals (control) survived consuming a diet closely resembling a typical Western diet (rich in n-6 PUFA) were replotted from a previous study(Reference Zaloga, Ruzmetov and Harvey27). Survival differences were tested using the log-rank test. Significant differences were observed between groups at P < 0·05. - - -, trans-fat diet; – –, n-6 diet; —, n-3 diet.
trans-Fatty acids cause atherosclerotic lesions in the vasculature
Surviving animals fed n-3 PUFA- or TFA-enriched diets were also examined for atherosclerotic lesions. The data shown in Fig. 2 are representative of these animals and indicate that animals fed n-3 diets did not exhibit any fatty streaks in their aortas; however, animals fed TFA-enriched diets exhibited variable degrees of fatty streaks in their aortas. We further investigated rat aortas for intimal thickness using H&E staining.

Fig. 2 Fatty streaks deposition in aortic arch. Aortic arches of rats were isolated and opened through the midline. The fatty streaks in aortas were visualised with Sudan IV stain for 10 min and then washed several times with PBS until the non-specific stain washed out. The aorta was visualised under a dissecting microscope (Leica S8APO, Leica Corporation), and photographs were captured with a digital camera (MagnaFire). Results are a representation of at least three animals in each group.
trans-Fatty acids induce inflammatory responses
Levels of sICAM-1 in plasma were assayed in order to determine whether the TFA-enriched diet induced a pro-inflammatory response in these animals. Data presented in Fig. 3 indicate that animals fed a TFA-enriched diet exhibited 45 % (P < 0·05) higher concentration of sICAM-1 compared with that of animals fed an n-3 PUFA-enriched diet.
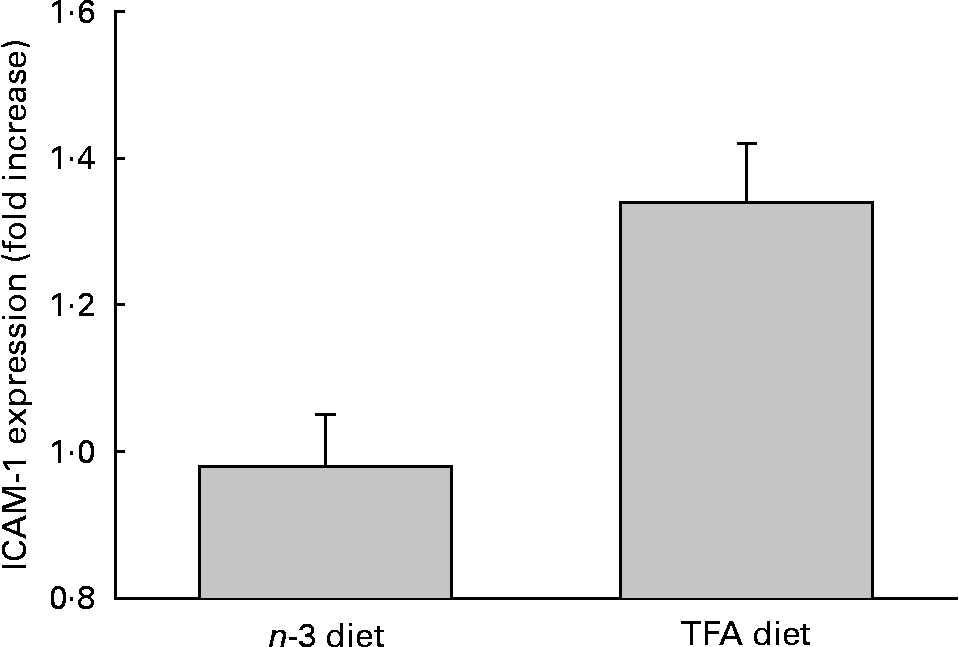
Fig. 3 Plasma soluble intercellular adhesion molecule-1 (sICAM-1) levels. Rat blood samples were collected in tubes containing the anticoagulant, EDTA. To quantify the sICAM-1 plasma content, the diluted samples were analysed in comparison to a rat sICAM-1 standard curve as per the manufacturer's (R&D Systems) protocol. Data are expressed as the means and standard deviations (n 5). Significant differences were observed between groups at P < 0·05. TFA, trans-fatty acid.
n-3 PUFA promotes vascular remodelling
To further study the effects of fatty acids on the vasculature, we examined collateral vascular growth induction in response to ischaemia utilising the well-established ischaemic hindlimb model that is created by ligation of the femoral artery. Fig. 4(a) represents a typical site that is used for hindlimb ligation and observation of collateral growth. The data in Fig. 4(b) are a typical representation of the vascular response to ischaemia in the TFA and n-3 PUFA groups. The results indicate that animals fed a TFA-enriched diet lacked the ability to develop collateral vascular remodelling about the site of occlusion, whereas animals fed n-3 PUFA were capable of collateral vascular remodelling.

Fig. 4 Induction of compensatory vascular remodelling following hindlimb ischaemia. Hindlimb of mice was ligated as shown by ![]() , and region (a) shown in the red box was examined for the induction of compensatory vascular growth. (b) Animals were fed diets enriched in n-3 PUFA or trans-fatty acid (TFA) for 3 weeks following ligation of the femoral artery, and induction of the collateral pathways was examined as described in the text. Results are a representation of at least three animals in each group.
, and region (a) shown in the red box was examined for the induction of compensatory vascular growth. (b) Animals were fed diets enriched in n-3 PUFA or trans-fatty acid (TFA) for 3 weeks following ligation of the femoral artery, and induction of the collateral pathways was examined as described in the text. Results are a representation of at least three animals in each group.
n-3 PUFA can prevent trans-fatty acids-induced pro-inflammatory responses
We further tested the effects of n-3 PUFA and TFA on ICAM-1 expression in vascular endothelial cells. Data presented in Table 2 demonstrate that DHA treatment significantly inhibited expression of ICAM-1 (P < 0·05), whereas trans-C18 : 2, a relevant trans-fatty acid, significantly stimulated (P < 0·05) ICAM-1 expression on endothelial cell surfaces. Furthermore, ICAM-1 expression was also significantly reduced (P < 0·05) when endothelial cells were initially treated with DHA and then subsequently challenged by TFA. However, when ICAM-1 expression was induced by pretreating cells with TFA (P < 0·05), DHA was not able to reduce the expression of ICAM-1. Furthermore, when cells were initially treated with DHA and then DHA was washed out, the inhibitory effects of DHA on ICAM-1 expression disappeared, but when cells were treated with TFA and then the TFA were washed out, elevated ICAM-1 expression still persisted.
Table 2 Effect of n-3 PUFA and trans-fatty acid on endothelial intercellular adhesion molecule-1 (ICAM-1) expression
(Mean fluorescent intensity values and standard deviations for at least three observations)
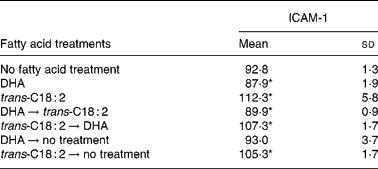
ICAM-1 surface expression was quantified on a FACSCalibur flow cytometer (Becton Dickinson) using a phycoerythrin-conjugated antibody.
* P value < 0·05 (Students' t test) compared with cells not supplemented with fatty acids.
trans-Fatty acids cause oxidative stress
We determined oxidative stress by assaying the status of nitrosylated proteins in endothelial cells after treating them with n-3 PUFA or TFA. Data shown in Fig. 5 demonstrate that DHA has no effect of nitrosylation of proteins (lane 2), whereas trans-C18 : 2 clearly induced nitrosylation of endothelial cells proteins (lane 3).

Fig. 5 Effect of n-3 PUFA and trans-fatty acid (TFA) on nitrosylation of proteins. Endothelial cells were cultured in media supplemented with n-3 PUFA or TFA, and nitrosylation of proteins was detected by Western analysis using anti-nitrotyrosine antibody (Upstate Biotechnology Incorporated). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) protein expression served as the loading control. Molecular weight markers are identified with arrow annotation. Lane assignments are as follows: (1) vehicle, (2) DHA (5 μm) for 48 h, (3) trans-C18 : 2 (5 μm) for 48 h. Results are a representation of at least three experiments in each group.
Discussion
Both epidemiological and prospective randomised clinical trials have reported a decrease in morbidity and mortality from heart disease in patients consuming diets supplemented with n-3 PUFA (reviewed in ref (Reference Siddiqui, Harvey and Zaloga1)). The GISSI Prevenzione Study(28) is the largest study to probe the cardiovascular benefits of n-3 PUFA. In this study, the group receiving n-3 PUFA alone had a significant reduction in the relative risk of death, non-fatal MI or non-fatal stroke. After 2 years of follow-up, this trial concluded that n-3 PUFA consumption significantly reduced mortality by 27 %.
In addition, four large cohort studies(Reference Ascherio, Hennekens and Buring2, Reference Oomen, Ocke and Feskens29–Reference Willett, Stampfer and Manson31) and a meta-analysis of sixty controlled trials in human subjects(Reference Mensink, Zock and Kester32) concluded that a higher dietary consumption of TFA was associated with a higher risk of CHD. As compared with the consumption of an equal number of calories from saturated or cis unsaturated fats, the consumption of TFA raises levels of LDL cholesterol, reduces levels of HDL-C and increases the ratio of total cholesterol to HDL-C, a powerful predictor of the risk of CHD(Reference Lichtenstein, Ausman and Jalbert16, Reference Mensink and Katan33). Although these effects would be expected to increase the risk of CHD, the relationship between the intake of trans-fats and the incidence of CHD reported in prospective studies has been greater than that predicted by changes in serum lipid levels alone, suggesting that TFA may also influence other risk factors for CHD(Reference Ascherio, Katan and Zock34, Reference Lemaitre, King and Mozaffarian35). In the present study, we investigated effects of n-3 PUFA and TFA in animal models of sudden cardiac death and vascular remodelling. We also performed studies in human vascular endothelial cells to investigate the effects of n-3 PUFA and TFA on pro-inflammatory responses.
Our data indicate that manipulation of dietary fatty acids content clearly affects tissue distribution of fatty acids, as the plasma levels of n-3 PUFA and TFA closely reflect their dietary content. It is interesting to note that feeding n-3 PUFA or TFA resulted in a decrease in MUFA. We did not measured tissue distribution of fatty acids in the present study, but the results are consistent with our earlier studies that n-3 PUFA and TFA incorporated in tissues at the expense of MUFA(Reference Harvey, Arnold and Rasool36). Incorporation of n-3 PUFA or TFA at the expense of MUFA suggests that these fatty acids may be acylated on the sn-2 position of phospholipids. This change may have an implication for cellular phospholipases that generate lipid mediators and as a consequence may influence downstream signalling(Reference Siddiqui, Harvey and Zaloga1). It is hypothesised that lipid mediators containing or derived from TFA act differently than lipid mediators containing or derived from n-3 PUFA.
Our data indicate that dietary TFA adversely affect survival, whereas dietary n-3 PUFA beneficially affect survival following MI. Importantly, the effects of these dietary fatty acids occurred over many weeks following MI and appeared to primarily affect arrhythmia generation (i.e. sudden death). Furthermore, we did not observe detectable differences in infarct size and heart weight. This observation prompted us to think that fatty acids may have induced other cellular and/or molecular changes particularly in the vasculature of these animals. We found that animals fed TFA-enriched diets exhibited variable degrees of fatty streaks in their aortas, whereas animals fed n-3 PUFA-enriched diets did not exhibit any fatty streaks in their aortas. We were surprised by these findings, since rats are known to resist the formation of atherosclerotic lesions(Reference Moghadasian37). Most investigators now agree that arterial fatty streaks represent the earliest stages of plaque development(Reference Masuda and Ross38, Reference Rosenfeld, Tsukada and Gown39). The relation of fatty streaks to more advanced atherosclerotic lesions has long been disputed and their full reversibility is generally accepted. Fatty streaks are areas of focal intimal thickening produced by the intimal accumulation of lipid-laden macrophages (foam cells) surrounded by extracellular matrix and a variable number of lymphocytes. As fatty streaks are not ‘classical’ atherosclerosis lesions, we further investigated the arteries from rats for intimal thickness using H&E staining. We did not see major differences in the intima. Presumably, TFA feeding could exacerbate the development of vascular pathology over time. Since more animals survived on an n-3 PUFA diet than on a TFA diet following MI, it suggests that n-3 PUFA may have promoted blood flow about occluded areas by inducing collateral or compensatory vascular remodelling. Initial attempts to look for compensatory vascular remodelling about coronary artery ligation sites were not successful, because the heart is full of vascular networks and finding novel vascular networks was extremely difficult. We therefore used a hindlimb ischaemia model to test the hypothesis that n-3 PUFA promote collateral vascular modelling whereas TFA fail to do so.
Collateral growth, or compensatory vascular remodelling, is a process whereby vessels from a pre-existing arteriolar network develop into collateral arteries, effectively bypassing the site of arterial occlusion(Reference Scholz, Cai and Schaper40). The development of collateral pathways, sometimes referred to as arteriogenesis, is a natural mechanism to compensate for the tissue ischaemia resulting from an arterial occlusion. Data in Fig. 4(b) indicate that animals fed TFA-enriched diets lacked the ability to develop collaterals about the site of occlusion, whereas animals fed n-3 PUFA-enriched diets induced compensatory vascular remodelling. These data suggest that animals consuming TFA have endothelial cells that were not able to induce compensatory vascular remodelling, whereas endothelial cells from animals fed n-3 PUFA responded to the increase in blood flow with the induction of compensatory vascular remodelling. However, it is not clear how these fatty acids influence the functional activities of endothelial cells, which cause resistance to or induction of compensatory vascular remodelling. The underlying cause of the impairment in collateral vascular development is not known, although it may be preceded by pro-inflammatory conditions and endothelial dysfunction.
We assessed the effects of dietary n-3 PUFA and TFA on pro-inflammatory conditions by measuring plasma levels of sICAM-1, which appears in the circulation from an inflamed endothelium. Our data presented in Fig. 3 indicate that animals fed a TFA-enriched diet have higher circulating levels of sICAM-1 and therefore may have dysfunctional endothelium. Under normal physiologic conditions, the vascular endothelium maintains homeostasis in response to blood-borne and locally generated stimuli by altering its functional state. However, sustained or abnormal stimuli, including inflammatory cytokines, can result in endothelial dysfunction(Reference De Caterina, Liao and Libby12). Endothelial dysfunction is characterised as altered control of vascular tone, altered permeability to plasma lipoproteins, hyperadhesiveness to blood leukocytes and increased cytokine and growth factor production(Reference Gimbrone41). Endothelial cell activation plays an important role in both the development and the prevention of atherosclerosis and CHD(Reference Gimbrone41). Furthermore, endothelial cells are involved in the development of vascular remodelling, conditions often associated with CHD. We therefore investigated the effect of n-3 PUFA and TFA on human endothelial cells. Some investigators suggest that trans-C18 : 2 are associated with a relatively higher relative risk of CHD than trans-C18 : 1(Reference Lemaitre, King and Mozaffarian35, Reference Baylin, Kabagambe and Ascherio42). We therefore used trans-18 : 2 as a representative TFA. Data presented in Table 2 demonstrate that DHA treatment significantly inhibits expression of ICAM-1, whereas trans-C18 : 2 significantly stimulates ICAM-1 expression on the endothelial cell surface. This observation suggests that n-3 PUFA are anti-inflammatory, whereas TFA are pro-inflammatory. Furthermore, ICAM-1 expression was also significantly reduced when endothelial cells were initially treated with DHA and then subsequently challenged by TFA, indicating a preventive effect of n-3 PUFA for TFA-mediated effects. However, when ICAM-1 expression was induced by pretreating cells with TFA, DHA was not able to reduce the expression of ICAM-1. This suggests that perhaps a higher concentration or longer treatment duration is required to inhibit TFA-mediated effects. When cells were initially treated with DHA and then washed out, the inhibitory effects of DHA on ICAM-1 expression disappeared, but when cells were treated with TFA and then washed out, stimulated expression of ICAM-1 still persisted. These data suggest that n-3 PUFA are depleted from cells quickly, whereas TFA stay in cells for a longer duration. Enhanced adhesion molecule expression is often associated with an inflammatory endothelial cell phenotype. This finding is important because it demonstrates that TFA treatment causes an inflammatory endothelial cell phenotype, which is regarded as the initial step for a dysfunctional endothelium.
Among other factors, altered generation of NO has been linked to a dysfunctional endothelium. Erdei et al. (Reference Erdei, Toth and Pasztor43) have shown that a high-fat diet in rats reduced NO-dependent arteriolar dilation. NO has been shown to be essential for collateral growth and is normally increased under conditions of increased flow and shear stress(Reference Matsunaga, Warltier and Weihrauch44). Thus, dysregulation of NO production could be responsible for impaired collateral growth and increased endothelial dysfunction, possibly due to a reduced response to increased shear, which may in turn alter endothelial NO synthase expression or activity. Several studies have implicated endothelial NO synthase uncoupling as a possible contributor to vascular function. Growth factors are ultimately involved in the vascular remodelling necessary for collateral growth, and many are dependent on NO(Reference Matsunaga, Warltier and Weihrauch44, Reference Baffour, Berman and Garb45). Several studies have identified inflammation-related cells and molecules as being important to collateral growth. This process includes activation of endothelial cells and release of cytokines such as MCP-1, GM-CSF and TGF-β1(Reference Grundmann, Hoefer and Ulusans46–Reference Seiler, Pohl and Wustmann48). In order to determine whether these fatty acids play any role upon NO regulation, we assayed nitrosylation of proteins in endothelial cells following n-3 PUFA or TFA treatments. Data shown in Fig. 5 demonstrate that DHA has no effect upon nitrosylation of proteins, whereas trans-C18 : 2 clearly induced nitrosylation of endothelial cell proteins. These data suggest that TFA have caused formation of peroxynitrite (ONOO− ) through reactions between superoxide (O2−) and NO resulted in nitrosylation of proteins. These data indicate that TFA generate oxidative stress, whereas n-3 PUFA prevent oxidative stress, presumably via their effects upon the NAD(P)H oxidase system. Clearly, additional data are needed to determine the effect of n-3 PUFA and TFA on the generation of O2 and NO through activation of NAD(P)H oxidase and endothelial NO synthase under induced oxidative stress conditions.
In conclusion, our data suggest that dietary n-3 fatty acids prevent, whereas TFA induce, sudden cardiac death following MI. It appears that TFA promote, whereas n-3 PUFA prevent, pro-inflammatory responses and vascular dysfunction. Furthermore, it appears that TFA-mediated endothelial cell dysfunction impairs induction of collateral growth at the site of occlusion, whereas n-3 PUFA induce collateral growth. Although the common perception is that the effects of TFA can be prevented by simply removing these fats from the diet, studies(Reference Przybylski49) have suggested that TFA are generated during everyday cooking in sufficient amounts to impact cardiovascular function. Furthermore, a recent study has demonstrated endogenous production of TFA through free radical-mediated pathway(Reference Zambonin, Prata and Cabrini50). It is therefore not possible to completely eliminate TFA; however, the present studies suggest that the effect of TFA may be reduced or prevented by consumption of n-3 PUFA.
Acknowledgements
The present work was supported by grants from the Showalter Foundation and the Methodist Research Institute. There is no potential conflict of interest by any of the authors. R. A. S. and G. P. Z. planned and supervised the study; N. R. performed coronary and femoral arteries ligation. K. A. H. performed in vivo and in vitro experiments; and S. J. M. help in studying vascular remodelling studies. The authors wish to thank Ms Diane Bond for animal care; Ms Charlene Shaffer for secretarial assistance and Mr Colin Terry for statistical analysis of the data.









