INTRODUCTION
GBV-C is a flavivirus that is closely related to hepatitis C virus (HCV) [Reference Stapleton1]. Transmission of GBV-C via parenteral, sexual, and vertical routes has been documented, and infection is common in many populations [Reference Dawson2–Reference Xiang7]. There are five major genotypes that are prevalent worldwide [Reference Muerhoff8, Reference Naito, Hayashi and Abe9]. Several studies have reported a strong association between the existence of GBV-C viraemia and the survival of HIV-infected patients [Reference Xiang7, Reference Lefrère10, Reference Tillmann11]. However, other studies do not support this association [Reference Birk, Lindbäck and Lidman12–Reference Van der Bij14]. In fact, two studies showed that patients co-infected with GBV-C and HIV-1 had an increased mortality rate [Reference Quiros-Roldan15, Reference Ryt-Hansen16]. Mechanisms underlying the positive effects on the progression of HIV infection include decreased expression of CC chemokine receptor 5 (CCR5) on CD4+ T cells and direct inhibition of HIV replication by GBV-C [Reference Nattermann17, Reference Xiang18]. Recent findings suggest that HIV-1 patients with active GBV-C infection had a reduced percentage of T cells positive for CD38+CD4+, CD38+CD8+, CCR5+CD4+, and CCR5+CD8+, which might provide a key mechanism involved in the protection conferred by GBV-C against HIV-1 disease progression [Reference Maidana-Giret19].
MSM are the second most vulnerable population for HIV-1 infections after intravenous drug users in China in recent years. In Beijing, 3·0–4·6% of MSM were found to be HIV-1 positive [Reference Zhang20]. A cross-sectional study has shown a high prevalence of sexually transmitted infections and HCV to be present in HIV-1-positive MSM [Reference Zhang21], but the infection rate of GBV-C in MSM has not been established. Here, we performed a cohort study to evaluate the prevalence of GBV-C viraemia in MSM for those with a clear date of HIV-1 infection and those without HIV-1 infection. We also analysed the association of GBV-C viraemia, syphilis, HCV and hepatitis B virus (HBV) with HIV-1 infection in this MSM cohort. Furthermore, we determined the potential effect of GBV-C viraemia on the progression of HIV-1 disease.
MATERIALS AND METHODS
Study sample
The subjects included in this study were selected from the participants in the AIDS High Risk Cohort Programme supported by the Beijing Science & Technology Committee and the study was approved by the Ethics Committee of the Beijing Youan Hospital. A total of 4236 MSM were enrolled in the study between 2007 and 2010. Written informed consent was obtained from all participants. At 2-month intervals, HIV-related clinical status was assessed, an interviewer-administered questionnaire was completed, and blood samples were obtained for detection of HIV-1 antibodies and HIV-1 RNA levels by immunoassay and RT–PCR (Roche, Sweden), respectively. Of 4236 MSM, 99 subjects who had a clear date of HIV-1 infection and 175 subjects without HIV-1 infection at the same visit as a control were selected from the cohort for the case-control study. The prevalence of GBV-C viraemia and antibodies against HCV, HBV surface antigen (HBsAg) and syphilis were compared between individuals with HIV-1 infection and controls without HIV-1 infection. In the cohort study, 99 HIV-1-positive subjects were detected for GBV-C viraemia at the acute stage of HIV infection and the chronic stage of HIV-1 infection. Acute stage is defined as negative HIV p24 antibody testing by enzyme-linked immunosorbent assay in the presence of detectable HIV-1 RNA or positive HIV p24 antibody testing and an evolving (⩽3 bands positive) HIV Western blot [Reference Streeck22]. Chronic stage is defined as having been infected for at least 6 months and usually for several years [Reference Moir23]. In our study, we collected the samples at 12 months post-HIV infection. CD4+ T-cell counts and HIV-1 viral loads were also determined at each time point.
RT-PCR for detection of GBV-C RNA
GBV-C RNA was extracted from the samples by the Qiagen viral RNA Mini kit (Qiagen, Germany). A Superscript First-Strand Synthesis kit (Invitrogen, USA) was used to create GBV-C complementary DNAs (cDNAs). Both first- and second-round PCRs were performed using primers that hybridize to 5′ non-translated regions of GBV-C (GenBank accession no. NC_001710.1, 31–376 nt and 121–333 nt, respectively). Primers for the first-round RT–PCR were GBV-F1 (5′-ACT GGG TGC AAG CCC CAG AAA CC) and GBV-R1 (5′-CTG GTC CTT GTC AAC TCG CCG). Primers for the second-round PCR were GBV-F2 (5′-GTG ATG ACA GGG TTG GTA GGT CGT) and GBV-R2 (5′-GAC ATT GAA GGG CGA CGT GGA). PCR products were detected on 1·5% agarose gels containing 0·5 μg/ml of ethidium bromide. The expected band sizes were 366 and 233 bp for the first- and second-round PCRs, respectively.
Quantification of GBV-C viral load
Absolute quantification requires a standard curve of known amounts of the amplicon per reaction. The substrates for standard curve were generated by subcloning the amplicon in a plasmid (pGEM-T easy, Promega). A standard curve of the GBV-C NCR plasmid was quantified using the universal QPCR method in a 25-μl reaction containing 1× Taqman Universal PCR Master Mix (Applied Biosystems, USA), 400 nm of primer gbv-p1 (5′-AGC GCA CGG TCC ACA GGT GTT-3′), 400 nm of primer gbv-p2 (5′-GAC ATT GAA GGG CGA CGT GGA-3′), and 200 nm of fluorescent probe gbv-probe (5′-FAM-CCC TAC CGG TGG GAA TAA GGG CCC GAC TAMRA-3′) (Invitrogen). Serial tenfold dilutions of GBV-C NCR plasmid were used to cover a range of 104–107 molecules per reaction. Triplicate reactions of each dilution were pipetted into a 96-well plate and amplified in an ABI Prism 7900HT real-time PCR system (Applied Biosystems) at 50 °C for 30 min and then 94 °C for 2 min for the inactivation of the AMV reverse transcription enzyme, followed by 40 cycles of 94 °C for 10 s and 60 °C for 1 min. The data was analysed with Sequence Detection Systems (SDS) software (Applied Biosystems).
Determination of nucleotide sequences and GBV-C genotypes
The 627-bp PCR outer fragments of 5′-UTR were obtained by the primer pairs of GBV-OF1 (5′-GTG CAA GCC CCA GAA ACC GAC-3′, 36 nt, NC_001710.1) and GBV-OR1 (5′-GGG GCG CAA CAG TTT GTG AGG-3′, 641 nt, NC_001710.1). Subsequently, the 366-bp PCR products were obtained by primer pairs GBV-F1 and GBV-R1. The nucleotide sequences were determined using an ABI PRISM3730 XL Genetic Analyzer (ABI Biosystems, USA). The sequences were aligned with the sequences from reference isolates of GBV-C genotype 1 (U36380), genotype 2 (U44402 and AF081782), genotype 3 (D90601) and genotype 5 (AY949711) by Mega software (version 5.0). The phylogenetic trees were constructed by neighbour-joining as implemented in the software and the evolutionary distances were computed using the maximum composite likelihood method.
Statistical analyses
The odds ratios (OR) and 95% confidence intervals (CI) for the association between the occurrence of GBV-C viraemia and HIV acquisition was calculated. The occurrence of GBV-C viraemia between subjects prior to HIV-1 infection and those after acquisition of HIV-1 infection were compared by Pearson's χ2 test. The GBV-C viral loads at two time points were compared by paired Student's t test. The median of HIV viral loads and CD4+ T-cell counts between the GBV-C viraemia group and the GBV-C RNA-negative group in HIV-1-positive subjects were compared by Mann–Whitney U test. The relationship between HIV-1 viral loads and GBV-C viral loads, and CD4+ T-cell counts were evaluated by linear regression analysis.
RESULTS
HIV-1 acquisition was independent of GBV-C viraemia
To determine the impact of GBV-C viraemia on HIV-1 transmission, we followed up 99 MSM subjects before and after they acquired HIV-1. Based on a cross-sectional analysis, we found that the prevalence of GBV-C viraemia in these 99 study subjects was 17·7% before HIV-1 infection, which was not significantly different from that (12·6%) of the 175 HIV-1-negative subjects (OR 1·36, P = 0·29) (Table 1), indicating that a pre-existing GBV-C infection probably did not play a significant role in preventing the acquisition of HIV-1. Similarly, the prevalence of HBV and HCV in the 99 HIV-1-positive subjects was not significantly different from that in the 175 HIV-1-negative subjects (Table 1). In contrast, the prevalence of syphilis in the 99 HIV-1-positive subjects (20·20%) was significantly higher than that in the HIV-1-negative subjects (3·42%, OR 5·89). These results indicated that HIV-1 acquisition was independent of the presence of HBV, HCV and GBV-C viraemia, but was highly associated with the presence of syphilis in the MSM population in Beijing.
Table 1. Association of HIV-1 co-infection with other pathogens including HBV, HCV, syphilis and GBV-C
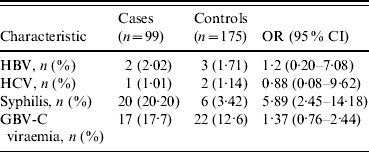
OR, Odds ratio, CI, confidence interval.
Occurrence of GBV-C viraemia was increased after HIV-1 acquisition
During follow-up studies, the occurrence of GBV-C viraemia in the 99 HIV-1-positive subjects increased from 17·7% prior to HIV-1 acquisition (visit 1) to 27·2% at the time of HIV-1 acquisition (visit 2) (P = 0·09). After 12 months (visit 3), GBV-C RNA was still positive in the 27 subjects that were positive at visit 2. Furthermore, an additional seven subjects had acquired GBV-C viraemia at visit 3, leading to a significant increase of GBV-C viraemia occurrence compared to visit 1 (34·3%, P < 0·01). However, for the HIV-negative subjects, newly acquired GBV-C viraemia was not observed after 12 months. The occurrence of GBV-C viraemia was significantly higher in the HIV-1-positive subjects than in the HIV-1-negative subjects at visits 2 and 3, respectively (Fig. 1). For HIV-1-positive subjects at visit 2, the distribution of GBV-C RNA (27·2%) was similar with respect to age, HBV, HCV, syphilis co-infection and HIV-1 subtypes. The baseline clinical characteristics of 99 MSM with HIV-1 infections are given in Table 2.

Fig. 1. The occurrence of GBV-C viraemia in subjects at different stages of HIV infection (n = 99) and HIV-negative subjects (n = 175). The x-axis represents the time during which the samples were collected. The y-axis represents the percentage of GBV-C viraemia. P values represent the statistical analysis between each comparison as shown in the figure. Compared by Pearson's χ2 test, the significance level was set at 0·05. Visit 1: before HIV infection; visit 2: near HIV infection; visit 3: 12 months post-HIV infection.
Table 2. Baseline clinical characteristics of 99 HIV-positive MSM with or without GBV-C infection

MSM, Men who have sex with men; IQR, interquartile range.
* Mann–Whitney test.
† Two-sided Pearson's χ2 test.
GBV-C genotypes
Following the determination of GBV-C prevalence in 99 HIV-1-positive and 175 HIV-1-negative subjects, the nucleotide sequences of PCR products were analysed to determine the GBV-C genotypes. Reference sequences of four genotypes (1, 2, 3, 5) were included in this analysis. Of the 34 samples from HIV-1-positive subjects, 30 (88·2%) were GBV-C genotype 3, while the remaining four (11·8%) were genotype 2 according to comparison with the reference strains (Fig. 2). Of the 22 samples from HIV-1-negative subjects, one (4·5%) sample was genotype 2, and 21 (95·5%) samples belonged to genotype 3. The genetic distances were small between the viruses from HIV-1-positive subjects and those from HIV-1-negative subjects (Figs 2, 3). These results indicated that the majority of GBV-C in MSM in Beijing belonged to genotype 3. During HIV-1 infection, both genotypes 2 and 3 were transmissible.

Fig. 2. Phylogenetic tree of 34 GBV-C strains from 34 HIV-infected Chinese MSM subjects (indicated by pt. prefix) based on the sequences of the 5′-UTR gene (300 bp). The phylogenetic tree was constructed by the neighbour-joining method and the evolutionary distances were computed using the maximum composite likelihood method of Mega software (version 5.0). Reference strains of genotypes 1 (U36380), 2 (U44402 and AF081782 from China), 3 (D90601) and 5 (AY949711) were obtained from GenBank. The scale bar represents 0·5% genetic distance (0·005 substitution per site).

Fig. 3. Phylogenetic tree of 22 GBV-C strains from 22 HIV-1-negative MSM subjects (indicated by NG prefix) based on the sequences of the 5′-UTR gene (300 bp). The phylogenetic tree was constructed by the neighbour-joining method and the evolutionary distances were computed using the maximum composite likelihood method of Mega software (version 5.0). Reference strains of genotypes 1 (U36380), 2 (U44402 and AF081782 from China), 3 (D90601) and 5 (AY949711) were obtained from GenBank. The scale bar represents 0·5% genetic distance (0·005 substitution per site).
GBV-C viral loads at the acute and chronic stages of HIV-1 infection
The GBV-C viral load in each of 26 HIV-1-positive subjects [one case started antiretroviral therapy (ART) 3 months after HIV-1 acquisition in the follow-up study and was excluded from the analysis] at two time points (acute and chronic stages) of HIV infection were quantified with a real-time PCR. The GBV-C viral loads at the acute stage of HIV-1 infection ranged from 1·62 × 103 to 2·82 × 106 copies/ml with a median of 5·24 log copies/ml. At 12 months post-HIV-1 acquisition (chronic stage), the viral loads ranged from 3·24 × 103 to 3·80 × 106 copies/ml with a median of 5·56 log copies/ml. No significant difference was found between the GBV-C viral loads at acute stage and those at the chronic stage (Fig. 4).

Fig. 4. Comparison of the GBV-C viral loads at the acute and chronic stages of HIV infection in HIV-1-positive subjects (n = 26, one case started ART 3 months after acquiring HIV-1 and was excluded from the analysis.). The x-axis represents the stage of HIV infection, ‘Early’ indicates the acute stage of HIV infection and ‘Late’ indicates the chronic stage of HIV infection. The y-axis represents the GBV-C viral loads (log copies/ml). Evaluated by paired Student's t test; significance level was set at 0·05.
Comparison of HIV-1 viral loads and CD4+ T-cell counts between subjects with and without GBV-C infection
To evaluate the potential effect of GBV-C co-infection on the replication of HIV-1, we analysed the HIV viral loads and CD4+ T-cell counts in the GBV-C-positive and -negative subjects at the acute and chronic stages of HIV-1 infection. In the GBV-C RNA-positive subjects, the median HIV viral load was 4·29 log copies/ml [25–75%, interquartile range (IQR) 3·69–5·51], and the median CD4+ T-cell count was 423 cells/mm3 (IQR 361–587) at the acute stage. In the GBV-C RNA-negative subjects, the median viral load was 4·81 log copies/ml (IQR 4·00–5·57), and the median CD4+ T-cell count was 502 cells/mm3 (IQR 327–622). Statistical analysis showed that both CD4+ T-cell counts and HIV-1 viral load were not significantly different between the two groups at the acute stage of HIV-1 infection (P = 0·34, P = 0·5, respectively) (Table 2).
In the follow-up study, one case in the GBV-C-positive group and two cases in the GBV-C-negative group started ART 3 months after acquiring HIV-1. Seven cases in the GBV-C-negative group acquired GBV-C viraemia 12 months post-HIV-1 acquisition. Thus, the case numbers in the GBV-C-positive and -negative groups became 26 and 63, respectively. The median of HIV-1 viral load for 26 GBV-C-positive subjects and 63 GBV-C-negative subjects was 4·65 log10 copies/ml (IQR 3·86–5·03) and 4·41 log10 copies/ml (IQR 3·99–4·97), respectively, at 12 months post-HIV-1 acquisition. Statistical analysis showed that HIV-1 viral load was not significantly different between these two groups (P = 0·47). The median CD4+ T-cell counts were 431 cells/mm3 (IQR 281–542) and 435 cells/mm3 (IQR 313–613) in the GBV-C positive and negative groups, respectively. Statistical analysis showed that there was no significant difference between CD4+ T-cell counts in the GBV-C-positive and GBV-C-negative subjects (P = 0·31).
We then conducted a correlation analysis between GBV-C viral load and HIV-1 viral load or CD4+ T-cell count at the acute or chronic stages of HIV-1 infection. At the acute stage, a significant positive correlation was observed between the GBV-C viral load and CD4+ T-cell count (r = 0·42, P = 0·03) (Fig. 5). HIV-1 viral load was inversely and not significantly correlated with the GBV-C viral load (r = − 0·17, P = 0·39). At the chronic stage (12 months post-HIV-1 acquisition), we performed the same analyses excluding the ART cases, and found a persistent positive correlation between GBV-C viral loads and CD4+ T-cell counts at this time point, but the correlation was not significant (r = 0·09, P = 0·67) (Fig. 6). There was a persistent negative, but not significant, correlation between GBV-C viral load and HIV-1 viral load (r = − 0·2, P = 0·34) (data not shown) at the chronic stage.

Fig. 5. Correlation between GBV-C viral loads and CD4+ T-cell counts at the acute stage of HIV-1 infection in ART-naive Chinese MSM subjects (n = 27). The x-axis represents the GBV-C viral loads (log copies/ml). The y-axis represents the CD4 cell counts (cells/mm3). The GBV-C viral loads showed a significant positive correlation (r = 0·42, P = 0·03) with the CD4+ T-cell counts. The significance level was set at 0·05 in the linear regression analysis.

Fig. 6. Correlation between GBV-C viral loads and the CD4+ T-cell counts at the chronic stage (12 months post-HIV-1 acquisition) of HIV-1 infection in Chinese MSM subjects (n = 26, one case started ART 3 months after acquiring HIV-1 and was excluded from the analysis). The correlation was still positive but no longer significant (r = 0·09, P = 0·67). The x-axis represents the GBV-C viral loads (log copies/ml). The y-axis represents the CD4 cell counts (cells/mm3). The significance level was set at 0·05 in the linear regression analysis.
DISCUSSION
Previous studies have shown that GBV-C is prevalent in haemodialysis patients, commercial blood donors [Reference Wang24], intravenous drug users [Reference Wu25] and female commercial sex workers [Reference Wu26] in China. To our knowledge, the current study is the first to describe the prevalence of GBV-C viraemia in the MSM population with or without HIV-1 infection in China. The positive rate of GBV-C viraemia in subjects with HIV-1 infections was 27·2% (27/99) at the time of HIV-1 acquisition, which was much higher than that in the HIV-1-negative subjects (P = 0·002). These results are consistent with previous reports showing that the prevalence of GBV-C viraemia in HIV-1-positive Americans and Europeans was higher than in HIV-1-negative subjects [Reference Van der Bij14, Reference Ibáñez27–Reference Berzsenyi29]. Our data also indicate that GBV-C infection is common in recently infected HIV-1 MSM populations in Beijing, China.
The purpose of this study was to determine if pre-existence of GBV-C viraemia confers beneficial effects for resistance to HIV-1 infection. Our case-control study showed that the positive rate of GBV-C in the 99 HIV-1-positive subjects prior to acquisition of HIV-1 infection was not significantly different from that in HIV-1-negative subjects (P = 0·29), suggesting that the pre-existence of GBV-C viraemia does not play a significant role in resistance to HIV-1 infection. These results are consistent with a previous study showing that the prevalence of GBV-C viraemia in HIV-uninfected individuals who did and did not acquire HIV was similar in American populations [Reference Bisson30]. Our results also showed that after HIV-1 acquisition, the positive rate of GBV-C significantly increased (Fig. 1), indicating GBV-C infection may occur concurrently with HIV-1 acquisition. In the follow-up study, seven cases acquired GBV-C viraemia among HIV-1-infected individuals at 12 months post-HIV-1 infection, resulting in an incidence density of 9·7/100 person-years. None of the subjects acquired new GBV-C viraemia in the HIV-negative group during this period, indicating the high incidence density of GBV-C viraemia in the MSM populations with HIV infection. In our study, the occurrence of syphilis was high in HIV-1-positive subjects. This result was in accord with a previous cross-sectional study on MSM in Beijing [Reference Zhang21]. In addition, we did not find a high prevalence of HCV and HBV in MSM with or without HIV-1 infections.
According to some reports of the past two decades, the major genotype of GBV-C is genotype 3. Few virus strains of genotypes 2 and 1 have been reported in different populations in China [Reference Cao31, Reference Yu32]. Genotypic analysis in this study showed that MSM subjects were mainly infected with the genotype 3 strain of GBV-C (88·2%). Only four subjects were infected with genotype 2 strain and no subjects were infected with genotype 1 strain. Therefore, we concluded that the major GBV-C genotype prevalent in the MSM population was similar to that in other populations in China. Our results also agree with the viral genotype prevalent in other Asian countries [Reference Yu32, Reference Hattori34]. We attempted to identify if the genotypes had any effect on the viral loads of GBV-C. Our results showed that there was no significant relationship between the genotype and viral loads of GBV-C (data not shown). This differs from a previous report [Reference Hattori34] showing that GBV-C viral loads were associated with genotype. This difference may be due to the small numbers of genotype 2 in our study or differences in the progress of HIV-1 within the study population.
Previous studies have shown that GBV-C replication in peripheral blood mononuclear cells decreases the expression of HIV-1 co-receptors (chemokine receptors CCR5 and CXCR4) on the surface of CD4+ T cells and increases the production of various chemokines that serve as competitive inhibitors of the HIV-1 co-receptors [Reference Xiang18, Reference Jung35, Reference Schwarze-Zander36]. It is known that there is vigorous viral replication at the acute stage during the natural history of HIV-1 infection. Thus, we speculate that there are some complicated interactions between the two viruses at the acute stage of HIV-1 infection. Our results showed that the GBV-C viral loads had an ascending trend from the acute (5·24 log copies/ml) to chronic (5·56 log copies/ml) stage (Fig. 4). Statistical analysis showed a sustained negative correlation between the GBV-C viral loads and HIV-1 viral loads in the acute and chronic stages of HIV-1 infection. Although, we were unable to conclude that the lower GBV-C viral loads at the acute stage of HIV-1 infection was directly caused by higher HIV-1 viral loads, our results provide some evidence that an inhibitory interaction between these two viruses might exist during the period of co-infection.
Previous studies have suggested that HIV RNA levels at later time points are better indicators of long-term disease progression compared to the levels when HIV-1 was acquired because the viral load reaches a stable mean or ‘set point’ around 1 year post-infection [Reference de Wolf37, Reference Hubert38]. In this study, both plasma HIV RNA and the CD4+ T-cell counts did not differ between GBV-C-positive and GBV-C-negative subjects at 1 year post-HIV infection. These results indicate that GBV-C viraemia could not increase CD4+ T-cell counts or reduce HIV-1 viral load during the 1-year period following HIV-1 infection. These results also suggest that there was no significant suppressive effect by GBV-C on HIV-1 replication in the year following HIV-1 infection. Williams et al. reported that GBV-C viraemia was significantly associated with the prolonged survival of HIV-positive men 5–6 years post-HIV-1 infection. However, the survival rate was not increased 12–18 months post-HIV infection [Reference Williams39]. Thus, a long-term follow-up study should be conducted on this cohort.
The molecular mechanisms by which GBV-C suppresses the replication of HIV-1 are poorly understood and the effect of GBV-C viraemia on the progress of HIV disease is currently controversial. Several studies have shown that GBV-C viraemia could assist the elevation of CD4+ T-cell counts because a positive correlation between GBV-C viral load and CD4+ T-cell counts was observed [Reference Smith40, Reference Giret41]. Interestingly, in our study, a significant positive correlation between CD4+ T-cell counts and GBV-C viral loads (r = 0·42, P = 0·03) (Fig. 5) was observed at the acute stage of HIV-1 infection after 1 year, and the correlation was still positive but no longer significant (r = 0·09, P = 0·67) (Fig. 6). Because GBV-C RNA can replicate in CD4+ T cells, the decrease of CD4+ T cells during the course of HIV-1 infection implies a loss of target cells for GBV-C RNA. This might explain why the correlation between CD4+ T cells and GBV-C viral loads was weakened 1 year post-HIV infection as a consequence of a decrease in CD4+ T-cell counts. Our study can only reflect on the instance of male homosexuals who sexually transmit GBV-C, and does not include bloodborne transmission of HIV-1. In addition, the period of the current study is not long enough to observe the loss of GBV-C viraemia and the effect of sustained GBV-C viraemia on the progress of HIV/AIDS. In conclusion, this is the first cohort study, to the best of our knowledge, on GBV-C co-infection with HIV-1 in the MSM population of Beijing, China. Long-term observation is needed on this cohort to further reveal the correlation between GBV-C and HIV infection.
ACKNOWLEDGEMENTS
This study was supported by Beijing Science and Technology Programme (D09050703590901 to H. Wu); Natural Science Foundation of China (30870853 to D. Chen); HKU-UDF and LSK Faculty of Medicine Matching Fund for financial supports to HKU AIDS Institute (to Z. Chen).
DECLARATION OF INTEREST
None.










